ABSTRACT
Obesity is a chronic metabolic disease that has become one of the leading causes of disability and death in the world, affecting not only adults but also children and adolescents. In Iraq, one third of the adult population is overweight and another third obese. Clinical diagnosis is accomplished by measuring body mass index (BMI) and waist circumference (a marker for intra-visceral fat and higher metabolic and cardiovascular disease risk). A complex interaction between behavioral, social (rapid urbanization), environmental and genetic factors underlies the etiology of the disease. Treatment options for obesity may include a multicomponent approach, involving dietary changes to reduce calorie intake, an increase in physical activity, behavioral modification, pharmacotherapy and bariatric surgery. The purpose for these recommendations is to develop a management plan and standards of care that are relevant to the Iraqi population and that can prevent/manage obesity and obesity-related complications, for the promotion of a healthy community.
1. Introduction/rationale
The obesity and overweight pandemic continues to afflict global populations across all socioeconomic settings. The World Health Organization (WHO) estimates that close to 40% (1.9 billion) of adults were overweight in 2016, 13% (650 million) of whom were obese [Citation1]. The dramatic rise in the rates of overweight and obesity was also reflected in younger individuals, with approximately 38.2 million children aged 5 years and below and more than 340 million children and adolescents aged 5–19 being overweight or obese in 2019 and 2016, respectively [Citation1].
Simply defined by the WHO as ‘abnormal or excessive fat accumulation that may impair health’ [Citation1], obesity and overweight were officially recognized by the American Medical Association (AMA) as a complex chronic disease in 2013 [Citation2]. On a pathophysiological level, obesity is now widely recognized as a chronic, relapsing multifactorial neurobehavioral disease with serious implications on the level of metabolic, biomechanical, and psychosocial wellbeing [Citation3].
The lethality of obesity has become more concerning than underweight and malnutrition seeing as obesity-related mortality has at least doubled since 1990 [Citation4]. With around 5.4 million global deaths attributed to high body mass index (BMI) in 2019 [Citation5], obesity remains one of the leading risk factors for premature deaths and is associated with a plethora of non-communicable diseases (metabolic diseases, cardiovascular diseases, and malignancies, etc.)[Citation6], leading to a decrease in life expectancy [Citation7,Citation8] while also causing an impairment of quality of life and loss of productivity [Citation9,Citation10].
The pandemic proportions of obesity have also been reported from the Middle East, particularly among countries with upper-middle and high income [Citation11]. Evidently, the high rates of obesity also extend to lower income countries such as Iraq, where the Non communicable Diseases Risk Factors Survey (STEPS) conducted in Iraq in 2015 showed that nearly one third (33.5%) of respondents were obese and 31.9% were overweight [Citation12]. Women were more likely to be obese whereas overweight supervened among men [Citation12]. These recommendations therefore aim to develop a management plan and standards of care that are relevant to the Iraqi population and can prevent/manage obesity and obesity-related complications for the promotion of a healthy community.
2. Methods
A multi-disciplinary panel of regional experts from different specialties (adult and pediatric endocrinologists, bariatric surgeons, nutritionists, family physicians, cardiologists, gynecologists and psychiatrists) who are involved in the management of obese patients convened in monthly meetings (from December 2019 till February 2021) to review the progress of the panel with ad-hoc meetings for each specialty. The latest literature and local studies were reviewed in writing these recommendations, which incorporated Canadian, European and WHO guidelines [Citation13,Citation14]. The recommendations are endorsed by the Iraqi Medical Association, the Iraqi Obesity Society, and the Iraqi Diabetes Association and will also be presented to the Iraqi ministry of health for endorsement.
3. Obesity management in adults
3.1. Screening
3.1.1. Rationale
Weight loss, whether via lifestyle/behavioral, medical therapy or surgical interventions, is recommended as it could lead to the reduction of a wide array of weight-related complications. Documented benefits of weight loss include a lower risk of developing diabetes, hypertension, and dyslipidemia as well as a reduction in the number of cardiovascular events, a lower risk of asthma, sleep apnea, and an improvement in quality of life [Citation15–18]. Intentional weight loss could also lead to a decrease in all-cause mortality, as well as mortality due to cancer and cardiovascular disease [Citation15]. Prevention and treatment of obesity is therefore much needed. Research from the Middle East lags far behind other regions in the world, despite the region’s high rates of obesity. The paucity and variability of available evidence in the Middle East prevents the establishment of the efficacy of dietary interventions among adults for weight loss [Citation19]. Screening is therefore essential to ensure that high-risk patients receive the necessary counselling about the health risks of being overweight/obese, and the available risk factor reduction, lifestyle changes and obesity treatment options.
3.1.2. Barriers
That being said, the diagnosis of obesity could lead to negative changes for the patient, particularly considering the stigmatization and discrimination they might face even in the healthcare setting [Citation20]. The obesity stigma should be eradicated, as it would most likely reflect in an improvement in the management of obesity by preventing the deleterious effects of stigmatization (i.e. weight gain and poor mental health) [Citation21–23]. Healthcare providers involved in the management and counseling of such patients should therefore adopt positive attitudes, welcoming patients and avoiding the use of insensitive and/or discriminatory terms. It is also important to address weight misperceptions and misconceptions, commonly observed among the general population [Citation24], in order to promote a proactive and healthy attitude towards weight loss and weight loss maintenance.
3.1.3. Measurements: BMI and waist circumference
BMI is a practical tool for evaluating patient weight (). Measuring waist circumference is also suggested in overweight and obese adults with a BMI ≤ 35 kg/m2 in order to assess abdominal obesity as an abnormal waist circumference correlates with a higher risk of metabolic and cardiovascular disease [Citation25,Citation26]. Internationally, high disease risk in relation to waist circumference was standardized at ≥102 cm for males and ≥88 cm for females [Citation14,Citation27]. Alternatively, local studies reported a waist circumference of ≥99 cm for Iraqi men and ≥97 cm for Iraqi women to be considered elevated and indicative of increased cardiometabolic risk [Citation28].
Table 1. BMI categories in the general population (including Middle-Eastern populations) (WHO 1997).
3.2. Evaluation
In patients found to be overweight (body mass index [BMI] ≥25 kg/m2) or abdominally obese, assessment of the etiology of the weight gain and its associated health risk should be undertaken. Specifically, evaluation of the overweight or obese patient includes establishing patient history (age at onset, weight loss attempts, current or previous medications, behavioral/dietary pattern changes), physical examination, and measurement of fasting glucose (or glycated hemoglobin [A1C]), thyroid-stimulating hormone (TSH), liver enzymes, and fasting lipids.
3.2.1. Determining etiology
The majority of obesity cases are related to behavioral factors, such as excessive calories intake and sedentary lifestyle. That being said, other factors can contribute to the development of obesity. Although uncommon, secondary causes of overweight/obesity should be considered and ruled out prior to proceeding with management strategies () [Citation29]. Physical examination findings that might point to a secondary cause of obesity include goiter (hypothyroidism), proximal muscle weakness, purple striae, osteoporosis (Cushing’s syndrome), and acne/hirsutism (polycystic ovary syndrome (PCOS). If needed, additional testing should be conducted for the detection of possible involvement of the hypothalamic-pituitary axis (Cushing’s syndrome, growth hormone deficiency, or hypothalamic obesity). While it is possible that monogenetic disorders could be responsible for obesity, these occurrences are rare and predominately limited to childhood. Routine genetic testing is therefore not recommended in adult patients. To note that pathogenic mutations in the hypothalamic appetite-regulating melanocortin-4 receptor (MC4R) have a prevalence of 6% globally and are the most common cause of monogenic juvenile-onset obesity [Citation30–32]. Developing countries such as Iraq exhibit a higher prevalence due to the existence of higher rates of consanguinity [Citation33]. Early-onset obesity, hyperphagia, increased linear growth are characteristic manifestations of pathogenic MC4R mutations [Citation34–36], in addition to a lesser risk of hypertension despite the presence of obesity [Citation37]. Pathogenic MC4R mutations are worth considering seeing as patients carrying these mutations can still benefit from Glucagon-like Peptide-1 Receptor Agonists [Citation38,Citation39]. Measurement of metabolic rate is also not recommended, as it is not widely available and may be misinterpreted.
Table 2. Differential diagnosis/secondary causes of obesity/overweight.
3.2.2. Assessing obesity-related CV risk and complications
Obesity is identified as a risk factor for cardiovascular disease and diabetes mellitus, among other metabolic and mechanical disorders. Cardiovascular risk factors should be identified and managed in order to reduce cardiovascular disease risk independently of weight loss. These risk factors include hypertension, dyslipidemia (reduced levels of high-density lipoprotein [HDL] or elevated levels of low-density lipoprotein [LDL]), elevated triglycerides, impaired fasting glucose or diabetes, obstructive sleep apnea, and cigarette smoking. Assessment of other obesity related disorders that do not increase cardiovascular risk but are associated with significant morbidity should also be undertaken. Examples include symptomatic osteoarthritis, cholelithiasis, nonalcoholic fatty liver disease, PCOS, depression, sleep apnea and impaired quality of life.
3.3. Approach to therapy
3.3.1. Goals of treatment
The management of obesity largely depends on available resources, clinical expertise as well as patient preference. It should be undertaken within the framework of a multidisciplinary team involving at least a primary health care physician, possibly a family physician, internist or an endocrinologist, a bariatric surgeon and a psychiatrist or psychologist. Management should not only target the prevention, treatment or reversal of the complications of obesity but also the improvement of quality of life. In fact, health benefits can be observed after weight loss as modest as 5% of body weight [Citation40,Citation41].
In general, management can rely on lifestyle/behavioral changes alone. In this case, 5–7% weight loss can be expected, but is difficult to maintain due to the great implication of patient adherence [Citation42,Citation43]. The supplementation of lifestyle interventions with pharmacologic therapy can also be considered. 5–10% weight loss can often be achieved with pharmacologic therapy [Citation44,Citation45], while the use of medical devices and bariatric surgery can ensure greater weight loss than medical therapy (> 10%) [Citation46–48].
3.3.2. Candidate identification
Patient management should be individualized and the best candidates for each approach should be identified and categorized based on the degree of obesity (BMI), the presence of abdominal obesity (waist circumference), the presence of cardiovascular disease risk factors (hypertension, diabetes and dyslipidemia) and the presence of other comorbidities (sleep apnea, nonalcoholic fatty liver disease) (). We suggest that all patients must be checked for H. pylori, as it is a common infection, and it is prevalent in patients with excess weight or obesity
Table 3. Classification of patients.
Management recommendations and treatment algorithm are provided in and , respectively.
Figure 1. Overweight and obesity treatment algorithm a: only after agreement of multidisciplinary team b: only if comorbidities are present along with BMI between 35 and 40 kg/m.
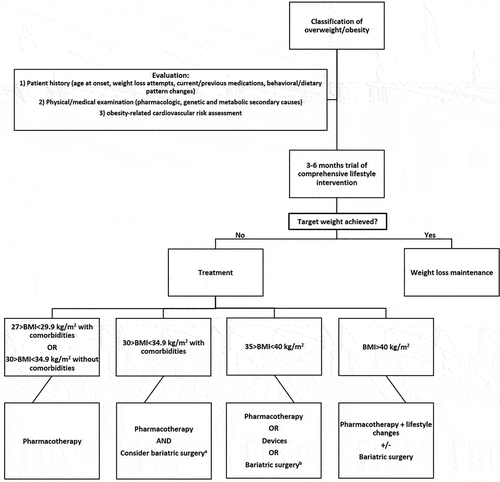
Table 4. Management recommendations.
3.3.3. Psychological assessment
Initial assessment of patients should include psychological assessment
Any patient with history of a psychiatric illness or psychotropic medication use should be referred to a psychiatrist.
Preoperative psychological assessment should be done and must include the following areas: consent, expectations, social support, mental health, chemical abuse/dependence, eating behaviour, adherence, and coping and stressors.
Before deciding on surgery, patients must try behavioural modification. This will give an idea about the patient’s commitment, which might influence relapse after surgery
Psychological opinion should be an integral part of a multidisciplinary team that manage obesity.
3.3.4. Initial treatment
3.3.4.1. Comprehensive lifestyle intervention
The cornerstone of obesity and overweight management is a comprehensive lifestyle intervention which combines diet, exercise and behavioral modification. Initial treatment includes regular self-monitoring of food intake, physical activity and weight. The modification of patient lifestyle facilitates adherence to physical activity and weight loss interventions/mainte[Citation49]nance. To that end, the adoption of lifestyle intervention program of the Diabetes Prevention Program (DPP) (targeting a minimum of 7% weight loss through a low-fat, hypocaloric diet and a minimum of 150 minutes of exercise per week as brisk walking) is recommended [Citation50]. Weight development can be predicted by behavioral (dietary intake and meal patterns) and psychological factors (motivation and self-efficacy) [Citation51]. For successful weight maintenance, appropriate eating behavior seems crucial and is evaluated by assessing eating restraint, disinhibition and hunger. Several psychometric measures of weight maintenance predictors consider control over eating as a crucial criteria such as the Three Factor Eating Questionnaire (TFEQ) [Citation52]. For reduction of caloric intake, self-control and careful decision making is necessary and are the building blocks of primary behavioral weight loss concepts such as restraint, disinhibition, self-efficacy [Citation53], and locus of control [Citation54]. Patient choice along with control is considered to be fundamental as patients have to exercise control in daily decisions on what foods to eat.
3.3.4.2. Dietary therapy
Adherence to dietary therapy is a more critical consideration for its success than the type of diet [Citation55], which could be a balanced low-calorie diet, a low-fat/low-calorie diet, a moderate fat/low-calorie diet, a low carbohydrate diets or a Mediterranean diet. The choice of diet should aim to reduce energy intake below energy expenditure while taking into consideration individual patient preferences in order to improve adherence, seeing as macronutrient composition is of lesser concern for weight loss [Citation56]. Diets are an effective strategy for the reduction of body mass [Citation57] and an energy deficit of 500 to 750 kcal/day should be targeted when individualizing diets (which could generally be restricted to 1,200–1,500 kcal/day for women and 1,500–1,800 kcal/day for men). More significant weight loss is achieved with diets of 1000 kcal/day or less, compared to higher energy intake (1500 Kcal/day) [Citation58]. Very-low-calorie diets (420 kcal or 530 kcal/day) and low-calorie diets (880 kcal/day) seem to yield comparable weight loss even on long-term follow-up [30]. Thus, weight loss can be expected even in patients concerned that they are ‘metabolically resistant’ to weight loss in case of compliance with a diet of 800 to 1200 kcal/day. Regardless, comorbidities and patient capacity to follow the diet should also be taken into account. Dietary counselling and psychological support could facilitate weight loss and long-term weight maintenance [Citation59]. Return visits with the dietitian or clinician should be scheduled at regular intervals to assess barriers, discuss next steps and offer encouragement. Pharmacotherapy should be considered when weight loss does not exceed 5% total body weight over 6 months.
3.3.4.3. Exercise
While exercise is a less potent approach for weight loss compared to dietary restriction, its addition to a diet (particularly resistance training) leads to more effective changes to body composition and biomarkers of metabolic tissue [Citation57]. In addition to weight loss[Citation60], exercise can attenuate muscle leas mass loss [Citation61], while preventing weight regain and improving cardiovascular health [Citation62,Citation63]. More significant weight loss is achieved with increasing amounts of exercise. In general, patients should be recommended to complete at least thirty minutes of a multicomponent program that includes aerobic and resistance training, five to seven days a week [Citation64]. Exercise should be of moderate intensity for meaningful weight and adiposity loss [Citation64]. Safety during exercise should be ensured and patients should be medically evaluated for physical fitness prior to initiation of physical exercise regimens.
3.3.4.4. Behavior modification
Clinicians should also aim to aid patients in implementing long-term changes to their eating behavior, promoting the modification and monitoring of food intake and physical activity. Moreover, eating triggers (environmental cues and stimuli) should be identified and controlled. Behavior modification should take into account several elements, as follows [Citation14]:
Setting initial goals: 0.5–1 kg/week or 5–10% within six months.
Self-monitoring: food diaries and self-regular weighing.
Controlling or modifying the stimuli that activate eating
Eating style: Slowing down the eating process.
Behavioral contracting and reinforcement: Providing rewards for weight loss.
Nutrition education and meal planning: Defined meal structure and use portion-controlled plates or diets.
Increasing physical activity
Social support
Cognitive restructuring: Adopting positive rather than negative self-talk.
Problem-solving: Developing strategies to manage food intake in difficult situations such as restaurants and parties.
3.3.5. Subsequent treatment
3.3.5.1. Drug therapy
Drug therapy is recommended for patients who have not met weight loss goals (loss of at least 5% of total body weight) 3–6 months after a comprehensive lifestyle intervention. This decision should be individualized according to patient profile (BMI and comorbidities) and made after a careful evaluation of the risks and benefits of all treatment options. Pharmacological therapy can be initiated in patients with a BMI above 30 Kg/m2 in the absence of comorbidities, while the BMI threshold is lowered to above 27 Kg/m2 in patients with comorbidities. Initiating therapy is recommended independently of waist circumference. Weight loss medication should be prescribed as a long term treatment as an adjunct to lifestyle modification, consistently with the management of other chronic conditions. If <5% weight loss is achieved after 12 weeks, medication should be withdrawn. Pharmacological options include 3 mg liraglutide (Saxenda®, daily injection), and 120 mg orlistat (Xenical®, Onlefit®, Refit®), both of which are approved by the Iraqi Ministry of Health to be used for obesity management. Other agents that are approved for chronic use in other countries are combination phentermine-extended release topiramate and combination naltrexone-bupropion. The choice of drug is governed by the comorbidities and relative contraindications in the individual patient. That being said, liraglutide is the preferred agent given its tolerability and beneficial effects on glycemia, cardiovascular risk reduction and modest weight loss even in a real-world setting [Citation16,Citation65–67]. Evidence suggests superior significant weight loss with liraglutide in comparison to orlistat [Citation44,Citation45]. However, temporary gastrointestinal side effects (nausea, vomiting), its daily administration, and its cost may limit its use. Orlistat also had proven benefits in regard to glycemia, blood pressure and lipid profile [Citation68]. However, it frequently causes gastrointestinal side effects and is often not tolerated by patients [Citation69]. Due to its limited tolerability, and the established safety and benefits of other available agent including liraglutide, we no longer consider orlistat to be a first-line agent for the treatment of obesity unless the cost of other therapy is problematic.
Risks of treatment.Most of the approved drugs have minor side effects that diminish with time (treatment). While a few serious side effects have been identified, these should not preclude the short-term use of pharmacologic therapy in the majority of overweight subjects. The occurrence of adverse events should be screened for at every visit, and patients should be carefully monitored every six weeks to establish weight-loss, blood pressure and heart rate. Blood sugar should also be assessed in patients with diabetes, especially in those on insulin or insulin secretagogues. Any modality that results in a significant weight loss may increase the likelihood of cholelithiasis due to the increase of the flux of cholesterol through the biliary system [Citation70,Citation71]. We therefore recommend diets with moderate amounts of fat that stimulate gallbladder contraction to reduce this risk [Citation72]. For subjects with a rapid weight loss (> 1.5 kg/week), bile acid (e.g. ursodeoxycholic acid) may be advisable [Citation72,Citation73].
3.3.5.2. Devices
Several approaches are available for the treatment of obesity, including electrical stimulation (vagal blockade) systems and gastric emptying (aspiration) systems. However, both of these modalities are not available in Iraq and cannot at present be recommended. Intragastric balloon systems have been advocated for use as a bridge to a more definitive surgical procedure and have been shown to be safe and effective for weight loss [Citation74,Citation75]. Three devices, Orbera, Obalon, and ReShape balloons, have been approved by the US Food and Drug Administration (FDA) to treat obesity in adults with a BMI of 30 to 40 kg/m2 with one or more comorbid conditions such as diabetes, hypertension, or hypercholesterolemia.
3.3.5.3. Bariatric surgery
Bariatric surgery should be considered for patients with a BMI of 35 kg/m2 or higher with obesity-related comorbidities that are expected to improve with weight reduction, as well as patients with one anastomosis gastric bypass (MGB/OAGB). Bariatric surgery is the intervention of choice for patients with a BMI of 40 kg/m2 or higher, AND:
No achievement or maintenance of clinically significant/beneficial weight loss for at least 6 months after trying all possible non-surgical measures.
General fitness for anaesthesia and surgery.
Commitment to long-term follow-up.
Skeletal and sexual maturity (14 years for girls and 15 years for boys)
No significant psychiatric disease.
The choice of operation type depends on the surgical team and patient preferences. When considering gastric surgery, patients should undergo perioperative assessment by a multidisciplinary team comprised of a bariatric surgeon, dietitian, endocrinologist, general physician or cardiologist, psychiatrist, anesthetist and physiotherapist, as well as a gastroenterologist, plastic surgeon, gynecologist and orthopedist when needed. Gastric surgery is best undertaken in specialized obesity centers with the necessary provisions and equipment (e.g. suitable seating, bed frames, pressure-relieving mattresses and other tools for bariatric surgery, in addition to trained staff in their use). The availability of competent staff and a qualified bariatric surgeon is important for the successful conduction of gastric surgery. During the surgical assessment and counselling, the potential benefits of the procedure should be weighed against perioperative morbidity and mortality as well as expected complications. Medical assessment is critical to establish cardiopulmonary status and fitness for general anesthesia. Endocrinological function should also be examined both before and after surgery to determine the degree of diabetes and level of control, gonadal axis and fertility, lipid disorders, and thyroid status. In addition to medical assessment, psychological factors are frequent in patients eligible for bariatric surgery and should also be considered [Citation76]. Psychological diseases contraindicating surgery should be determined prior to surgery and psychological support should be provided perioperatively to improve outcomes [Citation77]. Patients’ ability to comply with pre- and post-operative dietary, behavioral and lifestyle changes should also be assessed, while taking into account current nutrition status and medical/weight loss history, educational needs as well as micronutrient screening. Pre-operative workup should also include a series of laboratory tests to determine liver function, complete blood count with differential, serum levels of iron, ferritin, calcium, vitamin B12, Vitamin B1 (thiamin), folate, TIBC, alkaline phosphatase, PTH and 25-hydroxyvitamin D. Before bariatric surgery is attempted, micronutrient deficiencies and insufficiencies should be corrected (particularly iron, zinc, vitamin B12 and vitamin D). Moreover, appropriate preoperative diet should be initiated, and a high protein and/or liquid diet may be recommended [Citation78,Citation79] at least 2 weeks prior to the scheduled bariatric surgery to ensure adequate protein intake, hydration as well as to provide practice in the use of protein supplements. Weight loss of 5% to 10% is also recommended to decrease total liver volume, facilitate the laparoscopic procedure and improve surgical outcomes. After surgery, patient compliance to the prescribed diet should be assessed, and rehabilitation (training and exercises) should be initiated to achieve maximum health improvement [Citation80,Citation81].
3.3.6. Therapies not currently recommended
3.3.6.1. Laparoscopic adjustable gastric banding
This is a purely restrictive procedure that is no longer recommended due to its relatively modest amount of expected weight loss, coupled with high rates of revision and weight recidivism.
3.3.6.2. Liposuction
We do not currently suggest liposuction as a strategy for long-term weight loss. While it appears to potentially improve insulin sensitivity and cardiovascular disease risk, available evidence remains insufficient and conflicting [Citation82–84].
3.3.6.3. Dietary supplements
Over-the-counter dietary supplements are widely used by individuals attempting to lose weight and misinformation regarding them is rampant [Citation85]. We recommend against the use of dietary supplements (e.g. ephedra, green tea, guar gum) seeing as they are poorly regulated and evidence to support their efficacy and safety are limited and generally of low quality [Citation86,Citation87].
3.3.6.4. Acupuncture
Although it has also been studied for the treatment of obesity, evidence regarding the efficacy of acupuncture is limited and of insufficient quality as most of the studies have been either uncontrolled trials, or of small samples and short durations, or do not includes adequate placebo controls[Citation88].
3.3.6.5. Off label medication
We do not recommend use of off label medications for weight reduction due to lack of evidence of efficacy and safety for the treatment of obesity.
3.4. Maintenance of weight loss
Maintenance of weight loss is made difficult by the decrease in energy expenditure and the favor of weight reinstatement that is induced by weight loss [Citation89]. Weight regain (or recidivism) is a common problem after obesity treatment [Citation90]. Besides the long-term use of the drug therapy, we recommend continued behavioral interventions such as frequent self-weighing [Citation91,Citation92], consumption of a reduced-calorie diet [Citation93], and high levels of physical activity [Citation94]. Continued access to weight loss programs ensures slower weight regain [Citation95] and the combination of drug therapy (i.e. liraglutide) with exercise leads to better weight loss maintenance[Citation96].
3.5. Obesity in special groups
3.5.1. Obesity in children and adolescents
The global obesity and overweight epidemic also affects children of all ages, among whom it a serious health issue. Childhood obesity is highly likely to continue into adolescence and adulthood [Citation97,Citation98]. Early adiposity rebound is an established predictor of later obesity and overweight [Citation97,Citation99]. Parental obesity, maternal gestational weight gain and infant overnutrition/high birth weight affect the development of childhood obesity [Citation100–102]. Genetic (monogenic disorders of the energy balance pathway, race and ethnicity) and environmental factors (lifestyle, stress, sleep deprivation, screen time) also contribute to childhood and adolescent obesity [Citation100,Citation103]. Other factors that should be considered include endocrine disorders with an obesity phenotype (e.g. Hypothyroidism, Cushing syndrome, GH deficiency and Pseudohypoparathyroidism type 1a (PHP1a)), insulin dynamic disorders, syndromic obesity and the use of certain medications. That being said, early obesity diagnosis and treatment is faced with societal hindrances, poor parental recognition and inadequate healthcare provider training. This results in delayed interventions and advanced disease stages, and consequently, poorer long-term health outcomes [Citation104]. It is therefore critical to improve screening initiatives for childhood obesity and provide comprehensive management, as necessary.
Currently, pharmacological options include 3 mg liraglutide (Saxenda®, daily injection) which is approved for the children above the age of 12 with BMI corresponding to 30 or more.
3.5.1.1. Screening and Evaluation
The assessment of an obese child requires establishing a complete history as well as physical examination and health risk assessment.
BMI assessment is not applicable for infants younger than 2 years of age. Infants’ weight percentile is instead compared to length percentile, and weight group is determined based on applicable growth charts. If the weight for recumbent length is equal to or exceeds the 97.7th percentile of WHO growth standards, a child younger than 2 years of age is considered obese.
BMI charts are used for children aged 2–20 years (Center for Disease Control (CDC) charts (Appendixes 1 and 2) or WHO charts (Appendixes 3 and 4). Using the revised CDC charts from 2000, a BMI ≥ 85th percentile, but < 95th percentile is indicative of overweight, while obesity is diagnosed if the BMI is ≥ 95th percentile for age and gender. Extreme obesity is defined as a BMI ≥120% of the 95th percentile or ≥ 35 kg/m2 (also referred to as class 2 obesity), and a BMI ≥140% of the 95th percentile or ≥40 kg/m2 indicates class 3 obesity.
To note that BMI is age- and puberty-dependent in children, which is reflected in the higher accuracy of BMI z-score compared to standard BMI for the assessment of childhood adiposity (Appendixes 3,4). According to the WHO growth reference for school aged children and adolescents, overweight is indicated by one standard deviation BMI for age and sex, and obese is indicated by two standard deviations BMI for age and sex.
Waist circumference is also recognized as a strong predictor of the later development of metabolic disturbance in children and adolescents [Citation105,Citation106] and might be more predictive than BMI which fails to discriminate between muscle, bone and both subcutaneous and visceral fat [Citation107,Citation108].
Diagnostic evaluation should focus on establishing history, followed by physical examination and laboratory investigations. Guidance for the initial evaluation of children are listed in .
Table 5. Diagnostic Evaluation of Childhood Obesity and Its Comorbidities.
Moreover, it is important to note that many of the obesity-related comorbidities recognized in adulthood begin to develop in childhood and should also be considered during child assessment. These comorbidities include:
Metabolic syndrome (clustering of type 2 diabetes, hypertension and dyslipidemia)
Nonalcoholic fatty liver disease
PCOS
Earlier age of pubertal initiation.
Infertility in older adolescents due PCOS in females and excessive aromatization of androgen to estrogen by peripheral adipose tissue in male.
Gynecomastia in male
Decreased GH secretion.
Subclinical hypothyroidism
Elevated cortisol level
Vitamin D deficiency.
Pseudotumor cerebri.
Obstructive sleep apnea.
Orthopedic morbidity: slipped capital femoral epiphysis, Blount's disease, scoliosis
Cholelithiasis in obese adolescents especially females.
Psychological distress including clinical depression, eating disorders
3.5.1.2. Approach to therapy for childhood obesity
3.5.1.2.1. Comprehensive lifestyle modification
Comprehensive lifestyle modification is also the cornerstone of obesity therapy in children. Weight maintenance rather than weight loss is recommended in children among whom the prevention of obesity is more cost-effective and feasible compared to its treatment. In order to achieve this, establishing a healthy lifestyle through adequate diets and physical activity is effective and encouraged as early as possible [Citation109–111]. In addition to preventing the development of obesity, such lifestyle, dietary and behavioral interventions could also be reflected in immediate benefits during childhood/adolescence (e.g. better fitness, more energy, better nutrition) and long-term improvement of adult health and habits, and by extension, lower chronic disease risk. A weight-maintenance program is advised by the American Academy of Pediatrics for overweight children aged 2 and above for the limitation of weight gain and the progressive decrease of BMI. The modification of eating habits could be preferable for children that are obese, with a target of gradual weight loss of no more than around 0.5 Kg/month in ages 6–11, and 1 Kg/week for older children and adolescents. Weight maintenance programs consist of monitoring the type and amount of food intake in addition to the increase of physical activity. Family commitment remains essential for the successful implementation and adherence to weight management interventions among children.
Dietary intervention
The optimal modification of food intake during childhood remains debated with no universally accepted approach. That being said, clinical decisions should be based on a robust understanding of appropriate intake for a child of normal weight. Childhood obesity management is age-dependent. Exclusive breast feeding is the diet of choice in the first 6 months of life, with the avoidance of early complementary food introduction. The latter was found to be associated with increased BMI in childhood and adolescence. Moreover, sugar intake should be limited, with no sugar-containing beverages allowed for infants [Citation112]. Children and adolescents can be allowed 5% to 10% of total daily energy from sugar, with the exception of sugar-containing fruit and vegetable due to their higher fiber content. Successful approaches to encourage weight loss or maintenance are not limited to the elimination of sugar intake, but also shifting to a low glycemic load diet, reducing portion sizes, fast-food consumption and snacking, promoting breakfast as well as increasing the consumption of fruits and vegetables.
Physical activity intervention
Physical activity interventions should be prioritized, long-term, sustained and implemented into behavioral modifications at several levels, namely the individual, the family, the school and the community [Citation113].
The current recommendation of the WHO is moderate to vigorous intensity physical activity for at least 60 minutes every day for children 5–17 years old. Increasing the duration of physical activity is associated with increased health benefits. Daily activity should primarily be aerobic, with the integration of vigorous intensity activities targeting muscle and bone strength 3 or more times every week. As for infants, activity should be encouraged in addition to direct interaction with parents as much as possible.
Appropriate physical activity interventions include:
Making school-based physical education mandatory for every child and school.
Increasing access to after-school recreation.
Increasing culturally appropriate activities.
Reducing the competitive nature of sports so that more children will participate.
Increasing the incorporation of physical activity into daily life (e.g. stairs, walking to school as appropriate).
Increasing the participation of parents in physical recreation, for their own weight management and as role models for their children.
Other behavioral interventions
Increased screen time is a risk factor for obesity in childhood and adolescence [Citation114]. Children under 2 years of age must have no screen time, while those aged 2–5 years should have limited use of no more than an hour. Ideally, any screen time should be spent co-watching with engaged adults.
Enhancing children’s sleep may also be an effective strategy for preventing and treating pediatric obesity [Citation115]. Infants 1–2 years need 11–14 hours of nightly sleep, preschool children need 10–13 hours and school aged children need 9–11 hours with regular sleep and wake-up times.
Family intervention
Parental involvement is critical for the success of obesity management in children and adolescents [Citation116,Citation117]. Often enough, various caretakers (grandmothers, babysitters, etc.) will feed children and allow unrestricted television access, and some parents are known to use food as a reward to obtain the child’s love. The family itself must therefore be the target of lifestyle intervention and the concept of ‘food is not love’ must be emphasized. Eating meals together as a family and eating at the table as opposed to in front of the television, removing over controlling behaviors towards meal consumption, promoting slow eat and food enjoyment are some of most important family interventions.
3.5.1.2.2. Drug therapy
Drug-based therapy remains limited in children with obesity and is primarily restricted till after the failure of formal and intensive lifestyle modifications to reduce weight gain and improve health outcomes. The majority of available weight-loss medication do not have FDA approval for use in children. Available options for children include orlistat (>12 years of age) and phentermine (>16 years of age), both of which induce only small to moderate weight loss albeit with concerning side effects [Citation118,Citation119]. Orlistat is associated with oily stools while phentermine may cause anxiety, tremors, and slightly increased blood pressure. Other medications can be used in children but have not received approval for the treatment of obesity in children, namely: metformin (used for type 2 diabetes mellitus in children ≥ 12 years of age) [Citation119], octreotide (used for hypothalamic obesity) [Citation120], leptin (used only in leptin deficiency) [Citation121], Glucagon-like peptide 1 (recently approved for treatment type 2 diabetes mellitus in children ≥ 10 years of age, with some weight reduction effect) [Citation122] and growth hormone (used in Prader-Willi syndrome) [Citation123].
3.5.1.2.3. Surgery
Bariatric surgery should be reserved for the treatment of severe obesity in adolescents [Citation124,Citation125]. Current adolescent bariatric recommendations include[Citation112]:
BMI >35 kg/m2 with moderate to severe comorbidities
or
BMI >40 kg/m2 and skeletal and sexual maturity (generally age 14 for girls and 15 for boys).
3.5.2. Obesity in pregnancy
The impact of obesity on pregnancy outcome emerged along with the rise of obesity among the antenatal population. In addition to its risk of cardiovascular disease, obesity is associated with subfertility and higher rates of miscarriage [Citation126,Citation127]. Obesity and overweight also affect fetal growth [Citation128] and increase the risk of pregnancy-related adverse outcomes such as gestational diabetes, gestational hypertension, caesarean delivery, birth trauma, infections and still birth [Citation129,Citation130]. The deleterious effect of pre-conception maternal BMI extend after delivery, affecting offspring in the neonatal period well into their adult life. Children born to women who were overweight/obese are significantly more likely to be born large for gestational weight and to become overweight/obese at later stages in life [Citation131]. Congenital abnormalities, preterm birth, and higher risk of admission to the neonatal intensive care unit are also more frequent among women who are overweight or obese [Citation132–134].
The prevention and management of obesity in women of childbearing age is therefore a public health issue. Women should be advised to enter pregnancy with a normal BMI (20–24.9 Kg/m2). Overweight women (BMI of 25–29.9 Kg/m2) and obese women (BMI ≥ 30 Kg/m2) should be informed of the adverse pregnancy outcomes associated with obesity and should be counseled to lose weight by dieting and exercise before conceiving. Women with a BMI of 40 kg/m2 or more should be strongly advised to avoid pregnancy until they have lost weight. A multidisciplinary approach is generally preferable and mothers may require referral to a dietitian/nutritionist [Citation135] for counseling and behavioral interventions, which can reduce the risk of adverse pregnancy outcomes [Citation136].
Women should be weighed in preconception care visits and weight gain during pregnancy should be tightly controlled. Adequate energy and nutrient intake during pregnancy is essential to prevent excessive or suboptimal weight gain and related adverse outcomes. Excessive gestational weight gain and obesity are associated with the development of maternal diabetes and child overweight [Citation137], while higher rates of low birthweight and neonatal/intrauterine mortality can result from inadequate weight gain [Citation130,Citation138]. Maternal diet should be supplemented as necessary during pregnancy with protein, fat and vitamins (e.g. cheese, beans, eggs).
3.5.3. Overweight and obesity in the elderly
When screening elderly patients and evaluating their weight, it is important to take into consideration the physiological changes incurred by older age. BMI may actually underestimate cardiometabolic risk in the elderly [Citation139–141] possibly due to the decline in muscle mass and quality with age, and the increased burden of excess fat mass on weak muscles [Citation142]. Fat-free mass index and waist circumference are probably more accurate than BMI as a measure of obesity and predictor of mortality in the elderly [Citation143,Citation144]. While debatable, evidence suggests the protective effect of obesity in elderly patients, among whom BMI was inversely correlated with mortality in what became known as the ‘obesity paradox’ [Citation145]. Weight-loss therapy which involves lifestyle modifications (diet and exercise) should be offered for elderly people with the aim to reduce the risk of type 2 diabetes mellitus and hypertension, as well as to improve osteoarthritis, mobility, and physical function [Citation146]. Aerobic and resistance exercise should also be encouraged in the elderly as it will lead to improvements in physical function and to the amelioration of frailty [Citation147]. Exercise should be of moderate intensity, generally aiming at pulse rate of 100/min for patients aged 60–70 years. Osteosarcopenia is common in elderly people and should be assessed before considering initiating obesity therapy as this condition might worsen with weight loss therapy [Citation148]. Extra caution should be taken when prescribing weight-loss medications in elderly as they are more likely to have nutritional deficiencies, polypharmacy, abnormalities in renal and hepatic function, subclinical cardiovascular disease, and impaired cognition, which can increase the risks associated with various weight-loss interventions. Other pharmacological approaches include testosterone replacement in elderly men with hypogonadism, an intervention which produces long-term benefits on the level of weight loss [Citation149]. As for surgical interventions, data on the efficacy and safety of bariatric surgery in the elderly are limited. It seems that elderly patients could benefit from bariatric surgery, which are relatively safe and effective in this population for weight loss and improvement in weight-related comorbidities [Citation150–153], albeit to a potentially lesser extent when compared to younger patients.
4. Conclusions
One third of the adult population in Iraq is overweight and another third is obese. The contribution of obesity and overweight to morbidity and mortality in adults as well as children and adolescents call for serious concerted efforts towards its prevention and management. These recommendations outline a management plan and standards of care that are relevant to the Iraqi population and that can prevent/manage obesity and obesity-related complications. Clinical diagnosis depends on body mass index (BMI) and waist circumference. Exploring the etiology of this disease reveals a complex interaction between behavioral, social (rapid urbanization), environmental and genetic factors, which should be addressed. Treatment should initially rely solely on comprehensive lifestyle interventions involving dietary changes to reduce calorie intake, an increase in physical activity, and behavioral modification. Pharmacotherapy, medical devices and bariatric surgery can be considered as needed when non-pharmacological approaches fail.
Declaration of financial/other relationships
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
A reviewer on this manuscript has disclosed receiving a research grant from Novo Nordisk. Peer reviewers on this manuscript have no other relevant financial relationships or otherwise to disclose.
Author contributions
The main frame of the guidelines are written by Dr Hussein Ali Nwayyir; the introduction was written by Dr Faris Abdulkareem; pediatric sections were written by Esraa Majid Mutasher and revised by Munib AlZubaidi; obesity in the elderly was written by Dr Majid Hameed; nutrition sections were written by Muthana Abdulrazzaq Jabbar and Wefaq Hassan; surgical sections were written by Osama Muhammed Ali and agreed upon by Faleh Muhsen and Ali Dawood; pregnancy section was written by Muhammed Chabek; Ali Albayati, Hilal Alsaffar, Haidar Iyad, Ibtihal Shukri and Lajeen Al Khazrajiare members of the revision team; editing, framing, referencing and logistics were done by Alhassan Zalzala.
Acknowledgments
The authors would like to thank Professor Abbas Mahdi Rehma, assistant Professor Musa Qasim Husain and Consultant Dr Hani Alansari for providing a comprehensive review of the guideline.
The authors would also like to thank Racha Aaraj, Pharm D, MSc, MPH and Nancy Al Akkary, BSc, MSc from Phoenix Clinical Research, Lebanon for providing editorial and medical writing assistance for the preparation of this manuscript.
Additional information
Funding
References
- WHO. Obesity and overweight. Published 2021. Accessed September 13, 2021. https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight
- AMA. Report of the council on science and public health - report 3-A-13. Accessed September 13, 2021. https://www.ama-assn.org/sites/ama-assn.org/files/corp/media-browser/public/about-ama/councils/Council Reports/council Reports/councilonsciencepublichealth/a13csaph3.pdf
- Bays H, Seger J, Primack C, et al. Obesity Algorithm, presented by the Obesity Medicine Association. Accessed September 13, 2021. https://www.amga.org/amga/media/pdfs/performance improvement and publications/best practices and analytics/learning collaboratives/obesity care model/oma_obesity improvement and publications/best practices and analytics/learning collaboratives/obesity care model/oma_obesityalgorithm.pdf
- Dai H, Alsalhe TA, Chalghaf N, et al. The global burden of disease attributable to high body mass index in 195 countries and territories, 1990–2017: an analysis of the global burden of disease study. Wareham NJ, ed. PLOS Med. 2020;17(7):e1003198.
- Murray CJL, Aravkin AY, Zheng P, et al. Global burden of 87 risk factors in 204 countries and territories, 1990–2019: a systematic analysis for the global burden of disease study 2019. Lancet. 2020;396(10258):1223–1249.
- Felisbino-Mendes MS, Cousin E, Malta DC, et al. The burden of non-communicable diseases attributable to high BMI in Brazil, 1990–2017: findings from the global burden of disease study. Popul Heal Metrics. 2020;18(1):1–13.
- Bhaskaran K, dos-Santos-Silva I, DA L, et al. Association of BMI with overall and cause-specific mortality: a population-based cohort study of 3·6 million adults in the UK. Lancet Diabetes Endocrinol. 2018;6(12):944–953.
- Collaboration PS. Body-mass index and cause-specific mortality in 900 000 adults: collaborative analyses of 57 prospective studies. Lancet. 2009;373(9669):1083–1096.
- Rozjabek H, Fastenau J, LaPrade A, et al. and Health-Related Quality of Life, Patient Activation, Work Productivity, and Weight Loss Behaviors in the United States. Diabetes, Metab Syndr Obes Targets Ther. 2020;13:2049.
- Kolotkin RL, Andersen JR. A systematic review of reviews: exploring the relationship between obesity, weight loss and health‐related quality of life. Clin Obes. 2017;7(5):273.
- Nikoloski Z, Williams G. Metabolic Syndrome. In: Ahima RS, editor. Obesity in Middle East. Springer International Publishing; 2016. p. 55–72. DOI:10.1007/978-3-319-11251-0_6.
- Ministry of Health/Directorate of Public Health, Ministry of Planning/Central Statistical Organization, World Health Organization. Non-Communicable Diseases Risk Factors STEPS Survey Iraq 2015; 2015. Accessed September 13, 2021. https://untobaccocontrol.org/impldb/wp-content/uploads/iraq_2018_annex-1_STEPS_report_2015.pdf
- Wharton S, Lau DCW, Vallis M, et al. Obesity in adults: a clinical practice guideline. Can Med Assoc J. 2020;192(31):E875–E891.
- Yumuk V, Tsigos C, Fried M, et al. European Guidelines for Obesity Management in Adults. Obes Facts. 2015;8(6):402.
- Ma C, Avenell A, Bolland M, et al. Effects of weight loss interventions for adults who are obese on mortality, cardiovascular disease, and cancer: systematic review and meta-analysis. BMJ. 2017;359:j4849.
- Haase CL, Lopes S, Olsen AH, et al. Weight loss and risk reduction of obesity-related outcomes in 0.5 million people: evidence from a UK primary care database. Int J Obes. 2021;45(6):1249–1258.
- Kritchevsky SB, Beavers KM, Miller ME, et al. Intentional Weight Loss and All-Cause Mortality: a Meta-Analysis of Randomized Clinical Trials. PLoS One. 2015;10(3):3.
- LeBlanc ES, Patnode CD, Webber EM, et al. Behavioral and Pharmacotherapy Weight Loss Interventions to Prevent Obesity-Related Morbidity and Mortality in Adults: updated Evidence Report and Systematic Review for the US Preventive Services Task Force. JAMA. 2018;320(11):1172–1191. doi:10.1001/JAMA.2018.7777
- Zaghloul H, Elshakh H, Elzafarany A, et al. A systematic review of randomized controlled trials of dietary interventions for weight loss in adults in the Middle East and north Africa region. Clin Obes. 2021;11(2):2.
- Gupta N, Bombak A, Foroughi I, et al. Evidence-based recommendations to assist adults with depression to become lifelong movers. Heal Promot Chronic Dis Prev Canada. 2020;40(11/12):329–335.
- Althumiri NA, Basyouni MH, AlMousa N, et al. Exploring weight stigma in Saudi Arabia: a nationwide cross-sectional study. Int J Environ Res Public Health. 2021;18(17):9141.
- Rubino F, Puhl RM, Cummings DE, et al. Joint international consensus statement for ending stigma of obesity. Nat Med. 2020;26(4):485–497.
- Tomiyama AJ, Carr D, Granberg EM, et al. How and why weight stigma drives the obesity ‘epidemic’ and harms health. BMC Med. 2018;16(1):123.
- Althumiri NA, Basyouni MH, BinDhim NF, et al. Levels and Associations of Weight Misperception with Healthy Lifestyle among Adults in Saudi Arabia. Obes Facts. 2021 September 14;1–7. doi:10.1159/000518633 Published online
- Zhu SK, Wang ZM, Heshka S, et al. Waist circumference and obesity-associated risk factors among whites in the third National Health and Nutrition Examination Survey: clinical action thresholds. Am J Clin Nutr. 2002;76(4):743–749.
- Ross R, Neeland IJ, Yamashita S, et al. Waist circumference as a vital sign in clinical practice: a Consensus Statement from the IAS and ICCR Working Group on Visceral Obesity. Nat Rev Endocrinol. 2020;16(3):177–189.
- Heart N, Institute B. Managing Overweight and Obesity in Adults Systematic Evidence Review From the Obesity Expert Panel, 2013. Published online 2013.
- Mansour AA, Al-Hassan AA, Al-Jazairi MI. Cut-off values for waist circumference in rural Iraqi adults for the diagnosis of metabolic syndrome. Rural Remote Health. 2007;7(4):765.
- Valk ES, Akker ELT, Savas M, et al. A comprehensive diagnostic approach to detect underlying causes of obesity in adults. Obes Rev. 2019;20(6):795–804.
- Vaisse C, Clement K, Durand E, et al. Melanocortin-4 receptor mutations are a frequent and heterogeneous cause of morbid obesity. J Clin Invest. 2000;106(2):253–262.
- Farooqi IS, Yeo GSH, Keogh JM, et al. Dominant and recessive inheritance of morbid obesity associated with melanocortin 4 receptor deficiency. J Clin Invest. 2000;106(2):271–279.
- Hainerová I, Larsen LH, Holst B, et al. Melanocortin 4 Receptor Mutations in Obese Czech Children: studies of Prevalence, Phenotype Development, Weight Reduction Response, and Functional Analysis. J Clin Endocrinol Metab. 2007;92(9):3689–3696.
- Saeed S, Bonnefond A, Manzoor J, et al. Genetic variants in LEP, LEPR, and MC4R explain 30% of severe obesity in children from a consanguineous population. Obesity (Silver Spring). 2015;23(8):1687–1695.
- Farooqi IS, Keogh JM, Yeo GSH, et al. Clinical spectrum of obesity and mutations in the melanocortin 4 receptor gene. N Engl J Med. 2003;348(12):1085–1095.
- Vollbach H, Brandt S, Lahr G, et al. Prevalence and phenotypic characterization of MC4R variants in a large pediatric cohort. Int J Obes (Lond). 2017;41(1):13–22.
- Branson R, Potoczna N, Kral JG, et al. Binge eating as a major phenotype of melanocortin 4 receptor gene mutations. N Engl J Med. 2003;348(12):1096–1103.
- Greenfield JR, Miller JW, Keogh JM, et al. Modulation of blood pressure by central melanocortinergic pathways. N Engl J Med. 2009;360(1):44–52.
- Iepsen EW, Zhang J, Thomsen HS, et al. Patients with Obesity Caused by Melanocortin-4 Receptor Mutations Can Be Treated with a Glucagon-like Peptide-1 Receptor Agonist. Cell Metab. 2018;28(1):23–32.e3.
- Iepsen EW, Have CT, Veedfald S, et al. GLP-1 Receptor Agonist Treatment in Morbid Obesity and Type 2 Diabetes Due to Pathogenic Homozygous Melanocortin-4 Receptor Mutation: a Case Report. Cell Reports Med. 2020;1(1):1.
- Blackburn G. Effect of Degree of Weight Loss on Health Benefits. Obes Res. 1995;3(S2):211s–216s.
- Ryan DH, Yockey SR, Loss W. Improvement in Comorbidity: differences at 5%, 10%, 15%, and Over HHS Public Access. Current Obesity Reports. 2017;6(2):187–194.
- Unick JL, Beavers D, Jakicic JM, et al. Effectiveness of lifestyle interventions for individuals with severe obesity and type 2 diabetes: results from the look AHEAD trial. Diabetes Care. 2011;34(10):2152–2157.
- Singh N, Stewart RAH, Benatar JR. Intensity and duration of lifestyle interventions for long-term weight loss and association with mortality: a meta-analysis of randomised trials. BMJ Open. 2019;9(8):e029966.
- Khera R, Murad MH, Chandar AK, et al. Association of Pharmacological Treatments for Obesity With Weight Loss and Adverse Events. JAMA. 2016;315(22):2424.
- Singh AK, Singh R. Pharmacotherapy in obesity: a systematic review and meta-analysis of randomized controlled trials of anti-obesity drugs. Expert Rev Clin Pharmacol. 2020;13(1):53–64.
- Cosentino C, Marchetti C, Monami M, et al. Efficacy and effects of bariatric surgery in the treatment of obesity: network meta-analysis of randomized controlled trials. Nutr Metab Cardiovasc Dis. 2021;31(10):2815–2824.
- Ramai D, Singh J, Mohan BP, et al. Influence of the Elipse Intragastric Balloon on Obesity and Metabolic Profile: a Systematic Review and Meta-Analysis. J Clin Gastroenterol. 2021;55(10):10.
- Vantanasiri K, Matar R, Beran A, et al. Safety of a Procedureless Gastric Balloon for Weight Loss: a Systematic Review and Meta-Analysis. Obes Surg. 2020;30(9):3341–3346.
- Ogden J, Clementi C, Aylwin S. The impact of obesity surgery and the paradox of control: a qualitative study. Psychol Health. 2006;21(2):273–293.
- The Diabetes Prevention Program (DPP): description of lifestyle intervention. Diabetes Care. 2002;25(12):2165–2171.
- Elfhag K, Rössner S. Who succeeds in maintaining weight loss? A conceptual review of factors associated with weight loss maintenance and weight regain. Obes Rev. 2005;6(1):67–85.
- Stunkard AJ, Messick S. The three-factor eating questionnaire to measure dietary restraint, disinhibition and hunger. J Psychosom Res. 1985;29(1):71–83.
- Bandura A. Self-Efficacy. Corsini Encycl Psychol. 2010 January 30;1–3. doi:10.1002/9780470479216.CORPSY0836 Published online
- Wallston KA, Wallston BS, DeVellis R. Development of the Multidimensional Health Locus of Control (MHLC) Scales. Health Educ Monogr. 1978;6(2):160–170.
- Dansinger ML, Gleason JA, Griffith JL, et al. Comparison of the Atkins, Ornish, Weight Watchers, and Zone Diets for Weight Loss and Heart Disease Risk Reduction. JAMA. 2005;293(1):43.
- Ge L, Sadeghirad B, Ball GDC, et al. Comparison of dietary macronutrient patterns of 14 popular named dietary programmes for weight and cardiovascular risk factor reduction in adults: systematic review and network meta-analysis of randomised trials. BMJ. 2020;369. DOI:10.1136/BMJ.M696.
- Clark JE. Diet, exercise or diet with exercise: comparing the effectiveness of treatment options for weight-loss and changes in fitness for adults (18–65 years old) who are overfat, or obese; systematic review and meta-analysis. J Diabetes Metab Disord. 2015;14(1):31.
- Nackers LM, Middleton KR, Dubyak PJ, et al. Effects of Prescribing 1,000 versus 1,500 Kilocalories per Day in the Behavioral Treatment of Obesity: a Randomized Trial. Obesity (Silver Spring). 2013;21(12):2481.
- Iłowiecka K, Glibowski P, Skrzypek M, et al. Dietitian and Psychological Support of Obese Patients Who Have Reduced Their Weight Allows Them to Maintain the Effects. Nutrients. 2021;13(6):2020.
- Bellicha A, Baak MA, Battista F, et al. Effect of exercise training on weight loss, body composition changes, and weight maintenance in adults with overweight or obesity: an overview of 12 systematic reviews and 149 studies. Obes Rev. 2021;22(S4):S4.
- McCarthy D, Berg A. Weight Loss Strategies and the Risk of Skeletal Muscle Mass Loss. Nutrients. 2021;13(7):2473.
- Ho SS, Dhaliwal SS, Hills AP, et al. The effect of 12 weeks of aerobic, resistance or combination exercise training on cardiovascular risk factors in the overweight and obese in a randomized trial. BMC Public Health. 2012;12(1):704.
- Marc-Hernández A, Ruiz-Tovar J, Aracil A, et al. Effects of a High-Intensity Exercise Program on Weight Regain and Cardio-metabolic Profile after 3 Years of Bariatric Surgery: a Randomized Trial. Sci Rep. 2020;10(1):3123.
- Johnson NA, Sultana RN, Brown WJ, et al. Physical activity in the management of obesity in adults: a position statement from Exercise and Sport Science Australia. J Sci Med Sport. 2021;24(12):1245–1254.
- Wharton S, Liu A, Pakseresht A, et al. Real‐World Clinical Effectiveness of Liraglutide 3.0 mg for Weight Management in Canada. Obesity. 2019;27(6):917–924.
- le Roux CW, Astrup A, Fujioka K, et al. 3 years of liraglutide versus placebo for type 2 diabetes risk reduction and weight management in individuals with prediabetes: a randomised, double-blind trial. Lancet. 2017;389(10077):1399–1409.
- Moon S, Lee J, Chung HS, et al. Efficacy and Safety of the New Appetite Suppressant, Liraglutide: a Meta-Analysis of Randomized Controlled Trials. Endocrinol Metab. 2021;36(3):647–660.
- Sahebkar A, Simental-Mendía LE, Ž R, et al. Effect of orlistat on plasma lipids and body weight: a systematic review and meta-analysis of 33 randomized controlled trials. Pharmacol Res. 2017;122:53–65.
- Sumithran P, Benefit-Risk PJ. Assessment of Orlistat in the Treatment of Obesity. Drug Saf. 2014;37(8):597–608.
- Weinsier RL, Wilson LJ, Lee J. Medically safe rate of weight loss for the treatment of obesity: a guideline based on risk of gallstone formation. Am J Med. 1995;98(2):115–117.
- Andrés-Imaz A, Martí-Gelonch L, Eizaguirre-Letamendia E, et al. Incidence and risk factors for de novo cholelithiasis after bariatric surgery. Cir Esp. 2020;99(9):648–654.
- Stokes CS, Gluud LL, Casper M, et al. Ursodeoxycholic acid and diets higher in fat prevent gallbladder stones during weight loss: a meta-analysis of randomized controlled trials. Clin Gastroenterol Hepatol. 2014;12(7):7.
- Maa S, Salman A, US M, et al. Ursodeoxycholic acid for the prevention of gall stones after laparoscopic sleeve gastrectomy: a prospective controlled study. Surg Endosc. 2022. DOI:10.1007/S00464-021-08980-3. Published online.
- Moore RL, Seger MV, Garber SM, et al. Clinical safety and effectiveness of a swallowable gas-filled intragastric balloon system for weight loss: consecutively treated patients in the initial year of U.S. commercialization. Surg Obes Relat Dis. 2019;15(3):417–423.
- Sullivan S, Swain J, Woodman G, et al. Randomized sham-controlled trial of the 6-month swallowable gas-filled intragastric balloon system for weight loss. Surg Obes Relat Dis. 2018;14(12):1876–1889.
- Giulietti C, Menculini G, Brufani F, et al. Psychiatric Comorbidity in Bariatric Surgery: a Retrospective Study in a General Hospital. Psychiatr Danub. 2021;33(Suppl9): 75–79. Accessed September 30, 2021. https://pubmed.ncbi.nlm.nih.gov/34559782/
- Oliver A, Hooper S, Lau R, et al. Effect of a multidisciplinary rehabilitation program for patients receiving weight management interventions on eating behaviours and health-related quality of life. Obes Res Clin Pract. 2021;15(3):268–274.
- Yolsuriyanwong K, Thanavachirasin K, Sasso K, et al. Effectiveness, Compliance, and Acceptability of Preoperative Weight Loss with a Liquid Very Low-Calorie Diet Before Bariatric Surgery in Real Practice. Obes Surg. 2019;29(1):54–60.
- Van Nieuwenhove Y, Dambrauskas Z, Campillo-Soto A, et al. Preoperative very low-calorie diet and operative outcome after laparoscopic gastric bypass: a randomized multicenter study. Arch Surg. 2011;146(11):1300–1305.
- In G, Taskin HE, Al M, et al. Comparison of 12-Week Fitness Protocols Following Bariatric Surgery: aerobic Exercise Versus Aerobic Exercise and Progressive Resistance. Obes Surg. 2021;31(4):1475–1484.
- Gils Contreras A, Bonada Sanjaume A, Becerra-Tomás N, et al. Adherence to Mediterranean Diet or Physical Activity After Bariatric Surgery and Its Effects on Weight Loss, Quality of Life, and Food Tolerance. Obes Surg. 2020;30(2):687–696.
- Klein S, Fontana L, Young VL, et al. Absence of an Effect of Liposuction on Insulin Action and Risk Factors for Coronary Heart Disease. N Engl J Med. 2004;350(25):2549–2557.
- Gibas-Dorna M, Szulińska M, Turkowski P, et al. The Effect of VASER Abdominal Liposuction on Metabolic Profile in Overweight Males:. American Journal of Men’s Health. 2016;11(2):284–293.
- Giugliano G, Nicoletti G, Grella E, et al. Effect of liposuction on insulin resistance and vascular inflammatory markers in obese women. Br J Plast Surg. 2004;57(3):190–194.
- Ng JY, Ahmed S, Zhang CJ. Dietary and herbal supplements for weight loss: assessing the quality of patient information online. Nutr J. 2021;20(1):72.
- Batsis JA, Apolzan JW, Bagley PJ, et al. A Systematic Review of Dietary Supplements and Alternative Therapies for Weight Loss. Obesity. 2021;29(7):1102–1113.
- Kidambi S, Batsis JA, Donahoo WT, et al. Dietary supplements and alternative therapies for obesity: a Perspective from The Obesity Society’s Clinical Committee. Obesity. 2021;29(7):1095–1098.
- Chen J, Chen D, Ren Q, et al. Acupuncture and related techniques for obesity and cardiovascular risk factors: a systematic review and meta-regression analysis. Acupunct Med. 2020;38(4):227–234.
- Ravussin E, Smith SR, Ferrante AW. Physiology of Energy Expenditure in the Weight-Reduced State. Obesity. 2021;29(S1):S31–S38.
- Ben-Porat T, Mashin L, Kaluti D, et al. Weight Loss Outcomes and Lifestyle Patterns Following Sleeve Gastrectomy: an 8-Year Retrospective Study of 212 Patients. Obes Surg. 2021;31(11):4836–4845.
- Wilkinson L, Pacanowski CR, Levitsky D. Three-Year Follow-Up of Participants from a Self-Weighing Randomized Controlled Trial. J Obes. 2017;2017:1–7.
- Pacanowski CR, Bertz F, Levitsky DA. Daily Self-Weighing to Control Body Weight in Adults. SAGE Open. 2014;4(4):215824401455699.
- Correa LL, Moretti A, Pam DS, et al. Effectiveness and Safety of a Very Low-Calorie Ketogenic Diet on Weight Regain Following Bariatric Surgery. Obes Surg. 2021;31(12):5383–5390.
- van Baak MA, Hul G, Astrup A, Saris WH. Physical Activity, Weight Loss, and. Weight Maintenance in the DiOGenes Multicenter Trial. Front Nutr. 2021:8. DOI:10.3389/fnut.2021.683369
- Hartmann-Boyce J, Theodoulou A, Oke JL, et al. Association between characteristics of behavioural weight loss programmes and weight change after programme end: systematic review and meta-analysis. BMJ. 2021;374:n1840.
- Lundgren JR, Janus C, Jensen SBK, et al. Healthy Weight Loss Maintenance with Exercise, Liraglutide, or Both Combined. N Engl J Med. 2021;384(18):1719–1730.
- Brisbois TD, Farmer AP, McCargar LJ. Early markers of adult obesity: a review. Obes Rev. 2012;13(4):347–367.
- Gordon-Larsen P, The NS, Adair LS. Longitudinal Trends in Obesity in the United States From Adolescence to the Third Decade of Life. Obesity. 2010;18(9):1801–1804.
- Freedman DS, Goodman AB, King RJ, et al. The Relation of Adiposity Rebound to Subsequent BMI in a Large Electronic Health Record Database. Child Obes. 2021;17(1):51–57.
- Ziauddeen N, Wilding S, Roderick PJ, et al. Predicting the risk of childhood overweight and obesity at 4–5 years using population-level pregnancy and early-life healthcare data. BMC Med. 2020;18(1):1–15.
- Rooney BL, Mathiason MA, Schauberger CW. Predictors of Obesity in Childhood, Adolescence, and Adulthood in a Birth Cohort. Matern Child Health J. 2011;15(8):1166–1175.
- CS DS, Picoito J, Nunes C, et al. Early Individual and Family Predictors of Weight Trajectories From Early Childhood to Adolescence: results From the Millennium Cohort Study. Front Pediatr. 2020;8:8.
- Mǎrginean CO, Mǎrginean C, Meliţ LE. New Insights Regarding Genetic Aspects of Childhood Obesity: a Minireview. Front Pediatr. 2018;0:271.
- Goldhaber-Fiebert JD, Rubinfeld RE, Bhattacharya J, et al. The Utility of Childhood and Adolescent Obesity Assessment in Relation to Adult Health. Med Decis Mak. 2013;33(2):163–175.
- Spolidoro JV, Filho MLP, Vargas LT, et al. Waist circumference in children and adolescents correlate with metabolic syndrome and fat deposits in young adults. Clin Nutr. 2013;32(1):93–97.
- Jago R, Mendoza JA, Chen T, et al. Longitudinal associations between BMI, waist circumference, and cardiometabolic risk in US youth: monitoring implications. Obesity. 2013;21(3):E271–E279.
- Bassali R, Waller JL, Gower B, et al. Utility of waist circumference percentile for risk evaluation in obese children. Int J Pediatr Obes. 2010;5(1):97.
- Savva SC, Tornaritis M, Savva ME, et al. Waist circumference and waist-to-height ratio are better predictors of cardiovascular disease risk factors in children than body mass index. Int J Obes Relat Metab Disord. 2000;24(11):1453–1458.
- Podnar H, Jurić P, Karuc J, et al. Comparative effectiveness of school‐based interventions targeting physical activity, physical fitness or sedentary behaviour on obesity prevention in 6‐ to 12‐year‐old children: a systematic review and meta‐analysis. Obes Rev. 2021;22(2):2.
- Li B, Pallan M, Liu WJ, et al. The CHIRPY DRAGON intervention in preventing obesity in Chinese primary-school–aged children: a cluster-randomised controlled trial. Basu S, ed. PLOS Med. 2019;16(11):e1002971.
- Taghizadeh S, Farhangi MA. The effectiveness of pediatric obesity prevention policies: a comprehensive systematic review and dose–response meta-analysis of controlled clinical trials. J Transl Med. 2020;18(1):480.
- Cuda SE, Censani M. Pediatric Obesity Algorithm: a Practical Approach to Obesity Diagnosis and Management. Front Pediatr. 2018;6(JAN). DOI:10.3389/FPED.2018.00431
- Gutin B, Barbeau P, Owens S, et al. Effects of exercise intensity on cardiovascular fitness, total body composition, and visceral adiposity of obese adolescents. Am J Clin Nutr. 2002;75(5):818–826.
- Fang K, Mu M, Liu K, et al. Screen time and childhood overweight/obesity: a systematic review and meta‐analysis. Child Care Health Dev. 2019;45(5):744–753.
- Kawai M. Disruption of the circadian rhythms and its relationship with pediatric obesity. Pediatr Int. 2022;64(1):1.
- Askie LM, Espinoza D, Martin A, et al. Interventions commenced by early infancy to prevent childhood obesity—The EPOCH Collaboration: an individual participant data prospective meta‐analysis of four randomized controlled trials. Pediatr Obes. 2020;15(6):6.
- Tomayko EJ, Tovar A, Fitzgerald N, et al. Parent Involvement in Diet or Physical Activity Interventions to Treat or Prevent Childhood Obesity: an Umbrella Review. Nutrients. 2021;13(9):3227.
- Mead E, Atkinson G, Richter B, et al. Drug interventions for the treatment of obesity in children and adolescents. Cochrane Database Syst Rev. 2016;2016:11.
- Czepiel KS, Perez NP, Campoverde Reyes KJ, et al. Pharmacotherapy for the Treatment of Overweight and Obesity in Children, Adolescents, and Young Adults in a Large Health System in the US. Front Endocrinol (Lausanne). 2020;290.
- Lustig RH, Hinds PS, Ringwald-Smith K, et al. Octreotide Therapy of Pediatric Hypothalamic Obesity: a Double-Blind, Placebo-Controlled Trial. J Clin Endocrinol Metab. 2003;88(6):2586–2592.
- Zachurzok A, Ranke MB, Flehmig B, et al. Relative leptin deficiency in children with severe early-onset obesity (SEOO) – results of the Early-onset Obesity and Leptin – German-Polish Study (EOL-GPS). J Pediatr Endocrinol Metab. 2020;33(2):255–263.
- Ryan PM, Seltzer S, Hayward NE, et al. Safety and Efficacy of Glucagon-Like Peptide-1 Receptor Agonists in Children and Adolescents with Obesity: a Meta-Analysis. J Pediatr. 2021;236:137–147.e13.
- Liang S, Xue J, Li G. Effects of recombinant human growth hormone administration on cardiovascular risk factors in obese children with relative growth hormone deficiency. Lipids Health Dis. 2018;17(1):1.
- Black JA, White B, Viner RM, et al. Bariatric surgery for obese children and adolescents: a systematic review and meta-analysis. Obes Rev. 2013;14(8):634–644.
- Calcaterra V, Cena H, Pelizzo G, et al. Bariatric Surgery in Adolescents: to Do or Not to Do? Children. 2021;8(6):453.
- Lee JC, Bernardi LA, Boots CE. The association of euploid miscarriage with obesity. F&S Reports. 2020;1(2):142–148.
- Fabozzi G, Cimadomo D, Allori M, et al. Maternal body mass index associates with blastocyst euploidy and live birth rates: the tip of an iceberg? Reprod Biomed Online. 2021;43(4):645–654.
- van Duijn L, Rousian M, Laven JSE, et al. Periconceptional maternal body mass index and the impact on post-implantation (sex-specific) embryonic growth and morphological development. Int J Obes. 2021;45(11):2369–2376.
- Bautista-Castaño I, Henriquez-Sanchez P, Alemán-Perez N, et al. Maternal Obesity in Early Pregnancy and Risk of Adverse Outcomes. PLoS One. 2013;8(11):80410.
- Nohr EA, Wolff S, Kirkegaard H, et al. Cause-Specific Stillbirth and Neonatal Death According to Prepregnancy Obesity and Early Gestational Weight Gain: a Study in the Danish National Birth Cohort. Nutrients. 2021;13(5):1676.
- Chang R, Mei H, Zhang Y, et al. Early childhood body mass index trajectory and overweight/obesity risk differed by maternal weight status. Eur J Clin Nutr. 2022;76(3):450–455.
- Yang Z, Phung H, Freebairn L, et al. Contribution of maternal overweight and obesity to the occurrence of adverse pregnancy outcomes. Aust N Z J Obstet Gynaecol. 2019;59(3):367–374.
- Jadresić L, Au H, Woodhouse C, et al. Pre-pregnancy obesity and risk of congenital abnormalities of the kidney and urinary tract (CAKUT)—systematic review, meta-analysis and ecological study. Pediatr Nephrol. 2021;36(1):119–132.
- Zhang L, Zhang Y, Li Z, et al. Maternal periconceptional body mass index and risk for neural tube defects: results from a large cohort study in China. J Matern Neonatal Med. 2021;34(2):274–280.
- American College of Obstetricians and Gynecologists. Obesity in Pregnancy: ACOG Practice Bulletin, Number 230. Obstet Gynecol. 2021;137(6):e128–e144.
- Cantor AG, Jungbauer RM, McDonagh M, et al. Counseling and Behavioral Interventions for Healthy Weight and Weight Gain in Pregnancy. JAMA. 2021;325(20):2094.
- Gomes D, von Kries R, Delius M, et al. Late-pregnancy dysglycemia in obese pregnancies after negative testing for gestational diabetes and risk of future childhood overweight: an interim analysis from a longitudinal mother–child cohort study. Myers JE, ed. PLOS Med. 2018;15(10):e1002681.
- Sun Y, Shen Z, Zhan Y, et al. Effects of pre-pregnancy body mass index and gestational weight gain on maternal and infant complications. BMC Pregnancy Childbirth. 2020;20(1):390.
- Fan H, Li X, Zheng L, et al. Abdominal obesity is strongly associated with Cardiovascular Disease and its Risk Factors in Elderly and very Elderly Community-dwelling Chinese. Sci Rep. 2016;6(1):6.
- Leitzmann MF, Moore SC, Koster A, et al. Waist Circumference as Compared with Body-Mass Index in Predicting Mortality from Specific Causes. PLoS One. 2011;6(4):e18582.
- Batsis JA, Mackenzie TA, Bartels SJ, et al. Diagnostic Accuracy of Body Mass Index to Identify Obesity in Older Adults: NHANES 1999–2004. Int J Obes (Lond). 2016;40(5):761.
- Tyrovolas S, Panagiotakos D, Georgousopoulou E, et al. Skeletal muscle mass in relation to 10 year cardiovascular disease incidence among middle aged and older adults: the ATTICA study. J Epidemiol Community Health. 2020;74(1):26–31.
- Cerhan JR, Moore SC, Jacobs EJ, et al. A Pooled Analysis of Waist Circumference and Mortality in 650,000 Adults. Mayo Clin Proc. 2014;89(3):335.
- Knowles R, Carter J, Jebb SA, et al. Associations of Skeletal Muscle Mass and Fat Mass With Incident Cardiovascular Disease and All‐Cause Mortality: a Prospective Cohort Study of UK Biobank Participants. J Am Heart Assoc. 2021;10(9):9.
- Donini LM, Pinto A, Giusti AM, et al. Obesity or BMI Paradox? Beneath the Tip of the Iceberg. Front Nutr. 2020; 53.
- Brown RE, Kuk JL. Age-related Differences in the Consequences of Obesity on Cardiovascular Disease, Type 2 Diabetes, Osteoarthritis, Cancer, Physical Function, Osteoporosis, Cognitive Function, and Mortality Risk in the Elderly. Heal Aging Clin Care Elder. 2014;6:25–32.
- Liao C-D, Lee P-H, Hsiao D-J, et al. Effects of Protein Supplementation Combined with Exercise Intervention on Frailty Indices, Body Composition, and Physical Function in Frail Older Adults. Nutrients. 2018;10(12):1916.
- Colleluori G, Villareal DT. Aging, obesity, sarcopenia and the effect of diet and exercise intervention. Exp Gerontol. 2021 September; 155:111561.10.1016/j.exger.2021.111561 Published online
- Saad F, Yassin A, Doros G, et al. Effects of long-term treatment with testosterone on weight and waist size in 411 hypogonadal men with obesity classes I-III: observational data from two registry studies. Int J Obes. 2016;40(1):162–170.
- Vinan-Vega M, Diaz Vico T, Elli EF. Bariatric Surgery in the Elderly Patient: safety and Short-time Outcome. A Case Match Analysis. Obes Surg. 2019;29(3):1007–1011.
- Elbahrawy A, Bougie A, Loiselle S-E, et al. Medium to long-term outcomes of bariatric surgery in older adults with super obesity. Surg Obes Relat Dis. 2018;14(4):470–476.
- Martín AS, Sepúlveda M, Guzman F, et al. Surgical Morbidity in the Elderly Bariatric Patient: does Age Matter? Obes Surg. 2019;29(8):2548–2552.
- Victorzon M, Giordano S. Bariatric surgery in elderly patients: a systematic review. Clin Interv Aging. 2015;10:1627.
