Abstract
Glucagon-like peptide-1 receptor agonists (GLP-1RAs) are well established in clinical practice for the treatment of type 2 diabetes, and are approved and recommended for weight management in overweight or obesity. Gastrointestinal side effects are well known as the most common adverse effects of these agents and represent a potential barrier for use, particularly at higher doses. Drawing on both published evidence and our collective clinical experience, we aim to guide practitioners through managing these side effects with a view to optimizing therapeutic outcomes with GLP-1RAs.
© 2021 Dr Sean Wharton of Wharton Medical Clinic. Published by Informa UK Limited, trading as Taylor & Francis Group.
1. Introduction
Clinical guidelines for obesity typically recommend considering pharmacotherapy, such as treatment with a GLP-1RA, for achievement of weight loss and for weight loss maintenance in people with body mass index ≥30 kg/m2, or ≥27 kg/m2 with obesity-related complications, as an adjunct to lifestyle intervention [Citation10–12]. Pharmacotherapies may be initiated either after failure to lose weight or following weight regain during lifestyle intervention, or concurrently with initiation of lifestyle intervention, depending on the weight-loss needs of the patient [Citation11].
In clinical practice, the most common side effects of GLP-1RAs are gastrointestinal (GI), typically including upper-GI effects (e.g. nausea or vomiting) and/or lower-GI effects (diarrhea or constipation). These GI adverse effects appear to be dose-dependent and are likely a class effect [Citation1,Citation9]. They are typically transient, mild to moderate in severity, and mainly occur during initiation and up-titration of treatment. Constipation may last longer than other GI side effects [Citation4,Citation5], consistent with the more chronic nature of this condition. Although acute pancreatitis has been reported in patients treated with GLP-1RAs, and patients should be observed for any signs and symptoms of acute pancreatitis (e.g. persistent severe abdominal pain) [Citation2,Citation3,Citation8], large cardiovascular outcome trials have not shown an increased risk of pancreatitis with GLP-1RAs [Citation1]. Practitioners should also be aware that cholelithiasis (gallstones) may occur with rapid weight loss and an increased incidence has been reported in patients treated with GLP-1RAs for weight management – appropriate clinical follow-up is required if gallstones are suspected [Citation2–4,Citation7,Citation8,Citation13,Citation14].
Clinical trials in patients with T2D suggest that the incidence [Citation15] and time course [Citation16] of each type of GI side effect may vary between individual agents within the GLP-1RA class, but overall, nausea, diarrhea, and vomiting are most commonly reported, with incidences of ~15–30%, ~10–15%, and ~5–10% of participants, respectively [Citation15]. GI adverse events (AEs) lead to treatment discontinuation in a minority of patients, typically less than 5–10% in clinical trials in T2D [Citation16,Citation17] or overweight/obesity [Citation4–6,Citation14], though rates of discontinuation due to GI AEs are likely to be higher in clinical practice [Citation18]. Studies of GLP-1RAs in people with overweight/obesity suggest that GI AEs may occur more frequently with the higher doses of these agents used for weight management compared with the lower doses used for treatment of T2D, but the proportion discontinuing treatment owing to GI AEs remains low in overweight/obesity trials [Citation4,Citation5,Citation14]. Nausea is the most commonly reported GI symptom, although constipation appears more common in trials in obesity [Citation4–6] than in T2D [Citation16,Citation17].
The cause of GI side effects with GLP-1RAs is uncertain, but likely involves multiple different factors, with variation between GLP-1RAs and between patients in the contribution of each. Potential contributors include the duration of action of the GLP-1RA, with short-acting agents (currently only used for treatment of T2D) often considered to be associated with more nausea and vomiting and less diarrhea than longer-acting agents (used in T2D and for weight management) [Citation1,Citation9,Citation15]. Nausea is believed to arise from direct effects on the central nervous system, mediated by GLP-1 receptors in the area postrema [Citation1,Citation19]. Effects on GI function, such as delayed gastric emptying (primarily with short-acting agents) [Citation1,Citation9] and alterations in intestinal motility and transit time [Citation9,Citation20], may also contribute to the occurrence of GI side effects.
Real-world studies indicate a variable incidence of GI side effects, with some reporting similar incidences to those reported in clinical trials [Citation21] and others reporting lower incidences [Citation22]. However, GI side effects remain the most common reason for patients discontinuing treatment [Citation23]. To realize the benefits of GLP-1RA treatment for obesity, it is important that the potential GI side effects of these agents are explained to patients and mitigated with clinical strategies to enable treatment persistence. Herein, we provide guidance on how to manage these effects in clinical practice.
2. Management of GI side effects in clinical practice – the three ‘E’s
An individual patient’s experience of GI side effects (or lack thereof) with GLP-1RAs is unique, likely reflecting a combination of drug-specific and patient-related factors. A patient-centric approach to GI side effect management is therefore required, drawing on a range of potential strategies.
Randomized clinical trials with GLP-1RAs have typically not included any treatment strategies for management of GI side effects, with the exception of delayed dose escalation or dose reductions in some studies [Citation4,Citation24]. In addition, literature searching identified limited evidence from real-world studies on strategies for managing GI side effects during GLP-1RA treatment. Further clinical and real-world evidence would be beneficial to inform strategies for the management of such GI side effects. Given the limited clinical and real-world evidence, most recommendations described below are based on the clinical experience of the authors of this article. We recommend both pre-emptive techniques and severity-based stepwise management of any GI AEs that do arise, collectively encompassed by a proposed approach we describe as the three ‘E’s: Education and explanation, Escalation to an appropriate dose, and Effective management of GI side effects (discussed below and summarized in ).
Figure 1. Recommendations for managing gastrointestinal side effects with GLP-1RAs for weight management – the three ‘E’s.
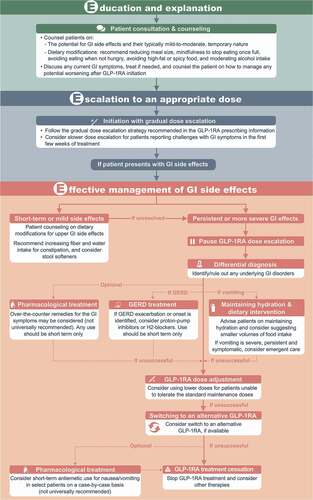
2.1. Education and explanation
All patients should be counseled about the possibility of side effects with GLP-1RA treatment to effectively manage their expectations. Discussions should highlight that most cases are mild to moderate, and nausea typically subsides after dose escalation is complete. We recommend discussing potential symptoms using non-technical terminology (e.g. stating that some patients report having nausea after taking the medication, but the nausea typically goes away in time and is usually not severe). Patients should be reassured that mild-to-moderate GI symptoms are not an indication that their treatment is going wrong.
Patients should be advised on potential management approaches that may be effective in mitigating side effects, including reducing meal size, mindfulness to stop eating once full, avoiding eating when not hungry, avoiding high-fat or spicy food (particularly during the initial dose-escalation period), and moderating intake of alcohol and fizzy drinks (particularly in the context of nausea and dyspepsia).
In addition, asking the patient about their bowel habits prior to treatment is recommended, particularly given GI disorders are common in people with overweight/obesity. If patients report GI disorders at baseline, consider addressing these (e.g. recommending increasing dietary fiber and water intake for patients with constipation) prior to starting GLP-1RA therapy and/or advising the patient on how to manage any potential worsening of such symptoms upon initiation of the GLP-1RA (e.g. over-the-counter medications for diarrhea). GLP-1RAs should typically be avoided in patients with pre-existing severe GI disease (e.g. gastroparesis) [Citation3].
2.2. Escalation to an appropriate dose
Gradual dose escalation is recommended in the prescribing information for most GLP-1RAs and may help reduce GI side effects [Citation1,Citation13], and should therefore be standard practice. While clinical trials with GLP-1RAs typically follow strict titration regimens to allow assessment of efficacy, clinical practice allows the flexibility to escalate doses more slowly, particularly for patients who report challenges with GI symptoms in the first few weeks of treatment. Although some studies suggest that more conservative or flexible dose escalation does not notably improve tolerability (e.g. Pieber et al. 2019 [Citation24]), such studies have not allowed for the completely open and flexible approach to dose escalation that can be used on an individualized basis in clinical practice. The outcome of individualized dose escalation relies on effective interaction between the patient and health-care provider, with the goal of attaining a dose that aids weight loss while also being tolerable for the patient.
2.3. Effective management of GI side effects
In patients who report troublesome GI symptoms, we recommend a stepwise, severity-based approach to management, as outlined below.
2.3.1. Management of short-term or mild GI side effects
If patients report upper GI side effects of short duration or mild severity, we suggest initial patient counseling on dietary modifications (e.g. decreasing the volume of food intake at each sitting and mindfulness to stop eating when full). Clinical and real-world studies indicate that constipation is common in patients with overweight/obesity treated with GLP-1RAs [Citation4–6,Citation14,Citation21], and therefore strategies to manage constipation are also important – increasing fiber and water intake can be advised, and stool softeners considered. Patients should be advised to monitor these side effects, and clinicians should consider reducing the GLP-1RA dose if symptoms worsen.
2.3.2. Management of persistent or more severe GI side effects
If patients report GI side effects that are more persistent or severe, we recommend pausing dose escalation and taking the following steps (see also )
1. Differential diagnosis
Clinicians should first consider any other conditions that may be causing these symptoms and influence the required treatment approach. For example, in clinical practice, we have seen patients who experience transient worsening or new onset of gastroesophageal reflux disease (GERD; a known complication of obesity) during GLP-1RA treatment – management of such cases requires treatment of the underlying GERD. Medications such as proton-pump inhibitors or H2-blockers can be considered on a temporary basis (or current treatment for pre-existing GERD increased where appropriate), if needed to manage exacerbation of GERD during the GLP-1RA dose-escalation phase. It should also be noted that GERD can improve with weight loss.
Patients presenting with abdominal pain should be fully evaluated as per standard clinical practice, irrespective of GLP-1RA use. The nature of the pain and physical examination findings should direct the scope of further work-up, potentially including laboratory tests and/or diagnostic imaging. In patients with symptoms that could indicate acute pancreatitis (persistent severe abdominal pain, sometimes radiating to the back, with or without vomiting), GLP-1RA treatment should be stopped and appropriate management for suspected pancreatitis should be undertaken [Citation2,Citation3,Citation8]. The GLP-1RA should not be restarted if pancreatitis is confirmed. In addition, as noted earlier, given cholelithiasis risk increases with rapid weight loss and has been reported with GLP-1RAs, if cholelithiasis is suspected appropriate gallbladder studies and clinical follow-up should be undertaken [Citation2–4,Citation8,Citation13,Citation14].
Clinicians should also consider whether any existing or recently initiated concomitant prescription or non-prescription medications could be responsible for, or contribute to, newly emerging GI disorders. For example, metformin can cause GI side effects and has been reported to be associated with an increased risk of GI AEs with GLP-1RAs in patients with T2D [Citation15]. In addition, all other approved anti-obesity medications are known to be associated with GI side effects [Citation11]. Evaluating the use of concomitant medications that could be a cause or contributor to GI side effects is therefore appropriate.
In particular, it is important to investigate the potential for alternative causes of GI disorders that present after a prolonged period of stable GLP-1RA treatment, as such cases are less likely to relate to the GLP-1RA. For example, viral gastroenteritis may occur and can be clearly identified with a careful medical history. A temporary adjustment in GLP-1RA dose may be required in this instance. In the event that symptoms indicate a need for upper endoscopy, consider stopping GLP-1RA treatment 1–2 weeks prior to endoscopy.
2. Maintaining hydration and dietary intervention
For patients with vomiting, maintaining hydration is important and patients should be advised accordingly. Dietary measures, such as smaller volumes of food intake, possibly more frequently if needed, may also be helpful. If vomiting persists and is excessive and also associated with dizziness, confusion, and fatigue, we recommend more emergent care, in line with standard clinical management of severe vomiting (which may require intravenous rehydration, although fortunately occurs rarely). Whether to decrease or stop GLP-1RA treatment in a patient who has experienced vomiting needs to be assessed on a case-by-case basis.
3. Pharmacological treatment
Over-the-counter remedies for the GI disorders may be considered, but are not universally recommended by the authors of this article.
Short-term use of antiemetics has been proposed as a potential strategy for managing nausea and vomiting with GLP-1RAs [Citation13], although evidence is limited. While some of the authors of this article occasionally use antiemetics for selected patients, typically we suggest attempting other approaches (e.g. dose reduction) in preference to antiemetics (). While evidence is lacking for treating GERD due to GLP-1RAs, anecdotally we have found temporary use of proton-pump inhibitors or H2-blockers to be useful in managing GERD, as noted above.
Any medications used to manage GI side effects during the titration phase should generally not be utilized as a long-term treatment strategy for chronic GLP-1RA-induced side effects. If side effects persist that require medication to manage them for over a month after achieving the desired maintenance dose, consideration should be given to decreasing the GLP-1RA to a dose that can be tolerated without needing additional medication to manage the side effects.
4. Dose adjustment
Given the dose-dependent nature of GI AEs [Citation1,Citation9], a lower GLP-1RA dosage (if available) could be considered for patients unable to tolerate GI AEs at recommended maintenance doses. An individualized assessment of the efficacy/tolerance balance is required, with the aim of achieving a dose that enables patients to gain at least some of the benefits of GLP-1RAs, with minimal GI side effects.
If GI symptoms occur and dose escalation is paused or the dose reduced, dose escalation can be retried once the patient is symptom-free at the lower dose, if required to aid weight loss. We would suggest any such escalation should be slower than previously used. Many patients who have experienced GI side effects are able to achieve the full dose of the medication in clinical practice, although the time required for titration to full dose can be longer than recommended in the prescribing information. In a real-world study with liraglutide in patients with obesity, the median time to maintenance dose (3.0 mg) was approximately 50 days [Citation25], considerably longer than the 28 days recommended in the prescribing information [Citation2,Citation3].
5. Switching to an alternative GLP-1RA
Occasionally, patients are not able to tolerate even a very low dose of a GLP-1RA [Citation1,Citation24], in which case treatment with the GLP-1RA should be stopped. Trying an alternative GLP-1RA may be a reasonable approach in such patients, given the variation in GI tolerability profile between GLP-1RAs [Citation1,Citation9,Citation15], and has been proposed by other authors in the context of switching between GLP-1RAs in T2D [Citation26,Citation27]. With semaglutide 2.4 mg now approved for weight management in the USA [Citation8], it is likely that some patients may be switched between once-daily liraglutide (the only other GLP-1RA approved for weight management) and once-weekly semaglutide, for efficacy, tolerability, or convenience reasons. Although evidence is currently lacking, in such circumstances a rational approach could be to discontinue the first GLP-1RA, wait for any GI symptoms (if present) to resolve, and then initiate the new GLP-1RA using the recommended dose-escalation regimen from the prescribing information – the time course for resolution of GI symptoms can be variable, from 1–2 days to 1–2 weeks.
Given differences in how semaglutide and liraglutide interact with the brain [Citation1], GI side effects may differ between the two agents. Greater insight into the relative efficacy and safety profiles of once-weekly semaglutide 2.4 mg and once-daily liraglutide 3.0 mg in patients with overweight/obesity is anticipated to be provided by the ongoing STEP 8 trial (Clinicaltrials.gov ID NCT04074161), which may help inform treatment decisions.
6. Treatment cessation and switching to a different class of obesity pharmacotherapy
If patients experience GI side effects with GLP-1RAs and are unable to tolerate them, despite best efforts to alleviate the symptoms, treatment should be stopped. Switching to a different class of obesity pharmacotherapy could be considered, if appropriate.
3. Conclusion
GI side effects are common with GLP-1RAs, usually present during initiation and titration, and are typically transient and mild to moderate in severity. We recommend the following approach: Education and explanation to help patients understand the potential for GI side effects and how to mitigate their impact; Escalation to an appropriate dose using a gradual individualized approach; and Effective management of troublesome GI side effects, should they arise, using a stepwise severity-based approach ().
Declaration of financial interests
Sean Wharton: research funding, advisory/consulting fees, and/or other support from AstraZeneca, Bausch Health Inc., Boehringer Ingelheim, CIHR, Janssen, Lilly, and Novo Nordisk.
Melanie Davies: consultant, advisory board member and speaker for Novo Nordisk, Sanofi, Lilly, and Boehringer Ingelheim, advisory board member and speaker for AstraZeneca, advisory board member for Janssen, Lexicon, Servier, and Gilead Sciences Ltd, and speaker for Napp Pharmaceuticals, Mitsubishi Tanabe Pharma Corporation, and Takeda Pharmaceuticals International Inc.; research funding from AstraZeneca, Boehringer Ingelheim, Janssen, Lilly, Novo Nordisk, and Sanofi-Aventis. Melanie Davies is co-funded by the NIHR Leicester Biomedical Research Centre.
Dror Dicker: consultant, advisory board member, and speaker for AstraZeneca, Boehringer Ingelheim, Eli Lilly, and Novo Nordisk; research funding from AstraZeneca, Boehringer Ingelheim, Eli Lilly, and Novo Nordisk.
Ildiko Lingvay: research funding, advisory/consulting fees, and/or other support from AstraZeneca, Bayer, Boehringer Ingelheim, Eli Lilly, GI Dynamics, Intarcia, Intercept, Janssen, Mannkind, Merck, Mylan, Novartis, Novo Nordisk, Pfizer, Sanofi, TARGETPharma, Valeritas, and Zealand Pharma.
Ofri Mosenzon: advisory board member for AstraZeneca, Boehringer Ingelheim, BOL Pharma, Eli Lilly, Merck Sharp & Dohme, Novo Nordisk, and Sanofi; research grant support through Hadassah Hebrew University Hospital from AstraZeneca and Novo Nordisk; speaker’s bureau for AstraZeneca, Boehringer Ingelheim, Eli Lilly, Janssen, Merck Sharp & Dohme, Novo Nordisk, and Sanofi.
Domenica M. Rubino: consultant, advisory board member, speaker, and clinical investigator for Novo Nordisk; clinical investigator for AstraZeneca and Boehringer Ingelheim; honoraria from Medscape; research funding from Obesinov and SARL; holds stock shares in Novo Nordisk.
Sue D. Pedersen: consulting fees and/or speaking honoraria from Abbott, AstraZeneca, Bausch, Bayer, Boehringer Ingelheim, Dexcom, HLS, Janssen, Lilly, Merck, Novo Nordisk, and Sanofi; research studies for Abbott, AstraZeneca, Bausch, Boehringer Ingelheim, Janssen, Lilly, Merck, Novo Nordisk, and Sanofi.
Acknowledgments
The authors thank Elham Kamran of the Wharton Weight Management Clinic (Toronto, ON, Canada) for research and administration support. In addition, the authors thank Laura Ward and Nicola Beadle of Axis, a division of Spirit Medical Communications Group Ltd., and Peter Birch, contract writer working on behalf of Axis, who provided medical writing assistance under the direction of the authors (funded by Novo Nordisk A/S, Denmark, which was provided with the opportunity to perform a medical accuracy review).
Disclosure statement
The authors have no other relevant financial or other conflicts of interest to disclose apart from those stated. Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
References
- Nauck MA, Quast DR, Wefers J, et al. GLP-1 receptor agonists in the treatment of type 2 diabetes - state-of-the-art. Mol Metab. 2021;46:101102.
- SAXENDA (liraglutide) injection prescribing information. 2020 [cited 2021 Apr 21]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/206321s012s013s014lbl.pdf
- Saxenda summary of product characteristics. 2021 [cited 2021 Apr 21] Available from: https://www.ema.europa.eu/en/documents/product-information/saxenda-epar-product-information_en.pdf
- Wilding JPH, Batterham RL, Calanna S, et al. Once-weekly semaglutide in adults with overweight or obesity. N Engl J Med. 2021;384(11):989.
- Davies M, Færch L, Jeppesen OK, et al. Semaglutide 2·4 mg once a week in adults with overweight or obesity, and type 2 diabetes (STEP 2): a randomised, double-blind, double-dummy, placebo-controlled, phase 3 trial. Lancet. 2021;397(10278):971–984.
- Wadden TA, Bailey TS, Billings LK, et al. Effect of subcutaneous semaglutide vs placebo as an adjunct to intensive behavioral therapy on body weight in adults with overweight or obesity: the STEP 3 randomized clinical trial. JAMA. 2021;325(14):1403–1413.
- Rubino D, Abrahamsson N, Davies M, et al. Effect of continued weekly subcutaneous semaglutide vs placebo on weight loss maintenance in adults with overweight or obesity: the STEP 4 randomized clinical trial. JAMA. 2021;325(14):1414–1425.
- WEGOVY (semaglutide) injection prescribing information. 2021 [cited 2021 Jun 9] Available from https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/215256s000lbl.pdf
- Nauck MA, Meier JJ. Management of endocrine disease: are all GLP-1 agonists equal in the treatment of type 2 diabetes? Eur J Endocrinol. 2019;181(6):R211–R234.
- Wharton S, Lau DCW, Vallis M, et al. Obesity in adults: a clinical practice guideline. CMAJ. 2020;192(31):E875–E891.
- Garvey WT, Mechanick JI, and Brett EM, et al. American Association of Clinical Endocrinologists and American College of Endocrinology comprehensive clinical practice guidelines for medical care of patients with obesity. Endocr Pract. 2016;22(Suppl 3):1–203.
- Yumuk V, Tsigos C, Fried M, et al. European guidelines for obesity management in adults. Obes Facts. 2015;8(6):402–424.
- Gill L, Mackey S. Obstetrician-gynecologists‘ strategies for patient initiation and maintenance of antiobesity treatment with glucagon-like peptide-1 receptor agonists. J Womens Health (Larchmt). 2021;30(7):1016–1027.
- Pi-Sunyer X, Astrup A, Fujioka K, et al. A randomized, controlled trial of 3.0 mg of liraglutide in weight management. N Engl J Med. 2015;373(1):11–22.
- Bettge K, Kahle M, Abd El Aziz MS, et al. Occurrence of nausea, vomiting and diarrhoea reported as adverse events in clinical trials studying glucagon-like peptide-1 receptor agonists: a systematic analysis of published clinical trials. Diabetes Obes Metab. 2017;19(3):336–347.
- Pratley R, Amod A, Hoff ST, et al. Oral semaglutide versus subcutaneous liraglutide and placebo in type 2 diabetes (PIONEER 4): a randomised, double-blind, phase 3a trial. Lancet. 2019;394(10192):39–50.
- Pratley RE, Aroda VR, Lingvay I, et al. Semaglutide versus dulaglutide once weekly in patients with type 2 diabetes (SUSTAIN 7): a randomised, open-label, phase 3b trial. Lancet Diabetes Endocrinol. 2018;6(4):275–286.
- Trujillo JM, Nuffer W, Smith BA. GLP-1 receptor agonists: an updated review of head-to-head clinical studies. Ther Adv Endocrinol Metab. 2021;12:1–15.
- Kanoski SE, Hayes MR, Skibicka KP. GLP-1 and weight loss: unraveling the diverse neural circuitry. Am J Physiol Regul Integr Comp Physiol. 2016;310(10):R885–R895.
- Thazhath SS, Marathe CS, Wu T, et al. The glucagon-like peptide 1 receptor agonist exenatide inhibits small intestinal motility, flow, transit, and absorption of glucose in healthy subjects and patients with type 2 diabetes: a randomized controlled trial. Diabetes. 2016;65(1):269–275.
- Wharton S, Kuk JL, Luszczynski M, et al. Liraglutide 3.0 mg for the management of insufficient weight loss or excessive weight regain post-bariatric surgery. Clin Obes. 2019;9(4):e12323.
- Gautier JF, Martinez L, and Penfornis A, et al. Effectiveness and persistence with liraglutide among patients with type 2 diabetes in routine clinical practice--EVIDENCE: a prospective, 2-year follow-up, observational, post-marketing study. Adv Ther. 2015;32(9):838–853.
- Sikirica MV, Martin AA, Wood R, et al. Reasons for discontinuation of GLP1 receptor agonists: data from a real-world cross-sectional survey of physicians and their patients with type 2 diabetes. Diabetes Metab Syndr Obes. 2017;10:403–412.
- Pieber TR, Bode B, Mertens A, et al. Efficacy and safety of oral semaglutide with flexible dose adjustment versus sitagliptin in type 2 diabetes (PIONEER 7): a multicentre, open-label, randomised, phase 3a trial. Lancet Diabetes Endocrinol. 2019;7(7):528–539.
- Wharton S, Haase CL, Kamran E, et al. Weight loss and persistence with liraglutide 3.0 mg by obesity class in the real-world effectiveness study in Canada. Obes Sci Pract. 2020;6(4):439–444.
- Almandoz JP, Lingvay I, Morales J, et al. Switching between glucagon-like peptide-1 receptor agonists: rationale and practical guidance. Clin Diabetes. 2020;38(4):390–402.
- Jain Ab, Ali A, Gorgojo Martínez JJ, et al. Switching between GLP-1 receptor agonists in clinical practice: expert consensus and practical guidance. Int J Clin Pract. 2021;75(2):e13731.
