ABSTRACT
Background
Acute pancreatitis (AP) is the most common pancreatic disease. Predicting the severity of AP is critical for making preventive decisions. However, the performance of existing scoring systems in predicting AP severity was not satisfactory. The purpose of this study was to develop predictive models for the severity of AP using machine learning (ML) algorithms and explore the important predictors that affected the prediction results.
Methods
The data of 441 patients in the Department of Gastroenterology in our hospital were analyzed retrospectively. The demographic data, blood routine and blood biochemical indexes, and the CTSI score were collected to develop five different ML predictive models to predict the severity of AP. The performance of the models was evaluated by the area under the receiver operating characteristic curve (AUC). The important predictors were determined by ranking the feature importance of the predictive factors.
Results
Compared to other ML models, the extreme gradient boosting model (XGBoost) showed better performance in predicting severe AP, with an AUC of 0.906, an accuracy of 0.902, a sensitivity of 0.700, a specificity of 0.961, and a F1 score of 0.764. Further analysis showed that the CTSI score, ALB, LDH, and NEUT were the important predictors of the severity of AP.
Conclusion
The results showed that the XGBoost algorithm can accurately predict the severity of AP, which can provide an assistance for the clinicians to identify severe AP at an early stage.
Introduction
Acute pancreatitis (AP) is one of the most common hospitalization diagnoses in the Gastroenterology Department, and its incidence has been reported increasing [Citation1,Citation2]. In 2019, about 2.8 million people worldwide were diagnosed with AP, and more than 110,000 deaths were caused by it [Citation3]. In the United Stated, there were about $2.5 billion used AP treatment and care each year, and the per capita hospitalization expenditure exceeded $30,000 [Citation1,Citation4]. The course of AP is mostly self-limiting, and the patients could recover within several days. However, about 20% of the patients will eventually develop into severe acute pancreatitis (SAP), accompanied by systemic inflammatory response syndrome (SIRS) and persistent multiple organ failure (OF), and the mortality rate is as high as 30% to 50% [Citation4,Citation5]. Therefore, early identification and classification of AP severity is of great significance to take measures to prevent SAP, which will improve the prognosis of the patients.
At present, several scoring tools such as the acute physiology and chronic health examination II (APACHE II) score, Ranson score, and the bedside index of severity in acute pancreatitis (BISAP) score have been applied to predict the severity of AP. However, they still have certain limitations. The APACHE II score is not specially developed for AP, and it is very complex, which contains some variables that need to be measured in ICU; the Ranson score needs to collect data in different time periods within 48 hours after admission to complete, which leads to the miss of critical treatment time window, so it is not suitable for early prediction; although the BISAP score is simpler and easy to quickly evaluate, its sensitivity is not ideal compared with other scores in predicting AP severity [Citation6–8]. In addition, studies have shown that the existing scoring tools only showed moderate predictive performance in predicting the severity of AP, and the AUC did not exceed 0.80, which indicated that simple scoring systems constructed by conventional statistical algorithms seemed to have reached their maximal utility [Citation9,Citation10].
As the core of artificial intelligence, machine learning (ML) has been increasingly used in disease diagnosis and classification, complication prediction, medication abnormality detection and other predictive tasks in recent years due to its ability to analyze the nonlinear interactions between predictive variables that superior to conventional statistical algorithms [Citation11]. Therefore, in this study, we aimed to use different ML algorithms and widely incorporate patients’ demographic characteristics, blood indexes and imaging score to develop predictive models, so as to obtain the best tool for early prediction of the severity of AP, and on this basis, explore the important predictors that affected the prediction results.
Material and methods
Patients
This retrospective observational study was conducted based on the electronic health records of the Affiliated Hospital of Yangzhou University. We collected the data of all patients diagnosed with AP and admitted to the Gastroenterology Department from January 2013 to December 2020. The diagnosis and severity classification of AP were based on the revised Atlanta classification of acute pancreatitis 2012. The diagnosis needed to satisfy at least two of the following features: (1) sudden abdominal pain, (2) the level of serum lipase or amylase exceeded three times the upper limit of normal, and (3) characteristic findings of AP on contrast enhanced computerized tomography, magnetic resonance, or transabdominal ultrasonographic [Citation5]. To ensure the homogeneity of patients’ underlying conditions, patients under 18 years old, pregnant, with pancreatic cancer, and with severe circulatory, respiratory, liver and kidney diseases were excluded from this study. All patients received standard treatments after admission according to the guidelines of AP management [Citation12]. This study was conducted in accordance with the principles of the Declaration of Helsinki. The protocol was approved by the Ethics Committee of the Affiliated Hospital of Yangzhou University (2018-YKL11-27). Due to the retrospective and anonymous nature, patients’ informed consent was not required.
Data collection
The selection of predictors used to construct models were based on literature search and experts’ suggestions. Predictive factors included demographic data (gender, age), etiology (biliary, alcoholic, hyperlipemia, and others), past history (diabetes, hypertension, smoking, and drinking), blood routine indexes (white blood cells (WBC), neutrophil (NEUT), platelet (PLT), hematocrit (HCT), hemoglobin(Hb), monocyte count (MONO)), blood biochemical indexes (serum amylase (AMY), total bilirubin (TB), serum sodium (Na+), serum potassium (K+), serum calcium (Ca2+), lactate dehydrogenase (LDH), blood urea nitrogen (BUN), high density lipoprotein cholesterol-to-low density lipoprotein cholesterol ratio (HDL/LDL), albumin (ALB), aspartate aminotransferase (AST), alanine aminotransferase (ALT), serum apolipoprotein B-to- apolipoprotein A1 ratio (apoB/apoA1), serum creatinine (sCr), triglycerides (TG), serum alkaline phosphatase (ALP), glucose (Glu)), and CT severity index (CTSI), a total of 30 predictors. Professional registered nurses used standardized questionnaires to inquire and record patients’ demographic data and past history at admission. A history of hypertension was defined as systolic blood pressure ≥140 mmHg or diastolic blood pressure ≥90 mmHg three times on different days in a resting state without taking antihypertensive drugs [Citation13]. Diabetes history was based on patients’ self-reports, or fasting plasma glucose ≥7.0 mmol/L, or 2-h plasma glucose ≥11.0 mmol/L during the oral glucose tolerance test [Citation14]. Smoking history was defined as smoking more than one cigarette per day for six consecutive months, and the drinking history referred to regular drinking habits at least once a week for more than six months [Citation15]. The blood routine tests were performed on the Sysmex XN-9000 (Sysmex Corporation, Kobe, Japan) analyzer, and the blood biochemical tests were performed using a spectrophotometric method on the Beckman Coulter AU 5800 (Beckman Coulter, Bear, CA, USA) analyzer. The blood index records of patients at admission were included in the dataset of this study, and if there were multiple test results, the highest value was taken. All patients underwent CT examination after admission, and the CTSI score was evaluated by two radiologists with more five years of experience. Any disagreement was resolved through negotiation.
Statistics
Continuous variables were expressed as median and interquartile range, and categorial data were expressed as frequency and proportion. Kolmogorov-Smirnov test was used to test the normality of data. The Student’s t-test, nonparametric Mann-Whitney test, and Pearson χ2 test were used to compare continuous variables with normal and skewed distribution, and categorical variables. Statistical analysis was conducted using SPSS 26.0 software. P value <0.05 was considered statistically significant.
Model construction
Five ML algorithms, logistic regression (LR), support vector machine (SVM), decision tree (DT), random forest (RF), and extreme gradient boosting (XGBoost), were chosen to develop models to predict the severity of AP. Before data preprocessing, we randomly divided the dataset into 70% training set and 30% testing set to prevent the leakage of data information. The training set was used for model construction and hyper-parameters tuning, and the testing set was used to assess the generalization performance of the models. Continuous variables were standardized using Minmaxscale, and categorical variables were coded by one-hot encoding. The MissForest package was used to fill missing values. Subsequently, a grid search with cross-validation was performed to adjust the hyper-parameters of the classifiers to obtain the models that performed best on the training set. The construction of ML models was conducted using Python 3.8.5 and the scikit-learn package 0.23.2.
Model evaluation
Based on the results of confusion matrix, the area under the receiver operating curve (AUC), accuracy, sensitivity, specificity, and F1 score were adopted to evaluate the performance of the models.
Results
Patient characteristics
A total of 441 patients were included in this study. According to the revised Atlanta classification criteria, 342 patients were mild acute pancreatitis (MAP), 70 patients were moderately severe acute pancreatitis (MSAP), and 29 patients were SAP. The proportion of male patients was 63.7%, and the age of the patients ranged from 18 to 91 years old, with a median age of 47 years old. Hypertriglyceridemia (49.2%) and biliary disease (23.6%) were the common causes of AP. We defined the MAP patients as the non-severe group (77.6%), and the MSAP and SAP patients were defined as the severe group (22.4%).
summarized the demographic and clinical characteristics of the patients of non-severe and severe groups. Compared with the non-severe group, the severe group had a higher proportion of patients with a history of diabetes (P = 0.038) and had a higher level of CTSI score. Among blood routine indexes, patients in the severe group had a higher level of WBC (P < 0.001) and NEUT (P < 0.001). Among blood biochemical indexes, severe group patients had a higher level of AMY (P = 0.026), TB (P = 0.040), LDH (P < 0.001), BUN (P = 0.004), AST (P = 0.014), ALP (P = 0.029), Glu (P < 0.001) and apoB/apoA1 (P = 0.002), and a lower level of Ca2+ (P = 0.001) and ALB (P = 0.003). summarized the data characteristics on the training and testing set. There was no statistical difference between the two datasets in all 30 variables (P > 0.05).
Table 1. The characteristics of the patients.
Table 2. Patients’ characteristics on the training and testing sets.
Model hyper-parameters
Based on the data of demographics, blood routine and biochemistry indexes, and CTSI score in patients, we developed LR, SVM, DT, RF and XGBoost models on the training set and determined the optimal hyper-parameters by grid search. Supplement Table S1 showed the parameter tuning process and specific hyper-parameters of each model. Default values were used for parameters not listed.
Performance of ML models
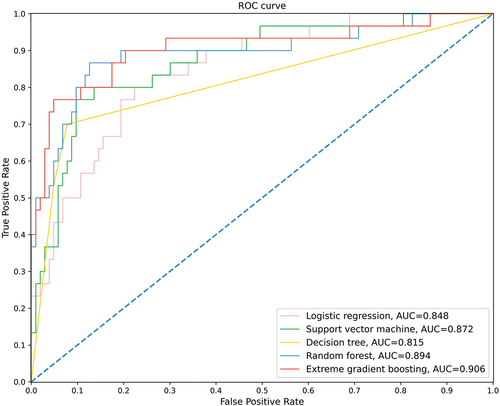
showed the receiver operating curves of the five ML models on the testing set. The XGBoost had the highest AUC of 0.906, followed by RF with an AUC of 0.894, the SVM with an AUC of 0.872, the LR with an AUC of 0.848, and the DT with an AUC of 0.815. summarized the performance of the models. Among the five classifiers, XGBoost had the best predictive performance, with the highest accuracy of 0.902, the highest sensitivity of 0.700, the highest F1 score of 0.764, and the second highest specificity of 0.961, indicating that XGBoost was superior to other ML models in predicting the severity of AP. Based on the coefficient of LR and the feature importance of RF and XGBoost, summarized the top six important predictive factors of the three ML models. The CTSI score, ALB, LDH, and NEUT were the common predictors of the three models.
Table 3. The performance of five machine learning models.
Table 4. The rank of top six predictors in LR, RF, and XGBoost.
In addition, based on the four common significant predictors, we reconstructed the ML models using the same data preprocessing and parameter tuning method to compare the impact of simplified data on model performance. Supplementary Table S2 and Supplementary Figure S1 demonstrated the performance of the simplified models.
Discussion
Early prediction of the severity of AP is of great significance for preventing SAP, which can improve the prognosis. However, due to the complexity and variability of AP, it was often difficult to predict the clinical course, leading to the miss of critical time window for preventive treatments. In order to develop a better predictive model and determine important predictors for AP severity, we developed five ML models. The results showed that the XGBoost algorithm can accurately predict high-risk patients with severe AP and identify risk predictors, which can provide the clinicians a reference for prevention strategies of AP.
Currently, several studies have developed ML models to predict AP severity. Jin et al. used the percentage of NEUTs, the NEUT count, the percentage of lymphocytes, AMY, pancreatic AMY, and C-reaction protein to establish an ANN model to predict SAP, which reached an AUC of 0.951 [Citation16]. Based on magnetic resonance imaging, Lin et al. constructed a SVM radiomics model with an AUC of 0.848, indicating the potential value of pancreatic local features for early prediction of AP severity [Citation17]. To the best of our knowledge, our study was the first to combine the CTSI score, which reflects the severity of local pancreatic injury, with blood indicators that reflect patient’s general condition to develop ML models to predict the severity of AP. Although this was a retrospective study based on single-center data, we believed that the combination of imaging data and blood indicators was a good attempt and enlightenment. It is hoped that the follow-up studies can construct more specialized and automated models based on patients’ radiomics and clinical data on the basis of our study to predict the severity of AP.
Based on the results of the comparation of LR, RF, and XGBoost model, we found that the CTSI score, ALB, LDH, and NEUT were the common predictors. The CTSI score was created by Balthazar et al. in 1990, which quantified the degree of pancreatic and peripancreatic tissue inflammation and pancreatic necrosis. The CTSI score can effectively reflect the severity of pancreatic local tissue injury. Previous studies showed that the CTSI score of SAP patients was significantly higher than that of MAP and MSAP patients (3.5 ± 2.2 vs. 2.2 ± 1.4, P < 0.001), and compared with the APACHE II score, BISAP score, and Ranson score, the CTSI score had better predictive performance [Citation18,Citation19]. ALB, 40% of which remains in plasma, is involved in substance synthesis and transport, anti-inflammatory, antioxidation, anticoagulation, antiplatelet aggregation, and maintains plasma colloidal osmotic pressure [Citation20]. When SIRS occurs, the up-regulation of cytokines and inflammatory mediators leads to the injury of capillary endothelium, increases its permeability, and then leads to plasma extravasation [Citation21,Citation22]. In addition, the low perfusion, leading to the weakening of the ability of liver to synthesize ALB, which, combined with fasting management, results in the decrease of ALB. Previous studies have shown that the low ALB levels can be used to assess the severity of AP and were related to the hospital mortality of SAP patients, and when using the ALB to predict persistent OF, with the cutoff value of 30.8 g/L, the AUC was 0.78 [Citation23–25]. LDH is the key enzyme of anaerobic glycolysis and gluconeogenesis. It widely exists in human tissues, and is most abundant in kidney, myocardium and skeletal muscle. Since the SAP patients are often accompanied by multiple organ dysfunction, which can lead to an increase in LDH levels, LDH can be used as an important evaluation indicator of the severity of AP [Citation26,Citation27]. Tian et al. found that the LDH level of SAP patients was significantly higher than that of MAP patients (398.80 ± 133.48 vs. 193.09 ± 44.97, P = 0.017). When the LDH was used to predict the severity of AP, the AUC was 0.919, the sensitivity was 0.827, and the specificity was 0.960 [Citation28]. The NEUTs are the most abundant immune cell in the blood circulation and pancreatic tissue. Consistent with the results of this study, some studies showed that the NEUTs played an important role in the development of SAP. When the autodigestion of pancreas started, tissue injury activated the immune system, the NETUs were recruited and activated, releasing various pro-inflammatory cytokines, which led to the deterioration of pancreatic local inflammation and systemic inflammation. Furthermore, the neutrophil extracellular traps after NEUTs activation were also involved in the progression of AP, further promoting pancreatic inflammation, pancreatic necrosis, and leading to SAP [Citation29,Citation30]. Therefore, monitoring the level of NEUTs and effectively controlling secondary infections plays an important role in the prevention of SAP. In addition, we found that serum calcium showed significant differences between the severe and non-severe group. In the course of AP, the autodigestion of mesenteric fat by pancreatic enzymes leads to the release of free fatty acids, which combine with calcium to form fatty acid calcium, resulting in a large consumption of calcium ions, and then hypocalcemia [Citation31,Citation32]. Current studies suggested that serum calcium has a great predictive performance for the severity of AP [Citation31,Citation33]. However, probably due to the data limitations, its value was not validated in our study. It is recommended that future studies could combine serum calcium with the predictors found in our study to develop better predictive models for AP severity.
Since the patients in the severe group accounted for only about 22% of all participants in this study, there was a certain degree of data imbalance. On this basis, we have tried the over-sampling method to improve imbalance, but it did not significantly improve the AUC of the models. As we guessed, the two ensemble models RF and XGBoost showed better performance, and among them, XGBoost performed even better. XGBoost is an ensemble model based on the gradient boosting algorithm. By constructing weak classifiers one by one and integrating several weak classifiers in multiple iterations, it obtains a more powerful model to make accurate prediction. Although the interpretability of LR model is better than XGBoost, ML algorithms have a strong ability to explain the nonlinear relationship between variables. Especially in the medical datasets, there are a large number of variables with complex interaction relationship, outliers, and missing values. Therefore, XGBoost is especially suitable for medical predictive tasks. When developing ML models using the simplified four predictors, we found a drop in the performance of RF and XGBoost compared to the previous model. This was not surprising to us, because as the dimension of data decreased, relatively complex ensemble models inevitably lost their advantages of dealing with complex relationships between variables. However, it was worth noting that the performance of LR and DT based on the simplified data was improved, which seemed to indicate that these models can obtain most of the information about the predicted outcome by learning the characteristics of the simplified data. This also confirmed from the side that CTSI, NEUT, ALB and LDH found in this study were indeed related to the severity of AP.
This study also had some limitations. First, this was a retrospective study based on a single center. The number of cases included was limited and only represented the characteristics of the AP among local residents. Second, we did not validate our model with external datasets. Therefore, whether the results of this study can be promoted in a larger population still needs validations in further studies. In addition, the feature engineering was based on existing studies on risk factors and experts’ suggestions. The indicators we chose may not be comprehensive enough. We hoped that future studies can incorporate more variables to build stronger predictive models.
Conclusion
Our research found that, compared to other ML models, the XGBoost algorithm can effectively predict the severity of AP using patients’ demographic data, blood routine and blood biochemical indexes, and the CTSI score. ML predictive models may provide an assistance for the clinicians to identify the severity of AP at an early stage. The CTSI score, ALB, LDH, and NEUT were potential predictors of severe AP.
Disclosure of financial/other conflicts of interest
The authors have no relevant conflicts of interest to disclose. Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author contributions
YZ, GTL, LHH and GHY designed this study; FH, XLS, JXZ, and GYL collected the data; CCY, JJP, and JXZ conducted formal analysis; YZ, CCY, and GYL developed machine learning models; YZ, FH, and XLS wrote the original draft; GTL, LHH, JJP, WMX, and GHY provided guidance and amendments; all authors approved the final manuscript.
Supplemental Material
Download MS Word (158.5 KB)Acknowledgments
The author thanked the Affiliated Hospital of Yangzhou University and all the authors of the original studies.
Data availability statement
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/00325481.2022.2099193
Additional information
Funding
References
- Iannuzzi JP, King JA, and Leong JH, et al. Global incidence of acute pancreatitis is increasing over time: a systematic review and meta-analysis. Gastroenterology. 2022;162(1):122–134. DOI: 10.1053/j.gastro.2021.09.043.
- Matta B, Gougol A, and Gao X, et al. Worldwide variations in demographics, management, and outcomes of acute pancreatitis. Clin Gastroenterol Hepatol. 2020;18(7):1567–1575.e2. DOI: 10.1016/j.cgh.2019.11.017.
- Li C-L, Jiang M, and Pan C-Q, et al. The global, regional, and national burden of acute pancreatitis in 204 countries and territories, 1990–2019. BMC Gastroenterol. 2021;21(1):332 DOI: 10.1186/s12876-021-01906-2.
- Forsmark CE, Vege SS, and Wilcox CM. Acute pancreatitis. N Engl J Med. 2016;375(20):1972–1981. DOI: 10.1056/NEJMra1505202.
- Banks PA, Bollen TL, and Dervenis C, et al. Classification of acute pancreatitis–2012: revision of the Atlanta classification and definitions by international consensus. Gut. 2013;62(1):102–111. DOI: 10.1136/gutjnl-2012-302779.
- Wu BU, Johannes RS, and Sun X, et al. The early prediction of mortality in acute pancreatitis: a large population-based study. Gut. 2008;57(12):1698–1703 DOI: 10.1136/gut.2008.152702 .
- Gao W, Yang H-X, and Ma C-E. The value of BISAP score for predicting mortality and severity in acute pancreatitis: a systematic review and meta-analysis. PloS one. 2015;10(6):e0130412. DOI: 10.1371/journal.pone.0130412.
- Yang Y-X LL. Evaluating the ability of the Bedside Index for severity of acute pancreatitis score to predict severe acute pancreatitis: a meta-analysis. Med Princ Pract. 2016;25(2):137–142. DOI: 10.1159/000441003.
- Mounzer R, Langmead CJ, and Wu BU, et al. Comparison of existing clinical scoring systems to predict persistent organ failure in patients with acute pancreatitis. Gastroenterology. 2012;142(7):1476–82. DOI: 10.1053/j.gastro.2012.03.005.
- Papachristou GI, Muddana V, and Yadav D, et al. Comparison of BISAP, Ranson’s, APACHE-II, and CTSI scores in predicting organ failure, complications, and mortality in acute pancreatitis. Am J Gastroenterol. 2010;105(2):435–41. DOI: 10.1038/ajg.2009.622.
- Saria S, Butte A, and Sheikh A. Better medicine through machine learning: what’s real, and what’s artificial? PLoS Med. 2018;15(12):e1002721. DOI: 10.1371/journal.pmed.1002721.
- Tenner S, Baillie J, and DeWitt J, et al. American College of Gastroenterology guideline: management of acute pancreatitis. Am J Gastroenterol. 2013;108(9):1400–15; 1416. DOI: 10.1038/ajg.2013.218.
- Liu M, Zhou C, and Zhang Z, et al. Inverse association between riboflavin intake and new-onset hypertension: a nationwide cohort study in China. Hypertension. 2020;76(6):1709–1716. DOI: 10.1161/HYPERTENSIONAHA.120.16211.
- American Diabetes Association. 2. Classification and Diagnosis of Diabetes. Diabetes Care. 2021;44(Suppl 1):S15–S33. DOI: 10.2337/dc21-S002.
- Shi L, Shu X-O, and Li H, et al. Physical activity, smoking, and alcohol consumption in association with incidence of type 2 diabetes among middle-aged and elderly Chinese men. PloS one. 2013;8(11):e77919. DOI: 10.1371/journal.pone.0077919.
- Jin X, Ding Z, and Li T, et al. Comparison of MPL-ANN and PLS-DA models for predicting the severity of patients with acute pancreatitis: an exploratory study. Am J Emerg Med. 2021;44:85–91. DOI: 10.1016/j.ajem.2021.01.044.
- Lin Q, Ji YF, and Chen Y, et al. Radiomics model of contrast-enhanced MRI for early prediction of acute pancreatitis severity. J Magn Reson Imaging. 2020;51(2):397–406. DOI: 10.1002/jmri.26798.
- Cho JH, Kim TN, and Chung HH, et al. Comparison of scoring systems in predicting the severity of acute pancreatitis. World J Gastroenterol. 2015;21(8):2387–2394. DOI: 10.3748/wjg.v21.i8.2387.
- Harshit Kumar A, and Singh Griwan M. A comparison of APACHE II, BISAP, Ranson’s score and modified CTSI in predicting the severity of acute pancreatitis based on the 2012 revised Atlanta classification. Gastroenterol Rep (Oxf). 2018;6(2):127–131. DOI: 10.1093/gastro/gox029.
- Arques S. Human serum albumin in cardiovascular diseases. Eur J Intern Med. 2018;52:8–12. DOI: 10.1016/j.ejim.2018.04.014.
- Komara NL, Paragomi P, and Greer PJ, et al. Severe acute pancreatitis: capillary permeability model linking systemic inflammation to multiorgan failure. Am J Physiol Gastrointest Liver Physiol. 2020;319(5):G573–G583. DOI 10.1152/ajpgi.00285.2020.
- Elder ASF, Saccone GTP, and Dixon D-L. Lung injury in acute pancreatitis: mechanisms underlying augmented secondary injury. Pancreatology. 2012;12(1):49–56. DOI: 10.1016/j.pan.2011.12.012.
- Xu X, Ai F, and Huang M. Deceased serum bilirubin and albumin levels in the assessment of severity and mortality in patients with acute pancreatitis. Int J Med Sci. 2020;17(17):2685–2695. DOI: 10.7150/ijms.49606.
- Wang X, Cui Z, and Li H, et al. Nosocomial mortality and early prediction of patients with severe acute pancreatitis. J Gastroenterol Hepatol. 2010;25(8):1386–1393. DOI: 10.1111/j.1440-1746.2010.06376.x.
- Hong W, Lin S, and Zippi M, et al. Serum albumin is independently associated with persistent organ failure in acute pancreatitis. Can J Gastroenterol Hepatol. 2017;2017:5297143. DOI: 10.1155/2017/5297143.
- Yin X, Xu J, and Zhang Q, et al. Quantification analysis of lactate dehydrogenase and C-reactive protein in evaluation of the severity and prognosis of the acute pancreatitis. Cell Mol Biol (Noisy-le-grand). 2020;66(1):122–125. PMID: 32359394.
- Zrnić IK, Milić S, and Fisić E, et al. [C-reactive protein and lactate dehydrogenase as single prognostic factors of severity in acute pancreatitis]. Lijec Vjesn. 2007;129(1–2):1–4. PMID: 17489509.
- Tian F, Li H, and Wang L, et al. The diagnostic value of serum C-reactive protein, procalcitonin, interleukin-6 and lactate dehydrogenase in patients with severe acute pancreatitis. Clin Chim Acta. 2020;510:665–670. DOI: 10.1016/j.cca.2020.08.029.
- Murthy P, Singhi AD, and Ross MA, et al. Enhanced neutrophil extracellular trap formation in acute pancreatitis contributes to disease severity and is reduced by chloroquine. Front Immunol. 2019;10:28. DOI: 10.3389/fimmu.2019.00028.
- Yang Z-W, and Meng X-X XP. Central role of neutrophil in the pathogenesis of severe acute pancreatitis. J Cell Mol Med. 2015;19(11):2513–2520. DOI: 10.1111/jcmm.12639.
- He -S-S, Li D, and He Q-Y, et al. Establishment of early multi-indicator prediction models of moderately severe acute pancreatitis and severe acute pancreatitis. Gastroenterol Res Pract. 2022;2022:5142473. DOI: 10.1155/2022/5142473.
- Liu G-H, Chen J, and Li L-Q, et al. Development and validation of a nomogram for early assessment the severity of acute pancreatitis. Scand J Gastroenterol. 2022:1–6. DOI: 10.1080/00365521.2022.2050293.
- He F, Zhu H-M, and Li B-Y, et al. Factors predicting the severity of acute pancreatitis in elderly patients. Aging Clin Exp Res. 2021;33(1):183–192. DOI: 10.1007/s40520-020-01523-1. .