Over the past several decades, we have witnessed a significant decline in cardiovascular (CVD) mortality in the United States. [Citation1,Citation2] The most significant reduction occurred in the prevalence of coronary heart disease through implementation of lifestyle changes, the use of statins and better blood pressure control. [Citation3–5] Consequently, mortality from ischemic heart disease mortality has continued to decline. [Citation6] However, in sharp contrast mortality from heart failure (HF) has significantly increased since 2011. [Citation7] Several explanations might account for this increased HF mortality including (a) rapid aging of the population, (b) increased prevalence of hypertension, (c) better treatment of coronary artery disease, (d) obesity, and (e) type 2 diabetes mellitus pandemics. [Citation8–17] Unfortunately, all these major risk factors not only increase the incidence of HF but also additive/synergistic consequences contribute to the progression of HF. [Citation8–11]
As a result of a better understanding of the complex HF pathophysiology, improvement in the management of HF evolved over the last few decades. Treatment is no longer simply limited to relieving edema and improving hemodynamics, but now targets a direct approach to reduce neuroendocrine activation and improve cardiac remodeling. [Citation12] Despite these approaches, the unrelenting nature of this clinical entity led to the development of disease modifying medical therapies that may require intravenous or mechanical therapies to stabilize clinical conditions and end organ function. [Citation13]
While key advancements were made in using temporary mechanical circulatory support devices, improvement in drug therapies lagged. The emergence of angiotensin receptor neprilysin inhibitors that HF medical therapy was a long awaited step in the right direction. [Citation14] Then another dramatic, but unexpected, milestone change came along with the sodium glucose cotransporter 2 (SGLT2) inhibitors. Although initially studied for management of diabetes mellitus, inhibitors of the sodium–glucose cotransporters SGLT1 and SGLT2 were considered new diabetes medications for glucose management. [Citation15] However, in the case of SGLT2 inhibitors, their effects on kidney function, natriuresis, and diuresis occurring even in the absence of hyperglycemia opened the door to considering their potential use in nondiabetic patients with HF or chronic kidney disease. [Citation15–17] In terms of the use of SGLT2 inhibitors in HF, as Søren Kierkegaard once said, ‘Life can only be understood backwards; but it must be lived forwards.’ Simply meaning that to we need to fully comprehend the efficacy of this drug class as well as its role within the quartet of HF medications so we can move forward and continue making progress in managing HF. Most importantly, we need to make sure that all deserving patients can access these medications.
In a matter of a few years, this class of drugs has now changed the landscape as a new HF medication. SGLT2 inhibitors were first introduced as a HF keystone treatment at the European Society of Cardiology meeting in 2021 and this was followed by the incorporation of these agents into the updated 2022 American College of Cardiology/American Heart Association Joint Committee's new clinical HF practice guidelines. [Citation18,Citation19]
Referred as ‘The Fantastic Four’ [Citation20] three of these drugs namely, angiotensin converting enzyme (ACE) inhibitors, angiotensin receptor/neprilysin inhibitors (ARNIs; sacubitril/valsartan), beta blockers, and mineralocorticoid receptor antagonists (MRAs) have been proven invaluable. Their combined use has resulted in an incremental benefit resulting in a marked reduction in all cause mortality, CVD mortality, all-cause hospitalizations, and HF hospitalizations. [Citation21]
The initial data that resulted in the acceptance of SGLT-2 inhibitors into the newly recognized HF quartet was further scrutinized by Vaduganathan and associates in a recent meta-analysis[Citation22]. These investigators performed a comprehensive analysis on the utility of SGLT-2 inhibitors for treating patients with varying degrees of HF. [Citation22] A total of five randomized controlled trials were included into this analysis, namely the DELIVER, EMPEROR-Preserved, DAPA-HF, and EMPEROR-Reduced trials and the SOLOIST-WHF trial. [Citation22] Numerous demographic and clinical variables were analyzed, and the median follow-up time ranged from 9 months to 28.1 months. [Citation23] The most significant finding that Vaduganathan et al. reported is that SGLT-2 inhibitor therapy led to a significant reduction in the primary endpoint of time to CV death or first hospitalization for HF. The benefit was revealed in both the combined analysis from DELIVER (with mildly reduced EF) and EMPEROR-Preserved trials and in the larger analysis (21,947 participants studied) of all five SGLT-2 inhibitor randomized controlled trials including patients with HFrEF and in the hospitalized setting. [Citation22] In addition, SGLT-2 inhibitor therapy also resulted in key reduction of secondary endpoints including CV death, first hospitalization for HF, and all-cause mortality. [Citation22] Finally, more participants using SGLT-2 inhibitors achieved clinically meaningful improvements in quality-of-life measures with less deterioration when objectively scored using the standard Kansas City Cardiomyopathy Questionnaire. [Citation22]
When the cumulative benefit of all four HF drug classes is calculated, the effect of these drugs becomes complementary to each other and because of this interaction the expected benefit of initiating such therapy on a typical 65-year-old HF patient would add 5 years of life. [Citation23] Even as these overwhelming overall benefits have been confirmed by data from several different clinical trials, a basic question remains unanswered: ‘by which mechanism(s) do SGLT2 inhibitors offer such unparallel benefit?’
Stimulation of osmotic diuresis and natriuresis was initially proposed as the main mechanism that would explain the beneficial effects of SGLT-2 inhibitors in HF. Even though osmotic diuresis and natriuresis would lower arterial pressure and overall stiffness, reducing afterload and thereby improving left ventricular loading conditions seems reasonable, it might not account for all the beneficial effects of SGLT2 inhibitors in HF. [Citation24,Citation25] If this was the case, how different would SGLT-2 inhibitors be from loop diuretics as we have already established that loop diuretics have no beneficial impact on HF clinical outcomes. [Citation26]
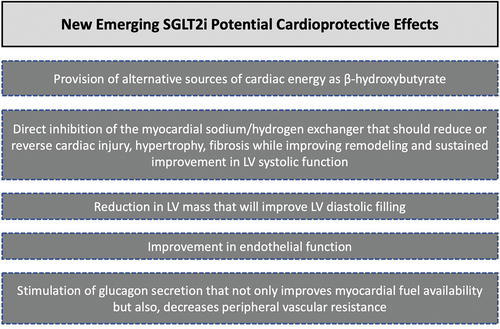
As shown in , emerging new data appear to shed new light into the unsolved mystery regarding potential SGLT2 inhibitors’ cardioprotective effects. [Citation27]
Subsequently, an even more provocative mechanism was recently proposed by Omar et al. as these investigators demonstrated that empagliflozin treatment resulted in a significant reduction in the amount of stressed blood volume. [Citation28]
Before we can appreciate the potential value of this contribution, it would be most relevant to review basic concepts that have been recently updated. Myocardial dysfunction and volume overload are well-established pathologic processes contributing to elevated cardiac filling pressures in HF; however, the concepts of stressed blood volume and unstressed blood volume have been recently introduced to explain in part how regulation of venous tone modifies the distribution of blood between the pulmonary and systemic circulations in HF. [Citation29]
Conceptually, the overall sum of stressed (SBV) and unstressed blood volume (UBV) accounts for the total blood volume (TBV). [Citation30] Furthermore, the distribution between stressed and unstressed blood volume components is not anatomically determined but instead functionally driven. [Citation30] Specifically, this distribution not only is critical in regulating systemic and pulmonary venous pressures but also, important as rapid changes between these two components are drastically seen either during exercise or acute HF. [Citation30]
In fact, in a recent review by Fudim and associates, methods for estimating stressed blood volume and how these can be clinically applicable to clinical studies would be of significant utility to better understand resting and exercise hemodynamics as well as therapeutics for HF. [Citation29]
To better explain stressed and unstressed blood volume better, let us review some physiology. Veins contain about 70% of TBV in comparison to arteries where it contains approximately 30%[Citation31]. Guyton el at. described a model of venous return in 1955 along with the factors that influence its physiology, he highlighted three variables that independently affect the venous return: (a) the vascular resistance, (b) the mean systemic pressure, and (c) the right atrial pressure[Citation32]. Among all these three variables, the mean systemic pressure is the least in getting the attention it deserves; this may be due to the complex and intricate system that makes the attempts to define its importance difficult. It is basically the pressure measured in the vascular system if the blood flow were to cease[Citation33]. It is determined by the TBV present in the venous system and the compliance of that vascular bed to dilate of constrict. Physiologically speaking, it is the required volume of fluid to fill the vascular bed where it exerts a force on the vessel walls, and this is what is known as UBV. Now, any volume that will exert a rising pressure (above that normal one) on the vascular bed is known as SBV[Citation33]. Therefore, it is related to venous constriction and dilation and that veno-constriction shifts blood volume from the unstressed to the stressed pool, resulting in increased pulmonary venous pressure, pulmonary edema, and thus HF.
With regard to SGLT2 inhibitors and the improvement of renal hemodynamics, the reduction in blood pressure in hypertensive patients with type 2 diabetes was noted[Citation34]. Besides this effect of lowering the blood pressure, SGLT2 inhibitors have a role in lowering the intraglomerular pressure through the activation of tubuloglomerular feedback response due to higher presence of sodium and chloride at the level of the macula densa. This was noticed after an acute ‘dip’ of glomerular filtration rate in patients with type 2 diabetes even after a single dose[Citation35]. This was also reversible after the discontinuation of the drug[Citation36].
As a proof of concept of these with regard to SGLT2-inhibitor therapy on estimated stress blood volume over a full range of submaximal exercise loads; Omar and associates demonstrated that empagliflozin resulted in a consistent reduction in stress blood volume, assessed by changes in pulmonary capillary wedge pressure compared with placebo after 12 weeks of treatment in these patients. [Citation28]
Even though we are certainly thrilled by these newly proposed mechanisms that might help us gain greater insight into SGLT-2 inhibitors for the treatment of HF; we do understand that much more needs to be learned. One thing we accept is another long period of HF treatment stagnation. Furthermore, we ought to find creative ways that approved HF treatments are available to all deserving patients, while eliminating management disparities.
Although McMurray and Packer had already outlined a proposed approach suggesting that all four medications could be used within 4 weeks with up-titration to target doses pursued in follow-up, [Citation37] we still need better guidance regarding the hierarchical order initiation of this quartet of HF medications, particularly, when this is not addressed in the latest guidelines. [Citation19]
Just as the lyrics from the song, Don’t Dream it’s Over by Crowded House states, ‘There’s a battle ahead, many battles are lost and you’ll never see the end of the road’. The same clearly applies to management of HF unless we make significant efforts to understand different phenotypic expression of the disease variants and clearly comprehend treatment options, drug interactions, and when implementation of their use is most beneficial.
Declaration of financial/other relationships
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Additional information
Funding
References
- Sidney S, Quesenberry CP, Jaffe MG, et al. Recent trends in cardiovascular mortality in the United States and public health goals. JAMA Cardiol. 2016;1(5):594–599.
- Shah NS, Lloyd‐Jones DM, O’Flaherty M, et al. Trends in cardiometabolic mortality in the United States, 1999–2017. JAMA. 2019;322:780–782c.
- Benjamin EJ, Muntner P, Alonso A, et al. American heart association council on epidemiology and prevention statistics committee and stroke statistics subcommittee. heart disease and stroke statistics-2019 update: a report from the American heart association. Circulation. 2019Mar5;139(10):e56–e528. Erratum in: Circulation. 2020 Jan 14;141(2):e33.PMID: 30700139.
- Maruthur NM, Wang NY, Appel LJ. Lifestyle interventions reduce coronary heart disease risk: results from the PREMIER trial. Circulation. 2009Apr21;119(15):2026–2031. Epub 2009 Apr 6. PMID: 19349322; PMCID: PMC2995494.
- Bots SH, Onland-Moret NC, Jancev M, et al. Statins are associated with a large reduction in all-cause mortality in women from a cardiac outpatient population. Open Heart. 2022Apr9;1:e001900. PMID: 35444049; PMCID: PMC9021779. 10.1136/openhrt-2021-001900
- Nowbar AN, Gitto M, Howard JP, et al. Mortality from ischemic heart disease. Circ Cardiovasc Qual Outcomes. 2019;12:e005375.
- Glynn P, Lloyd‐Jones DM, Feinstein MJ, et al. Disparities in cardiovascular mortality related to heart failure in the United States. J Am Coll Cardiol. 2019;73:2354–2355.
- Sidney S, Go AS, Jaffe MG, et al. Association between aging of the US Population and heart disease mortality from 2011 to 2017. JAMA Cardiol. 2019;4:1280–1286.
- Velagaleti RS, Vasan RS. Heart failure in the twenty-first century: is it a coronary artery disease or hypertension problem? Cardiol Clin. 2007Nov;25(4):487–495. PMID: 18063154; PMCID: PMC2350191.
- Ebong IA, Goff DC Jr, Rodriguez CJ, et al. Mechanisms of heart failure in obesity. Obes Res Clin Pract. 2014 Nov-Dec 6;8(6):e540–8. Epub 2014 Jan 6. PMID: 25434909; PMCID: PMC4250935.
- Dunlay SM, Givertz MM, Aguilar D, et al. American heart association heart failure and transplantation committee of the council on clinical cardiology; council on cardiovascular and stroke nursing; and the heart failure society of America. type 2 diabetes mellitus and heart failure: a scientific statement from the American heart association and the heart failure society of America: this statement does not represent an update of the 2017 ACC/AHA/HFSA heart failure guideline update. Circulation. 2019Aug13;140(7):e294–e324. Epub 2019 Jun 6. Erratum in: Circulation. 2019 Sep 17;140(12):e692.PMID: 31167558.
- Lechat P. The evolution of heart failure management over recent decades: from CONSENSUS to CIBIS. Eur Heart J Suppl. 2006;8(supplC):C5–C12.
- Truby LK, Rogers JG. Advanced heart failure: epidemiology, diagnosis, and therapeutic approaches. JACC Heart Fail. 2020Jul8;7:523–536. Epub 2020 Jun 10. PMID: 32535126. 10.1016/j.jchf.2020.01.014
- Docherty KF, Vaduganathan M, Solomon SD, et al. Sacubitril/Valsartan: neprilysin Inhibition 5 years after PARADIGM-HF. JACC Heart Fail. 2020Oct8;10:800–810. Erratum in: JACC Heart Fail. 2020 Dec;8(12):1057.PMID: 33004114; PMCID: PMC8837825. 10.1016/j.jchf.2020.06.020
- Rieg T, Vallon V. Development of SGLT1 and SGLT2 inhibitors. Diabetologia. 2018;61(10):2079–2086.
- Vallon V, Thomson SC. Targeting renal glucose reabsorption to treat hyperglycaemia: the pleiotropic effects of SGLT2 inhibition. Diabetologia. 2017Feb;60(2):215–225. Epub 2016 Nov 22. PMID: 27878313; PMCID: PMC5884445.
- Heerspink HJ, Desai M, Jardine M, et al. Canagliflozin slows progression of renal function decline independently of glycemic effects. J Am Soc Nephrol. 2017Jan;28(1):368–375. Epub 2016 Aug 18. PMID: 27539604; PMCID: PMC5198289.
- TA M, Metra M, Adamo M, et al. ESC Scientific document group. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021Sep21;42(36):3599–3726. Erratum in: Eur Heart J. 2021 Oct 14;: PMID: 34447992.
- Heidenreich PA, Bozkurt B, Aguilar D, et al. AHA/ACC/HFSA guideline for the management of heart failure: executive summary: a report of the American college of cardiology/American heart association joint committee on clinical practice guidelines. J Am Coll Cardiol. 2022May3;79(17):1757–1780. Epub 2022 Apr 1. PMID: 35379504.
- Bauersachs J. Heart failure drug treatment: the fantastic four. Eur Heart J. 2021Feb11;42(6):681–683. PMID: 33447845; PMCID: PMC7878007.
- Komajda M, Böhm M, Borer JS, et al. Incremental benefit of drug therapies for chronic heart failure with reduced ejection fraction: a network meta-analysis. Eur J Heart Fail. 2018;20:1315–1322.
- Vaduganathan M, Docherty KF, Claggett BL, et al. inhibitors in patients with heart failure: a comprehensive meta-analysis of five randomized controlled trials. Lancet. 2022Sep3;400(10354):757–767. Epub 2022 Aug 27. PMID: 36041474.
- Vaduganathan M, Claggett BL, Jhund PS, et al. Estimating lifetime benefits of comprehensive disease-modifying pharmacological therapies in patients with heart failure with reduced ejection fraction: a comparative analysis of three randomised controlled trials. Lancet. 2020;396:121–128.
- Verma S, JJV M, Cherney DZI. The metabolodiuretic promise of sodium-dependent glucose cotransporter 2 inhibition: the search for the sweet spot in heart failure. JAMA Cardiol. 2017;2:939–940.
- Hallow KM, Helmlinger G, Greasley PJ, et al. Why do SGLT2 inhibitors reduce heart failure hospitalization? A differential volume regulation hypothesis. Diabetes Obes Metab. 2018;20:479–487.
- Kapelios CJ, Malliaras K, Kaldara E, et al. Loop diuretics for chronic heart failure: a foe in disguise of a friend? Eur Heart J Cardiovasc Pharmacother. 2018;4(1):54–63.
- Lam CSP, Chandramouli C, Ahooja V, et al. Inhibitors in heart failure: current management, unmet needs, and therapeutic prospects. J Am Heart Assoc. 2019 Oct 15;8(20):e013389. Epub 2019 Oct 12. PMID: 31607208; PMCID: PMC6818035.
- Omar M, Jensen J, Burkhoff D, et al. Effect of empagliflozin on blood volume redistribution in patients with chronic heart failure and reduced ejection fraction: an analysis from the empire HF randomized clinical. Trial. Circ Heart Fail. 2022Mar;15(3):e009156. Epub 2021 Nov 8. PMID: 34743533.
- Fudim M, Kaye DM, Borlaug BA, et al. Venous tone and stressed blood volume in heart failure: JACC review topic of the week. J Am Coll Cardiol. 2022May10;79(18):1858–1869. PMID: 35512865; PMCID: PMC9097251.
- Fallick C, Sobotka PA, Dunlap ME. Sympathetically mediated changes in capacitance: redistribution of the venous reservoir as a cause of decompensation. Circ Heart Fail. 2011;4:669–675.
- Fudim M, Patel MR, Boortz-Marx R, et al. Splanchnic nerve block mediated changes in stressed blood volume in heart failure. JACC Heart Fail. 2021Apr9;4:293–300. Epub 2021 Mar 10. PMID: 33714749. 10.1016/j.jchf.2020.12.006
- AC GUYTON. Determination of cardiac output by equating venous return curves with cardiac response curves. Physiol Rev. 1955Jan;35(1):123–129. PMID: 14356924.
- Funk DJ, Jacobsohn E, Kumar A. The role of venous return in critical illness and shock-part I: physiology. Crit Care Med. 2013Jan;41(1):255–262. PMID: 23269130.
- Sj M, Brierley L, TM C, et al. Scottish diabetes research network epidemiology group. The effect of dapagliflozin on glycaemic control and other cardiovascular disease risk factors in type 2 diabetes mellitus: a real-world observational study. Diabetologia. 2019Apr;62(4):621–632. Epub 2019 Jan 10. PMID: 30631892.
- Bjornstad P, Laffel L, Tamborlane WV, et al. Acute effect of empagliflozin on fractional excretion of sodium and eGFR in YOUTH WITH TYPE 2 diabetes. Diabetes Care. 2018Aug;41(8):e129–e130. Epub 2018 Jun 25. PMID: 29941496; PMCID: PMC6054503.
- Thomson SC, Rieg T, Miracle C, et al. Acute and chronic effects of SGLT2 blockade on glomerular and tubular function in the early diabetic rat. Am J Physiol Regul. Integr Comp Physiol. 2012Jan1;302(1):R75–83. Epub 2011 Sep 21. PMID: 21940401; PMCID: PMC3349378.
- JJV M, Packer M. How should we sequence the treatments for heart failure and a reduced ejection fraction?: a redefinition of evidence-based medicine. Circulation. 2021Mar2;143(9):875–877. Epub 2020 Dec 30. PMID: 33378214.