ABSTRACT
Diabetes is the leading cause of end-stage kidney disease (ESKD), accounting for approximately 50% of patients starting dialysis. However, the management of these patients at the stage of chronic kidney disease (CKD) remains poor, with fragmented care pathways among healthcare professionals (HCPs). Diagnosis of CKD and most of its complications is based on laboratory evidence. This article provides an overview of critical laboratory evidence of CKD and their limitations, such as estimated glomerular filtration rate (eGFR), urine albumin-to-creatinine ratio (UACR), Kidney Failure Risk Equation (KFRE), and serum potassium. eGFR is estimated using the CKD-EPI 2009 formula, more relevant in Europe, from the calibrated dosage of plasma creatinine. The estimation formula and the diagnostic thresholds have been the subject of recent controversies. Recent guidelines emphasized the combined equation using both creatinine and cystatin for improved estimation of GFR. UACR on a spot urine sample is a simple method that replaces the collection of 24-hour urine. Albuminuria is the preferred test because of increased sensitivity but proteinuria may be appropriate in some settings as an alternative or in addition to albuminuria testing. KFRE is a new tool to estimate the risk of progression to ESKD. This score is now well validated and may improve the nephrology referral strategy. Plasma or serum potassium is an important parameter to monitor in patients with CKD, especially those on renin-angiotensin-aldosterone system (RAAS) inhibitors or diuretics. Pre-analytical conditions are essential to exclude factitious hyperkalemia. The current concept is to correct hyperkalemia using pharmacological approaches, resins or diuretics to be able to maintain RAAS blockers at the recommended dose and discontinue them at last resort. This paper also suggests expert recommendations to optimize the healthcare pathway and the roles and interactions of the HCPs involved in managing CKD in patients with diabetes.
1. Introduction
Diabetes is estimated to affect more than 8% of the global population (more than 450 million people) and is expected to affect over 700 million people by 2045. Worldwide, the rising prevalence of type 2 diabetes (T2D) is mainly driven by obesity, sedentary lifestyle, and population aging [Citation1–3]. Up to 40% of people with diabetes will likely develop chronic kidney disease (CKD) [Citation1–3].
CKD often manifests a decade post T1D diagnosis but can be present at T2D onset [Citation4]. In France, 2022 data indicates that 6% of adults have diabetes [Citation5], with a 29% CKD prevalence among T2D patients [Citation6]. T2D patients constitute half of those with end-stage kidney disease (ESKD) [Citation7], with renal failure being a significant contributor to cardiovascular (CV) morbidity and mortality in diabetics [Citation8,Citation9]. For diabetics, CKD elevates risks like cardiovascular disease (CVD), cardiorenal syndrome, expensive kidney replacement therapy (KRT), and shortened lifespan [Citation8,Citation10,Citation11].
The primary goals in CKD management for T2D patients are to mitigate ESKD risks, preserve kidney function, and decrease CV events and mortality. Numerous international guidelines advocate a combination of lifestyle changes and pharmacological measures to curb CKD progression and CV events in T2D patients [Citation1,Citation12–15]. Given the efficacy of these interventions, CKD screening is paramount. Public health policies should emphasize CKD screening and risk stratification. KDIGO endorses assessing the estimated glomerular filtration rate (eGFR) and urine albumin-to-creatinine ratio (UACR) [Citation16]. This article offers insights into laboratory evidence of CKD and guidelines to enhance CKD diagnosis and management in T2D patients.
2. Methodology
During 2022, an external task force of pharmacists, representatives of patient groups, and seven practitioners from France in general medicine, cardiology, biology, diabetology and nephrology experts attended three virtual meetings organized by Bayer as part of the ‘Carré Biologie’ program. The participants practice in various settings, including private and public clinics, university departments and government hospitals. The overarching aim of this program was to optimize the detection, early diagnosis, follow-up and prevention of CKD in patients with T2D in France.
The objectives of these medical boards were (1) to harmonize biological analyses allowing early diagnosis and monitoring of CKD in France; (2) to improve the readability of the biological results with clear alerts and definition of established high and low limits; (3) to improve knowledge on the interpretation of these values and the actions to be implemented to ‘correct’ them; (4) to discuss the need to monitor renal function with follow-up of eGFR over time; and (5) to optimize the referral of patients to nephrologists, by stratifying renal and CV risk. The task force discussed and provided opinions on each of the four biological parameters: eGFR, UACR, Kidney Failure Risk Equation (KFRE), and serum potassium, and their preventive value to improve and standardize the management of CKD in patients with T2D in France.
3. Results and discussion
3.1. Estimated glomerular filtration rate (eGFR)
Assessing renal function is a crucial problem in cardiology and nephrology because the decline in renal function is one of the strongest predictors of the risk of progression to ESKD and CV events. In addition, reduced renal function implies dosage adjustment for many drugs, the dose of which must be reduced (e.g. digoxin) or, on the contrary, increased (e.g. diuretics). Also, renal failure is associated with a change in the behavior of physicians (called ‘renalism’), including the underuse of examinations involving the injection of iodinated contrast products or revascularization procedures, and the use of sub-maximal dosages of CV or renal protective drugs (angiotensin-converting-enzyme inhibitors, statin, aspirin), probably due to side effects or drug interactions [Citation17].
Assessment of renal function in clinical practice is usually based on the estimation of glomerular filtration rate (eGFR). In France and as early as 2012, in parallel with the KDIGO 2012 guidelines, the French National Authority for Health (Haute Autorité de Santé - HAS) proposed estimating the GFR using the CKD-Epidemiology Collaboration (CKD-EPI) 2009 equation, recommending the isotope dilution mass spectrometry (IDMS) calibrated assay of plasma creatinine, but also favoring the use of an enzymatic technique [Citation18–22]. This enzymatic assay eliminates chromogen interference and ensures better accuracy and reproducibility among laboratories. The post-analytical report must mention the creatinine assay technique and give a GFR estimated by the CKD-EPI 2009 formula, expressed in ml/min indexed for a body surface area of 1.73 m2. The ‘racial’ correction proposed in the U.S.A. for African Americans is generally not used in Europe: this ‘corrective’ factor was mainly established from the MDRD and CKD-EPI studies. In the CKD-EPI study, a vast majority of black people were issued from the AASK study with possible methodological flaws due to the high prevalence of obesity and low GFR [Citation23]. Recent European data suggest a minimal difference on average between white subjects and people of African descent living in Europe [Citation24–26].
The CKD-EPI formula is less well validated in specific patient populations: (1) subjects of Asian origin, children and people aged over 75 years, for whom other equations are more suitable, for example, European Kidney Function Consortium (EKFC) [Citation27]; (2) people of extreme weight (obesity, malnutrition) or whose muscle mass is high or low (bodybuilding, sarcopenia, amputation); and (3) vegetarians (lower plasma creatinine at the same level of renal function).
The KDIGO 2012 guidelines proposed a classification of renal function in 6 stages of severity (G1, G2, G3a, G3b, G4 and G5), the value of 60 ml/min/1.73 m2 defining the threshold for renal failure independently of age and gender [Citation18]. This epidemiological definition is based on an increase in the relative risk of ESKD and CV events in proportion to the reduction in eGFR below 60 ml/min/1.73 m2. It was necessary to subdivide the G3 stage into G3a (45 to 59 ml/min/1.73 m2) and G3b (30 to 44 ml/min/1.73 m2) to refine the prognostic stratification due to significant epidemiological differences. Stage G3a represents the vast majority (70%) of patients with reduced eGFR, and these patients have a relatively low risk of renal progression and a predominant CV risk, especially when albuminuria is normal. Patients at stage G3b, much less numerous (24%), present much higher renal and CV risks.
Keeping a single threshold regardless of age or considering the decline in GFR, which is frequent with age (but not ‘physiological’ since it does not affect all individuals), was controversial. Some authors suggest different thresholds for renal failure at 75 ml/min/1.73 m2 in people below 55 years and, conversely, 45 ml/min/1.73 m2 in people older than 65 years [Citation28]. Introducing such a differentiation into clinical practice is a potential source of confusion. The CKD Prognosis Consortium, which defined the threshold at 60 ml/min/1.73 m2 maintains its universal position by arguing that the relative risk of CV and renal complications is similar at identical eGFR regardless of age [Citation29].
The dose adjustment indicated in the summary of product characteristics (SmPC Vidal) is currently done according to the clearance of creatinine estimated using the Cockcroft-Gault (CG) equation (HAS). However, SmPCs do refer specifically to the CG equation for dose adjustment in only 15% of cases [Citation30].
In France, the National Authority for Health still recommends dose adjustment to the values of the CG equation [Citation31], which estimates creatinine clearance and notoriously underestimates the GFR [Citation20]. This recommendation requires biology laboratories to continue to report this result, which is confusing, especially when the CKD-EPI and CG values diverge sharply. Some pharmacologists and geriatricians defend the CG equation since they are very sensitive to the iatrogenic risk in their populations of elderly patients. The underlying idea is that a formula which underestimates the GFR avoids overdosing. This is misleading reasoning because systematic under-dosing carries increased risks of ineffectiveness for many therapeutic classes, or even their contraindications due to a threshold effect (eGFR <30 ml/min/1.73 m2), for example, for metformin or direct anticoagulants. Another issue is that the colorimetric reagents available at the time of Cockcroft have not been marketed for several decades. An enzymatic assay for creatinine using the CG formula underestimates the eGFR by an additional 10–15%. Therefore the clinical use of this formula should be formally discouraged, in our opinion.
Another problem of practical importance pertains to the CKD-EPI formula, which gives an eGFR value indexed to a theoretical body surface area of 1.73 m2, leading to significant deviations in obese or underweight patients [Citation32,Citation33]. The National Kidney Disease Education Program (NKDEP) in the U.S.A. recommends drug dosage adjustment in adults, according to GFR estimated using either the Modification of Diet in Renal Disease (MDRD) or CG formulas; this approach is more straightforward but carries a risk of error as highlighted above. For people with extreme body weight, the NKDEP recommends de-indexing eGFR to account for the patient’s body surface area to obtain a ‘raw’ eGFR in ml/min. The new KDIGO CKD 2023 guidelines suggest, as practice points, that ‘for most people and clinical settings, validated eGFR equations using serum creatinine are appropriate for drug dosing.’ They also propose that, ‘where accuracy is required for dosing (e.g. due to narrow therapeutic or toxic range) and/or estimates may be unreliable, the use of equations that combine both creatinine and cystatin C or measured GFR may be indicated.’
They also suggest that “in people with extremes of body weight, eGFR unadjusted for body surface area (BSA) may be indicated, especially for medications with a narrow therapeutic range or requiring a minimum concentration to be effective. Use of non-indexed eGFR values (ml/min) should be considered for drug dosing decisions.
The authors suggest de-indexing eGFR to have a more accurate estimate of renal function of drug clearance. The de-indexed eGFR is given by the formula: de (eGFR) = eGFR x A/1.73 m2, where de (eGFR) is the de-indexed eGFR, and A is the body surface area of the patient. A is readily available online or on smartphones (QxMed application, for example) but requires weight and height, which are not always reported in the lab reports. Ideally, the biology laboratory should render the de-indexed CKD-EPI directly instead of the CG formula.
3.2. Urine albumin-to-creatinine ratio (UACR)
Urinary protein excretion, or proteinuria, is an early biomarker of CKD. Albumin, the most abundant circulating plasma protein, is also the most abundant protein in the urine, with a physiological level of less than 10 mg/day in most healthy individuals. A urinary albumin excretion higher than 30 mg/day is considered pathological and is associated with podocyte alterations, glomerular capillary pressure rise [Citation35] but also diffuse endothelial dysfunction and widespread microvascular disease in the eyes, brain, and heart [Citation36].
The evaluation of urinary albumin excretion or albuminuria is preferable to that of protein because measuring serum albumin by immunological methods is more sensitive than proteinuria in case of mild to moderate increases. In addition, urinary albumin excretion is a more specific marker of changes in glomerular permeability.
The general limits of the evaluation of albuminuria, like proteinuria, are related to its significant intra-individual variability, up to 30%, which requires repeating this examination 2 or 3 times over 3 to 6 months to confirm abnormal values [Citation4]. The leading causes of variability are physical exercise, fever, hyperglycemic or blood pressure peaks, some medications, and changes in dietary sodium intake.
The mode of assessment of urine albumin is essential. Classically, a 24-h urine sample is collected for urine albumin measurement, but collecting urine over 24 hours is cumbersome. UACR is reasonably well correlated with 24-hour urinary albumin excretion [Citation37] and can be done on a urine sample, best on morning urine, or otherwise at random [Citation4,Citation18,Citation38]. As early as 2008, French clinical practice guidelines were published [Citation39], and in 2011, the French National Authority for Health proposed using UACR for the diagnosis and monitoring of CKD [Citation38]. However, this practice remains insufficiently widespread, including among people with diabetes, because general practitioners (GPs) do not always know the simplicity of UACR compared to 24-hour proteinuria, and biology laboratories continue to report urinary protein values, particularly when urinary albumin values exceed 300 mg/g, for reimbursement reasons.
Another strong argument for using UACR in renal risk stratification is that this parameter has been used as an inclusion criterion in many clinical trials and that many therapeutic indications and marketing authorizations are now based on UACR values, such as gliflozins or non-steroidal mineralocorticoid antagonists (nsMRAs).
The authors suggest expressing UACR in milligrams of albumin per gram of urinary creatinine (mg/g) rather than the SI units (milligrams of albumin per millimoles of urinary creatinine, mg/mmol). This suggestion is made to avoid the risk of confusion between the two systems of units (which vary from 1 to 10) and because the value in mg/g is roughly equivalent to that in milligrams per 24 hours and, therefore, more intuitive for the practitioners.
The international KDIGO classification distinguishes three stages of albuminuria: A1 for physiological values below 30 mg/g, A2 for values from 30 to 300 mg/g, and A3 for values above 300 mg/g [Citation40]. This classification can be easily used to stratify the risk, but these threshold values are arbitrary since the increase in risk is continuous and proportional to the value of the UACR and without a threshold [Citation40,Citation41]
Overall, albuminuria is a risk marker, for renal progression, CV events and mortality [Citation40,Citation41], in addition to its role in risk profiling for new-onset heart failure [Citation42].
Noteworthy, changes in albuminuria or uACR have predictive value. For example, in diabetic nephropathy, a 50% reduction in albuminuria is associated with an equivalent decrease in the risk of CKD progression and CV events [Citation43]. In its latest 2023 recommendations, the American Diabetes Association (ADA) suggests that reducing albuminuria by at least 30% should be a therapeutic target in CKD patients with diabetes [Citation4]. The authors emphasize that the therapeutic objective is to reduce albuminuria to its lowest possible value, considering the direct relationship with the reduction of renal and CV risk [Citation44,Citation45]. In addition, the FDA now recognizes a reduction in UACR of more than 30% as a relevant surrogate for renal and CV events [Citation46].
Follow-up of people with diabetes should include the measurement of UACR and eGFR at least once a year, independently of treatment, following French [Citation31] and international guidelines [Citation1,Citation4]. In people with diabetes and CKD, these two biomarkers should be monitored more frequently to guide therapeutic decisions [Citation1,Citation4]. The GA classification can be represented in a heat map. Each part of the heat map is associated with a discrete risk of CKD progression, CV events and all-cause mortality [Citation1] (). In addition, the GA heat map specifies the treatment and referral indications [Citation1].
Figure 1. KDIGO GA matrix according to eGFR and albuminuria stages. [Citation1]. Heat map reflects the risk of progression by intensity of coloring (green: low risk [if no other markers of kidney disease, no CKD]; yellow: moderately increased risk; orange: high risk; red/deep red, very high risk). The numbers in the boxes are a guide to the frequency of monitoring (number per year). Treatment and referral indications are given in plain text (with the publisher permission).
![Figure 1. KDIGO GA matrix according to eGFR and albuminuria stages. [Citation1]. Heat map reflects the risk of progression by intensity of coloring (green: low risk [if no other markers of kidney disease, no CKD]; yellow: moderately increased risk; orange: high risk; red/deep red, very high risk). The numbers in the boxes are a guide to the frequency of monitoring (number per year). Treatment and referral indications are given in plain text (with the publisher permission).](/cms/asset/00ac4fb7-3fdd-4bd6-a84c-cc62f481ee3f/ipgm_a_2256208_f0001_c.jpg)
3.3. Scoring and risk of progression
Most cases of CKD are progressive, and the progression rate has a predictive value indicating, in particular the risk of ESKD and the time to KRT as well as a higher risk of CV events [Citation47]. Identifying patients at risk of CKD progression allows optimal nephrology care, including the early start of treatment for nephroprotection, triage for nephrology referrals and appropriate timing of dialysis access and living donor kidney transplantation. Early nephrology referral has been shown to improve overall survival, survival on dialysis [Citation48], and to reduce healthcare costs in patients requiring KRT [Citation49,Citation50].
The slope of the inverse of plasma creatinine, have been proposed for several decades as tools for assessing the progressive risk of CKD. Using a graphic representation with a semi-logarithmic scale, a slope of decrease in plasma creatinine can be drawn to roughly estimate the time duration up to KRT. Tools are available to calculate annual GFR loss online (https://www.sfdiabete.org/renalfunctiondeclinecalculator). A 5 ml/min/1.73 m2 or more loss over one year is considered rapid progression and requires specialist advice [Citation18]. The advantage of this representation is linked to its ease of implementation and its easy-to-understand visual aspect. The main limitation is that the evolution of renal function loss is not linear in approximately 30% of individuals. The second limitation is that several points spaced months or years apart are necessary to draw a slope, which can lead to delaying the referral to a nephrologist [Citation51].
KDIGO guidelines have proposed risk stratification with the GA heat map [Citation18]. The GA heat map visually represents the risk with a colorimetric scale representing both the renal and CV risks or the risk for all-cause mortality (). Each GA stage indicates a relative risk of renal or CV event compared to a G1A1 reference. This GA representation is modeled on the CV risk scales used in cardiology to stratify the risk of CV events. The significant advantage of this representation is to stratify the risk in all individuals with an easy-to-understand visual representation. The major limitation is that this heat map only indicates a relative risk, making it possible to stratify individuals among themselves but not to predict the individual absolute risk.
While lower eGFR and higher UACR identify patients with CKD, these biomarkers only provide a ‘snapshot’ of the disease. Risk scores have therefore been developed to consider multiple progression factors dynamically. The Kidney Failure Risk Equation (KFRE) was developed as a risk-predictive model to measure the 2-year and 5-year risk of developing ESKD in people with CKD stages G3-G4 (eGFR 15–60 ml/min/1.73 m2). The KFRE score was developed in Canada on a large cohort of patients with CKD stages 3 to 5 [Citation52]. After comparing numerous models, two equations were retained: a score of 4 variables including gender, age, eGFR and UACR, and a score using variables adding four serum parameters (albumin, calcium, phosphate, and bicarbonate). The 8-variable model performed marginally better in net reclassification improvement and calibration. Later on, a meta-analysis extended the validity of the 4-variable score from 31 international cohorts covering almost 720,000 participants from 4 continents [Citation53]. The score was calibrated for non-North American cohorts due to a lower basal risk of 32.9% at 2 years and 16.5% at 5 years. This meta-analysis found C statistics very satisfactory (4-variable score: 0.90 at 2 years and 0.88 at 5 years; 8-variable score: 0.89 at 2 year, and 0.86 at 5 years) [Citation53].
The score (4 variables) has now been externally validated in numerous multiethnic populations in Europe, North America and Asia [Citation54–56]. The score has also been validated in various types of kidney diseases and is very performant in diabetic nephropathy and chronic glomerular diseases but slightly less efficient in interstitial nephropathy and polycystic kidney disease, for which other tools are available [Citation57,Citation58]. KFRE is convenient, easy to use and reliable in most populations over a wide age range (KFRE @ qxmed). However, the KFRE has not been studied or validated in people with an eGFR >60 ml/min/1.73 m2 and may be slightly less effective in pediatric patients [Citation59] or in very elderly subjects (>80 years) due to the competitive risk of CV mortality [Citation60,Citation61]. Another drawback of this risk equation is that it does not account for post-intervention changes in UACR. Finally, this risk equation may require a recalibration in France and other low-risk countries [Citation62].
In Canada, Tangri et al. proposed a 5% risk threshold at five years for nephrology referral, while lower risk patients were referred to the treating physician for follow-up [Citation63]. This triage strategy reduced the wait time for the first nephrology visit from 9 months to less than 4 months. This research group also proposed a KFRE threshold of 40% at two years for planning vascular access and transplantation. However, this threshold has not been formally validated nor universally adopted. In the United Kingdom, the 2021 NICE guidelines for managing CKD assessed KFRE as a triage and referral tool and recommended rapid implementation in all electronic medical records [Citation64]. The National Institute for Health and Care Excellence (NICE) found that the best referral rule was ‘KRFE ≥5% at 5 years or UACR ≥650 mg/g,’ which allows to identify patients with CKD but whose eGFR is >60 ml/min/1.73 m2. In France, the French National Authority for Health has recognized KRFE as a predictive tool to optimize triage and referral to a nephrologist but has not made it mandatory [Citation31]. As part of an experiment in some regions of France, KRFE has been routinely implemented in laboratory reports for practitioners and patients with a comment to consider referral to the nephrologist when the 5-year KFRE is ≥ 5%. The authors suggest extending this practice to all laboratory reports, including eGFR and UACR measurements, as part of the post-analytical reporting.
3.4. Blood potassium
Factors associated with an increased likelihood of developing hyperkalemia include predisposing conditions (diabetes, CKD, heart failure), medications, potassium-rich foods, and potassium-enriched salt substitutes. CKD as early as G3a and G3b stages are one of the most important predictors of hyperkalemia [Citation65]. Potassium-sparing diuretics, MRAs, and RAAS inhibitors are the drugs most frequently associated with hyperkalemia, but diabetes itself may promote hyperkalemia in some patients resulting from hyporeninemic hypoaldosteronism [Citation66]. Other common drugs can interfere with renal potassium excretion and worsen hyperkalemia, including non-steroidal anti-inflammatory drugs (NSAIDs), COXIBs, cotrimoxazole, heparin and low molecular weight heparins, drospirenone, and calcineurin inhibitors (ciclosporin and tacrolimus).
Measuring serum or plasma potassium for diagnosis is acceptable, but the laboratory reports should state clearly which method was used, a practice not currently done in France. Since potassium is released from platelets during coagulation, serum potassium is higher than plasma potassium by 0.1 to 0.7 mmol/L) [Citation67]. Point-of-care devices have until now had limited accuracy and precision, which may have limited their widespread adoption [Citation65], but new devices look promising [Citation68]. A falsely elevated potassium level (pseudohyperkalemia) may occur with a clenched fist during phlebotomy, mechanical trauma, small gauge needle, use of a tourniquet for more than 1 minute, clotting, or hyperleukocytosis or thrombocythemia. A significant factor of pseudohyperkalemia is samples taken at home and measured too late, a frequent but not recommended practice. Generally, any factor tending to promote hemolysis or even minimal passive diffusion during the pre-analytical phase of the assay falsely increases blood potassium.
The definition of hyperkalemia is based on the distribution of potassium values in the general population. Hyperkalemia is generally defined as a plasma or serum potassium level higher than 5.0 mmol/L and is further classified as mild hyperkalemia (5.0 to 5.9 mmol/L), moderate hyperkalemia (6.0 to 6.4 mmol/L), and severe hyperkalemia (≥6.5 mmol/L) in the absence of suggestive ECG signs [Citation65].
There is no international consensus on the magnitude, duration and frequency of elevated potassium values that define chronicity. A consensus was achieved by a Delphi panel of French nephrologists defining ‘Persistent hyperkalemia as the recurrence of an episode of hyperkalemia two times or more within a year, despite the use of chelating resins or available loop diuretics in the same year’ [Citation69].
A definition based on prognosis should reflect the gradual association with adverse events, but the risk increases continuously with higher potassium levels, and CKD alters both the distribution of potassium levels and the associated risk [Citation70]. Hyperkalemia is associated with an increased risk of adverse events and mortality [Citation71,Citation72]. The likelihood of the association is explained by the electrophysiological role of potassium and the known cardiac abnormalities that can be induced by very high or very low potassium levels, especially after rapid changes.
Incorporating risk factors into predictive models can contribute to better individual risk stratification. In the Swedish SCREAM cohort, 70000 patients were evaluated after taking a RAAS inhibitor, and the incidence of hyperkalemia increased continuously as kidney function declined [Citation73]. These authors developed a hyperkalemia risk score integrating eGFR, baseline potassium level, gender, diabetes, heart failure, and concurrent use of potassium-sparing diuretics in new users of RAS blockers. This score accurately predicted the risk of hyperkalemia at one year in the SCREAM cohort (area under the curve: 0.85, 95% CI: 0.84 to 0.87) and in a validation cohort. This score is promising for predicting the risk of hyperkalemia before starting an RAAS inhibitor, but it is not yet available as a calculator. The more convenient 45/4.5 rule predicts a 4- to 8-fold increased risk of hyperkalemia on RAAS inhibitors in patients with a baseline eGFR ≤45 ml/min/1.73 m2 and serum potassium >4.5 mmol/l under diuretics at adequate doses [Citation74].
Chronic hyperkalemia is usually asymptomatic and more likely to be detected in patients with more frequent dosing [Citation65]. In patients at risk of hyperkalemia, it is recommended that serum potassium be measured before and 1 to 2 weeks after initiation and each titration of RAAS inhibitors, based on expert opinion in several guidelines [Citation65]. However, epidemiological data show that adherence to these recommendations is limited. To prevent hyperkalemia in patients with advanced CKD, recommendations based on expert opinion call for low-diet potassium, with reduced intake of fruits, vegetables, and plant-derived bioactive compounds, such as fiber, vitamin C and carotenoids. However, randomized data on the effectiveness of this approach are lacking, and observational studies in people with CKD or ESKD report weak associations between dietary potassium intake and potassium levels [Citation75,Citation76], which challenges the belief that dietary potassium intake strongly influences potassium levels.
Numerous observational studies on patients with different degrees of CKD severity have explored the association between dietary potassium intake and prognosis [Citation75,Citation76]. In most of these studies, markers of high potassium intake were associated with a lower risk of death or progression of kidney disease. It is unclear whether the observed associations are explained by potassium intake or by greater consumption of specific plants or diets associated with a better prognosis in people with or without kidney disease [Citation70]. Few trials, limited to short-term and small cohorts, have assessed the impact of altering dietary potassium in people with CKD and generally found no alterations in plasma potassium and no adverse hyperkalemic events [Citation65].
The current recommendation to limit dietary potassium in patients with CKD is not supported by any direct evidence. However, no evidence supports the safety of increasing potassium intake or liberalizing potassium restrictions in patients with advanced CKD.
RAAS inhibition improves prognosis in patients with heart failure with reduced ejection fraction, including patients with advanced CKD (eGFR <30 ml/min/1.73 m2) [Citation77], as well as in patients with albuminuric kidney disease, including diabetes. A recent meta-analysis has established the benefit of RAAS inhibition in advanced stages of CKD with albuminuria [Citation78]. Improved control of serum potassium could allow higher use of RAAS blockers in eligible patients. In observational cohorts, hyperkalemia is the leading cause of reduction or discontinuation of RAAS inhibitors [Citation79,Citation80], which are associated with an increased risk of CKD progression and mortality [Citation81,Citation82].
Recent retrospective and observational studies have suggested that continuing RAAS blockers may help to reduce the need for KRT [Citation83,Citation84]. In the randomized STOP-ACEi study, in 410 participants with CKD stages G4-G5 receiving RAAS inhibitors, continuation or cessation of RAAS inhibitors had no impact on the rate of CKD progression and the risk of hyperkalemia [Citation85], however, the small sample size limited the power of this study.
KDIGO 2022 guidelines for the management of diabetes in CKD recommend initiating therapy with RAAS inhibitors and titrating to the highest approved dose tolerated by hypertensive and albuminuric diabetic patients [Citation1]. Hyperkalemia induced by RAAS inhibitors can usually be corrected by dietary or pharmacological measures (increase/introduction of the dose of loop diuretics, correction of acidosis or chelators) rather than by dose reduction or stopping the RAAS inhibitors, and discontinuation should only be considered as a last resort. Randomized trials support that chronic hyperkalemia can be corrected in people with normal or reduced eGFR, with durations up to one year for the new agents, patiromer and sodium zirconium cyclosilicate, and a less proven level of evidence from short-term studies (up to one week) for sodium polystyrene sulfonate (SPS) [Citation86–88]. A recent editorial controversy discussed the arguments for or against the use of these new chelators vs SPS [Citation89]. Gliflozins, which are now part of the standard of care in patients with diabetes and CKD, could mitigate the risk of hyperkalemia when combined with other RAAS inhibitors, such as ACE inhibitors, ARB, spironolactone, eplerenone, finerenone, and sacubitril-valsartan [Citation90]. Therapeutic algorithms have been recently developed to implement new potassium chelators on the cardiorenal continuum [Citation65], although these new potassium chelators are not yet available in France.
Finally, it should be noted that drug interactions are possible, resulting from a direct binding (patiromer and SPS) or a change in gastric pH (sodium zirconium cyclosilicate). These interactions have led manufacturers to recommend taking all other drugs orally at least 3 hours before or after patiromer and SPS, and at least 2 hours before or after sodium zirconium cyclosilicate for drugs whose absorption depends on gastric pH, such as atorvastatin, azole antifungals, dabigatran, furosemide, protease inhibitors and tyrosine kinase inhibitors [Citation65].
3.5. ‘Tuning’ the healthcare pathway
The prevalence of CKDs is escalating, largely attributed to the rise in diabetic nephropathy and, secondarily, to an aging population. In France, major chronic diseases, including diabetes and CKDs, are fully reimbursed under the ‘ALD 30’ provision. Patients have direct GP access, but this is constrained by GP availability and the expansion of ‘medical deserts’ in underserved regions. Notably, 11000 patients initiate dialysis annually, with half being diabetic [Citation7]. This disparity between a comprehensive health system and persistent chronic disease complications underscores the need for healthcare system refinement.
GPs are the central point of the patient’s care pathway for screening and diagnosing chronic diseases, implementing the first treatments, and organizing the follow-up in coordination with the specialists. The French National Authority for Health has issued recommendations for screening CKD () and referring patients to a specialist (). These recommendations are poorly known and applied, as evidenced by the persisting 30% of patients starting chronic dialysis in emergency conditions [91].
Table 1. Situations in which CKD screening is recommended by HAS 2021 [28]. Screening includes plasma creatinine assay to estimate GFR and urine albumin-to-creatinine ratio (UACR). Screening is recommended once a year for all patients with diabetes and, at the time of diagnosis, then every five years (if initially normal) in hypertensive patients. The three first conditions (bold text) represent ~ 90% of patients diagnosed with CKD.
Table 2. Referral to a nephrologist based on the guidelines for CKD management established by the French National Authority for Health [28]. These recommendations generally suggest referring patients at late stages of CKD (1-5-8), which misses opportunities for optimal pharmacological interventions. The use of the KFRE could replace recommendations 1, 3 and 4.
The practice of GPs is still too often isolated and not relayed by other healthcare professionals (HCPs). The health authorities encourage GPs to provide primary care in ‘multi-professional health centers (Maison de Santé Pluriprofessionnelle – MSP)’ with the possibility of optimizing the care pathway thanks to team coordinators and advanced practice registered nurses (APRNs) or therapeutic education nurses. Involving users (such as expert patients, health mediators, receptionists, peer helpers, and adapted physical education specialists) to support patients with diabetes within these MSPs appears promising.
Effective management of complex diabetes necessitates multidisciplinary collaboration. Yet, care remains disjointed due to specialists’ disparate locations. Financial incentives could foster collaboration in multidisciplinary clinics, aligning with KDIGO 2022 recommendations [1]. The COORDINATE study highlighted the efficacy of such clinics, noting a 21% reduction in cardiovascular complications within a year [Citation91].
Other HCPs may be called upon to intervene in the care pathway of patients with diabetes and CKD, such as clinical biologists, pharmacists, dietitians, and nurses, including APRNs.
Other HCPs, including clinical biologists and pharmacists, play integral roles in diabetes and CKD management. Clinical biologists can proactively measure albuminuria annually in at-risk patients, ensuring systematic CKD screening. Pharmacists, with frequent patient interactions, can advise on drug interactions and monitor medication adherence. Advanced practice nurses (APRNs), post their specialized training, have demonstrated significant patient care improvements [Citation92–94].
With the development of new computer technologies and the large number of smartphone users, the role of telemedicine needs to be unraveled. Telemedicine includes tele-expertise to quickly answer a specific question from the GP to the specialist. It can also involve remote monitoring for blood pressure, glycemia, continuous interstitial glucose measurement, weight or edema. APRNs or therapeutic education nurses play a central coordinating role in this telemonitoring.
Telemedicine’s potential, bolstered by technological advancements, remains untapped. It encompasses tele-expertise, facilitating swift GP-specialist consultations, and remote monitoring, with nurses central to this coordination.
This manuscript delineates a model encapsulating HCP roles in diabetes and CKD management () and proffers recommendations for enhanced patient care ().
Figure 2. Coordinated care pathway model for CKD in patients with diabetes.
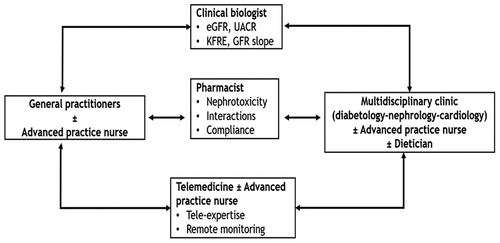
Table 3. Expert recommendations to improve the management of CKD in patients with T2D.
Controversy Which formula for estimating eGFR?:
GFR estimation is a complex and controversial subject around the world.
The original 2009 CKD-EPI formula using the racial correction factor generates a minimal bias in White people and an average overestimation of +4 ml/min in African-Americans. This bias was recognized as a factor of inequity in the diagnosis of renal insufficiency and registration on the transplant list. The new 2021 CKD-EPI formula that has no race coefficient (‘race-free’) has been validated and quickly recommended in the U.S.A. [Citation34]. This new formula performs slightly worse as it underestimates GFR in African-Americans, overestimates it in White populations by 4 ml/min, and does not provide additional benefits in European populations where it is not recommended [Citation24].
Other formulas than CKD-EPI have recently been proposed and seem promising, particularly the EKFC formula named after the European Kidney Function Consortium. The EKFC equation is a new equation developed and validated on all age groups (from 2 to 90 years) and levels of renal function (40 to 490 µmol/L) [Citation25] The originality of this equation is that it recalibrates the plasma creatinine value (by dividing the plasma creatinine by the median creatinine value in the general control population) to adjust for variations linked to differences in age, sex or race. This method makes it easy to transpose specific reference values to various populations [Citation26].
Justification for Incorporating Cystatin C in Equations for Chronic Kidney Disease (CKD) Staging
Creatinine, which is directly linked to muscle mass, may be misleading at extremes of body composition, or in specific conditions (spinal cord injuries, sarcopenia). Conversely, cystatin C is influenced by distinct factors, including steroid administration, thyroid disorders, and malignancies. Since neither is a perfect marker for estimating clearance, their combined assessment provides a more precise estimation of the GFR relative to measured values.
Very low serum creatinine levels frequently signify adverse health conditions, including frailty or sarcopenia, which curtail creatine production. This characteristic of serum creatinine, i.e. relation with muscle mass, has limited its prognostic relevance. and results in reducing the risk associations for eGFRcr 45 to 60 ml/min/1.73 m2 and elevating risks for eGFRcr >110 ml/min/1.73 m2. These limitations are not observed when risk is assessed using combined creatinine and cystatin C-based eGFR (eGFRcr-cys) or cystatin C-based eGFR (eGFRcys).
Comparing GFR estimations derived from these two filtration markers, risk gradients for eGFRcys consistently exhibit greater strength for the majority of outcomes compared to eGFRcr. Hence, for the purpose of evaluating the association of eGFR with outcomes (i.e. projecting prognosis for people with CKD), the eGFRcys or eGFRcr-cys can be considered most accurate.
In the French medical landscape, the measure of cystatin C for GFR estimation remains minimal. This is primarily due to the lack of standardization in assay methodologies and the absence of reimbursement for this assay. However, in light of the enhanced stratification potential of equations incorporating cystatin C, and the recent KDIGO guidelines, we strongly advocate for a comprehensive national reassessment of the cost-effectiveness of cystatin C measurement, with a view to broadening reimbursement provisions.
4. Conclusion
The progressive nature of CKD in patients with T2D and its association with an increased risk of CV and adverse kidney outcomes leads to shortened life expectancy, poor quality of life for these patients and sky-rocketing management costs. This paper raises awareness and informs HCPs on the importance of early diagnosis, monitoring renal function/damage (GPs and non-nephrologist specialists) using four biological parameters (eGFR UACR, KFRE, and blood potassium) to ensure an early biological diagnosis of CKD in patients with diabetes in France. New tools such as KFRE represent a step forward for proper referral and optimal therapeutic intervention in most patients with renal impairment. Moreover, this project leverages input of HCPs (biologists, pharmacists, and nurses). It highlights the need to improve the coordination and communication between all HCPs involved in managing renal disease (specialists, interdisciplinary and multidisciplinary approaches).
Declaration of financial/other relationships
T Hannedouche reports consulting honoraria or travel grants from Amgen, Astellas, AstraZeneca, Alnylam, Bayer, Boehringer, Fresenius Medical Care, GSK, Lilly, Meditor, Novo-Nordisk, Pfizer, Sanofi, and Vifor. He also received research grants from Agence de Biomédecine. P Rossignol reports consulting for Idorsia and G3P. He received honoraria from Ablative Solutions, AstraZeneca, Bayer, Boehringer-Ingelheim, Cincor, Corvidia, CVRx, Fresenius, Grunenthal, KBP Biosciences, Novartis, NovoNordisk, Relypsa, Sanofi, Sequana Medical, Servier, Stealth Peptides, and Vifor Fresenius Medical Care Renal Pharma; and travel grants from AstraZeneca, Bayer, CVRx, Novartis, and Vifor Fresenius Medical Care Renal Pharma. He is a co-founder of a company developing sensors for the home monitoring of potassium and creatinine, which sponsored the ALPHA trial. P Darmon reports consulting honoraria and/or travel grants from AstraZeneca, Bayer, Boehringer-Ingelheim, Eli Lilly, Novo Nordisk, Menarini, Mundipharma, Sanofi Aventis, Merck Sharp & Dohme, Abbott, LVL medical, IBSA, and Nutricia. J-M Halimi reports honoraria/travel grants from Alexion, AstraZeneca, Bayer, Boehringer Ingelheim France, Mundipharma, Novartis, Novo Nordisk, Sanofi, Servier, and Vifor Pharma. A Hagege has received consultancies fees from Boehringer Ingelheim and Bayer. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed. Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author contributions
T Hannedouche: review conception, literature review, analysis and interpretation of results, and manuscript preparation. P Rossignol, P Darmon, J-M Halimi, P Vuattoux, A Hagege, L Videloup and F Guinard: literature review, analysis and interpretation of results, and manuscript review.
Acknowledgments
The authors wish to thank Content Ed Net PSI SAS (France) for the support in the preparation of this manuscript. The authors retained the editorial process, including the discussion, at all times. Content Ed Net PSI SAS (France) provided editorial assistance for preparing this manuscript based on the Good Publication Practice (GPP 2022) and the ICMJE requirements; Bayer Healthcare SAS funded this assistance.
Additional information
Funding
References
- Rossing P, Caramori ML, Chan JCN, et al. Executive summary of the KDIGO 2022 clinical practice guideline for diabetes management in chronic kidney disease: an update based on rapidly emerging new evidence. Kidney Int. 2022;102(5):990–999. PMID: 36272755. doi: 10.1016/j.kint.2022.06.013
- Wu B, Bell K, Stanford A, et al. Understanding CKD among patients with T2DM: prevalence, temporal trends, and treatment patterns-NHANES 2007-2012. BMJ Open Diabetes Res Care. 2016;4(1):e000154. doi: 10.1136/bmjdrc-2015-000154
- Kainz A, Hronsky M, Stel VS, et al. Prediction of prevalence of chronic kidney disease in diabetic patients in countries of the European Union up to 2025. Nephrol Dial Transplant. 2015;30(4):iv113–8. doi: 10.1093/ndt/gfv073
- American Diabetes Association. Standards of medical care in diabetes-2022 abridged for primary care providers. Clin Diabetes. 2022;40(1):10–38. doi: 10.2337/cd22-as01
- Assurance Maladie. Fiche pathologie. Last Updated on 18 Jan 2023. Available at: https://www.ameli.fr/sites/default/files/2020_fiche_diabete_0.pdf Last access: 05 Jul 2023
- Assogba GF, Couchoud C, Roudier C, et al. Prevalence, screening and treatment of chronic kidney disease in people with type 2 diabetes in France: the ENTRED surveys (2001 and 2007). Diabetes metab. 2012;38(6):558–566. doi: 10.1016/j.diabet.2012.08.004
- Lassalle M, Monnet E, Ayav C, et al. REIN registry. 2017 annual report digest of the renal epidemiology Information Network (REIN) registry. Transplant Int. 2019;32(9):892–902. Epub 2019 Jul 4. PMID: 31148236. doi: 10.1111/tri.13466
- Afkarian M, Sachs MC, Kestenbaum B, et al. Kidney disease and increased mortality risk in type 2 diabetes. J Am Soc Nephrol. 2013;24(2):302–308. Epub 2013 Jan 29. PMID: 23362314; PMCID: PMC3559486. doi: 10.1681/ASN.2012070718
- Assogba FG, Couchoud C, Hannedouche T, et al. Trends in the epidemiology and care of diabetes mellitus-related end-stage renal disease in France, 2007-2011. Diabetologia. 2014;57(4):718–728. doi: 10.1007/s00125-014-3160-9
- Rangaswami J, Bhalla V, de Boer IH, et al. Cardiorenal protection with the newer antidiabetic agents in patients with diabetes and chronic kidney disease: a scientific statement from the American heart association. Circulation. 2020;142(17):e265–e86. doi: 10.1161/CIR.0000000000000920
- Maisons V, Halimi JM, Fauchier G, et al. Type 2 diabetes and cardiorenal syndromes. A nationwide French hospital cohort study. Diabetes metab. 2023;49(3):101441. Epub 2023 Mar 15. PMID: 36931430. doi: 10.1016/j.diabet.2023.101441
- ElSayed NA, Aleppo G, Aroda VR, et al. On behalf of the American Diabetes Association. 11. Chronic kidney disease and risk management: standards of care in diabetes-2023. Diabetes Care. 2023;46(Suppl 1):S191–S202. PMID: 36507634; PMCID: PMC9810467. doi: 10.2337/dc23-S011
- de Boer IH, Khunti K, Sadusky T, et al. Diabetes management in chronic kidney disease: a consensus report by the American diabetes association (ADA) and kidney disease: improving Global outcomes (KDIGO). Kidney Int. 2022;102(5):974–989. Epub 2022 Oct 3. PMID: 36202661. doi: 10.1016/j.kint.2022.08.012
- Chronic kidney disease: assessment and management. NICE guideline [NG203]. Published: 25 Aug 2021. Last updated: 24 Nov 2021. Available at: https://www.nice.org.uk/guidance/ng203 Last access: 05 Jul 2023
- Shubrook JH, Neumiller JJ, Wright E. Management of chronic kidney disease in type 2 diabetes: screening, diagnosis and treatment goals, and recommendations. Postgrad Med. 2021;134:1–12. doi: 10.1080/00325481.2021.2009726
- Shlipak MG, Tummalapalli SL, Boulware LE, et al. Conference participants. The case for early identification and intervention of chronic kidney disease: conclusions from a kidney disease: improving Global outcomes (KDIGO) controversies Conference. Kidney Int. 2021;99(1):34–47. 18.998 Q1. Epub 2020 Oct 27. PMID: 33127436. doi: 10.1016/j.kint.2020.10.012
- Gaut JP. Time to abandon renalism: patients with kidney diseases deserve more. Clin J Am Soc Nephrol. 2023;18(4):419–420. Epub 2023 Mar 10. PMID: 36913251; PMCID: PMC10103202. doi: 10.2215/CJN.0000000000000127
- Kidney Disease Improving Global Outcomes. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl. 2013;3:1–163.
- French National Authority for Health (Haute Autorité de Santé - HAS). Rapport d’évaluation technologique : Evaluation du débit de filtration glomérulaire et du dosage de la créatininémie dans le diagnostic de la maladie rénale chronique chez l’adulte. Jan 2012. (in French). PDF available at: https://www.has-sante.fr/portail/upload/docs/application/pdf/2012-10/evaluation_du_debit_de_filtration_glomerulaire_et_du_dosage_de_la_creatininemie_dans_le_diagnostic_de_la_maladie_renale_chronique_chez_ladulte_-_fiche_buts.pdf Last access: 5 Jul 2023
- Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate [published correction appears in Ann Intern Med. 2011;155(6): 408]. Ann Intern Med. 2009;150(9):604–612. doi: 10.7326/0003-4819-150-9-200905050-00006
- Levey AS, de Jong PE, Coresh J, et al. The definition, classification, and prognosis of chronic kidney disease: a KDIGO controversies Conference report [published correction appears in kidney int. 2011;80(9): 1000] [published correction appears in kidney int. 2011;80(9): 1000]. Kidney Int. 2011;80(1):17–28. doi: 10.1038/ki.2010.483
- Inker LA, Schmid CH, Tighiouart H, et al. CKD-EPI investigators. Estimating glomerular filtration rate from serum creatinine and cystatin C. Erratum in: N Engl J Med. 2012;367(7):681. Erratum in: N Engl J Med. 2012 Nov 22;367(21):2060. PMID: 22762315; PMCID: PMC4398023. N Engl J Med. 2012;367(1):20–29. doi: 10.1056/NEJMoa1114248
- Delanaye P, Mariat C, Cavalier E, et al. The « race » correction in estimating glomerular filtration rate: an European point of view. Curr Opin Nephrol Hypertens. 2021;30(6):525–530. PMID: 34456237. doi: 10.1097/MNH.0000000000000739
- Gansevoort RT, Anders HJ, Cozzolino M, et al. What should European nephrology do with the new CKD-EPI equation? Nephrol Dial Transplant. 2023;38(1):1–6. doi: 10.1093/ndt/gfac254
- Zingano CP, Escott GM, Rocha BM, et al. 2009 CKD-EPI glomerular filtration rate estimation in black individuals outside the United States: a systematic review and meta-analysis. Clin Kidney J. 2023;16:322–330. doi: 10.1093/ckj/sfac238
- Delanaye P, Vidal-Petiot E, Björk J, et al. Performance of creatinine-based equations to estimate glomerular filtration rate in White and black populations in Europe, Brazil and Africa. Nephrol Dial Transplant. 2023;38(1):106–118. 7.186 Q1. PMID: 36002032. doi: 10.1093/ndt/gfac241
- Pottel H, Björk J, Courbebaisse M, et al. Development and validation of a modified full age spectrum creatinine-based equation to estimate glomerular filtration rate: a cross-sectional analysis of pooled data. Ann Intern Med. 2021;174(2):183–191. doi: 10.7326/M20-4366
- Delanaye P, Jager KJ, Bökenkamp A, et al. CKD: a call for an age-adapted definition. J Am Soc Nephrol. 2019;30(10):1785–1805. Epub 2019 Sep 10. PMID: 31506289; PMCID: PMC6779354. doi: 10.1681/ASN.2019030238
- Coresh J, Gansevoort RT, Prognosis Consortium CKD, et al. Current CKD definition takes into account both relative and absolute risk. J Am Soc Nephrol. 2020;31(2):447–448. doi: 10.1681/ASN.2019101049. Epub 2019 Dec 23. PMID: 31871274; PMCID: PMC7003310
- Keller N, Ruppert M, Fourtage M, et al. Médicaments du système cardiovasculaire et fonction rénale : les pièges de l’adaptation rénale [Cardiovascular drugs and renal function: Pitfall of renal adaptation]. Nephrol Ther. 2019;15(2):97–103. in FrenchEpub 2019 Mar 1. PMID: 30827822. doi: 10.1016/j.nephro.2018.11.005
- French National Authority for Health (Haute Autorité de Santé - HAS). Guide du parcours de soins – Maladie rénale chronique de l’adulte (MRC). 1 Jul 2021. (in French). PDF available at: https://www.has-sante.fr/upload/docs/application/pdf/2021-09/guide__mrc.pdf Last access: 05 Jul 2023
- Geddes CC, Woo YM, Brady S. Glomerular filtration rate–what is the rationale and justification of normalizing GFR for body surface area? Nephrol Dial Transplant. 2008;23(1):4–6. Epub 2007 Oct 2. PMID: 17913737. doi: 10.1093/ndt/gfm662
- Delanaye P, Radermecker RP, Rorive M, et al. Indexing glomerular filtration rate for body surface area in obese patients is misleading: concept and example. Nephrol Dial Transplant. 2005;20(10):2024–2028. Epub 2005 Jul 19. PMID: 16030047. doi: 10.1093/ndt/gfh983
- Inker LA, Eneanya ND, Coresh J, et al. New creatinine- and cystatin C-Based equations to estimate GFR without race. N Engl J Med. 2021;385(19):1737–1749. doi: 10.1056/NEJMoa2102953
- Heyman SN, Raz I, Dwyer JP, et al. Diabetic proteinuria revisited: updated physiologic perspectives. Cells. 2022;11(18):2917. PMID: 36139492; PMCID: PMC9496872. doi: 10.3390/cells11182917
- Stehouwer CD, Smulders YM. Microalbuminuria and risk for cardiovascular disease: analysis of potential mechanisms. J Am Soc Nephrol. 2006;17(8):2106–2111. Epub 2006 Jul 6. PMID: 16825333. doi: 10.1681/ASN.2005121288
- Miller WG, Bruns DE, Hortin GL, et al. National Kidney Disease Education Program-IFCC Working Group on Standardization of Albumin in Urine. Current issues in measurement and reporting of urinary albumin excretion. Clin Chem. 2009;55(1):24–38. Epub 2008 Nov 21. PMID: 19028824. doi: 10.1373/clinchem.2008.106567
- French National Authority for Health (Haute Autorité de Santé – HAS 2011). Évaluation du rapport albuminurie/créatininurie dans le diagnostic de la maladie rénale chronique chez l’adulte - Rapport d’évaluation. 28 Dec 2011. (in French). Available at: https://www.has-sante.fr/jcms/c_1169049/fr/evaluation-du-rapport-albuminurie/creatininurie-dans-le-diagnostic-de-la-maladie-renale-chronique-chez-l-adulte-rapport-d-evaluation Last access: 05 Jul 2023
- Halimi JM, Hadjadj S, Aboyans V, et al. Microalbuminuria and urinary albumin excretion: French clinical practice guidelines. Diabetes metab. 2007;33(4):303–309. doi: 10.1016/j.diabet.2007.06.001
- Fox CS, Matsushita K, Woodward M, et al. Associations of kidney disease measures with mortality and end-stage renal disease in individuals with and without diabetes: a meta-analysis [published correction appears in Lancet. 2013 Feb 2;381(9864): 374]. Lancet. 2012;380(9854):1662–1673. doi: 10.1016/S0140-6736(12)61350-6
- Matsushita K, Coresh J, Sang Y, et al. Estimated glomerular filtration rate and albuminuria for prediction of cardiovascular outcomes: a collaborative meta-analysis of individual participant data. Lancet Diabetes Endocrinol. 2015;3(7):514–525. doi: 10.1016/S2213-8587(15)00040-6
- Khan MS, Shahid I, Anker SD, et al. Albuminuria and heart failure: JACC state-of-the-art review. J Am Coll Cardiol. 2023;81(3):270–282. doi: 10.1016/j.jacc.2022.10.028
- de Zeeuw D, Remuzzi G, Parving HH, et al. Proteinuria, a target for renoprotection in patients with type 2 diabetic nephropathy: lessons from RENAAL. Kidney Int. 2004;65(6):2309–2320. doi: 10.1111/j.1523-1755.2004.00653.x
- Coresh J, Heerspink HJL, Sang Y, et al. Chronic kidney disease prognosis Consortium and chronic kidney disease epidemiology collaboration. Change in albuminuria and subsequent risk of end-stage kidney disease: an individual participant-level consortium meta-analysis of observational studies. Lancet Diabetes Endocrinol. 2019;7(2):115–127. Epub 2019 Jan 8. PMID: 30635225; PMCID: PMC6379893. doi: 10.1016/S2213-8587(18)30313-9
- Palmer BF. Change in albuminuria as a surrogate endpoint for cardiovascular and renal outcomes in patients with diabetes. Diab Obes Metab. 2023;25(6):1434–1443. Epub 2023 Mar 8. PMID: 36809555. doi: 10.1111/dom.15030
- Pafundi PC, Garofalo C, Galiero R, et al. Role of albuminuria in detecting cardio-renal risk and outcome in diabetic subjects. Diagnostics. 2021;11(2):290. PMID: 33673215; PMCID: PMC7918197. doi: 10.3390/diagnostics11020290
- Ragot S, Saulnier PJ, Velho G, et al. Dynamic changes in renal function are associated with major cardiovascular events in patients with type 2 diabetes. Diabetes Care. 2016;39(7):1259–1266. doi: 10.2337/dc15-2607
- Pinier C, Gatault P, François M, et al. Renal function at the time of nephrology referral but not dialysis initiation as a risk for death in patients with diabetes mellitus. Clin Kidney J. 2018;11(6):762–768. doi: 10.1093/ckj/sfy032
- Smart NA, Dieberg G, Ladhani M, et al. Early referral to specialist nephrology services for preventing the progression to end-stage kidney disease. Cochrane Database Syst Rev. 2014 CD007333 PMID: 24938824 doi: 10.1002/14651858.CD007333.pub2
- Smart NA, Titus TT. Outcomes of early versus late nephrology referral in chronic kidney disease: a systematic review. Am j med. 2011;124(11):1073–80.e2. PMID: 22017785. doi: 10.1016/j.amjmed.2011.04.026
- Collier W, Inker LA, Haaland B, et al. Chronic kidney disease epidemiology collaboration (CKD-EPI)*. Evaluation of variation in the performance of GFR slope as a surrogate endpoint for kidney failure in clinical trials that differ by severity of CKD. Clin J Am Soc Nephrol. 2023;18(2):183–192. Epub 2023 Jan 19. PMID: 36754007. doi: 10.2215/CJN.0000000000000050
- Tangri N, Stevens LA, Griffith J, et al. A predictive model for progression of chronic kidney disease to kidney failure. JAMA. 2011;305(15):1553–1559. doi: 10.1001/jama.2011.451
- Tangri N, Grams ME, Levey AS, et al. Multinational assessment of accuracy of equations for predicting risk of kidney failure: a meta-analysis [published correction appears in JAMA. 2016;315(8): 822]. JAMA. 2016;315(2):164–174. doi: 10.1001/jama.2015.18202
- Peeters MJ, van Zuilen AD, van den Brand JA, et al. Validation of the kidney failure risk equation in European CKD patients. Nephrol Dial Transplant. 2013;28(7):1773–1779. doi: 10.1093/ndt/gft063
- Grams ME, Li L, Greene TH, et al. Estimating time to ESRD using kidney failure risk equations: results from the African American study of kidney disease and hypertension (AASK). Am J Kidney Dis. 2015;65(3):394–402. doi: 10.1053/j.ajkd.2014.07.026
- Wang Y, Nguyen FNHL, Allen JC, et al. Validation of the kidney failure risk equation for end-stage kidney disease in Southeast Asia. BMC Nephrol. 2019;20(1):451. doi: 10.1186/s12882-019-1643-0
- Hundemer GL, Tangri N, Sood MM, et al. Performance of the kidney failure risk equation by disease etiology in advanced CKD. Clin J Am Soc Nephrol. 2020;15(10):1424–1432. Epub 2020 Sep 14. PMID: 32928746; PMCID: PMC7536763. doi: 10.2215/CJN.03940320
- Lennartz CS, Pickering JW, Seiler-Mußler S, et al. External validation of the kidney failure risk equation and re-calibration with addition of ultrasound parameters. Clin J Am Soc Nephrol. 2016;11(4):609–615. doi: 10.2215/CJN.08110715
- Winnicki E, McCulloch CE, Mitsnefes MM, et al. Use of the kidney failure risk equation to determine the risk of progression to end-stage renal disease in children with chronic kidney disease. JAMA Pediatr. 2018;172(2):174–180. doi: 10.1001/jamapediatrics.2017.4083
- Hundemer GL, Tangri N, Sood MM, et al. The effect of age on performance of the kidney failure risk equation in advanced CKD. Kidney Int Rep. 2021;6(12):2993–3001. PMID: 34901569; PMCID: PMC8640561. doi: 10.1016/j.ekir.2021.09.006
- Al-Wahsh H, Tangri N, Quinn R, et al. Accounting for the competing risk of death to predict kidney failure in adults with stage 4 chronic kidney disease. JAMA Netw Open. 2021;4(5):e219225. PMID: 33944922; PMCID: PMC8097501. doi: 10.1001/jamanetworkopen.2021.9225
- Major RW, Shepherd D, Medcalf JF, et al. The kidney failure risk equation for prediction of end stage renal disease in UK primary care: an external validation and clinical impact projection cohort study. PLOS Med. 2019;16(11):e1002955. Erratum in: PLoS Med. 2020 Jul 24;17(7):e1003313. PMID: 31693662; PMCID: PMC6834237. doi: 10.1371/journal.pmed.1002955
- Hingwala J, Wojciechowski P, Hiebert B, et al. Risk-based triage for nephrology referrals using the kidney failure risk equation. Can J Kidney Health Dis. 2017;4:2054358117722782. PMID: 28835850; PMCID: PMC5555495. doi: 10.1177/2054358117722782
- NICE 2021. Chronic kidney disease: assessment and management. Available at: https://www.nice.org.uk/guidance/NG203 Last access: 5 Jul 2023
- Clase CM, Carrero JJ, Ellison DH, et al. Conference participants. Potassium homeostasis and management of dyskalemia in kidney diseases: conclusions from a kidney disease: improving Global outcomes (KDIGO) controversies Conference. Kidney Int. 2020;97(1):42–61. Epub 2019 Oct 10. PMID: 31706619. doi: 10.1016/j.kint.2019.09.018
- Sousa AG, Cabral JV, El-Feghaly WB, et al. Hyporeninemic hypoaldosteronism and diabetes mellitus: pathophysiology assumptions, clinical aspects and implications for management. World J Diabetes. 2016;7(5):101–111. PMID: 26981183; PMCID: PMC4781902. doi: 10.4239/wjd.v7.i5.101
- Cooper LB, Savarese G, Carrero JJ, et al. Clinical and research implications of serum versus plasma potassium measurements. Eur J Heart Fail. 2019;21(4):536–537. Epub 2018 Nov 28. PMID: 30485595. doi: 10.1002/ejhf.1371
- Gruson G, Jadoul M, Deltombe M, et al. MO739 evaluation of a capillary blood potassium measurement method in healthy volunteers and in chronic haemodialysis patients. Available at; https://academic.oup.com/ndt/article/37/Supplement_3/gfac079.018/6577586 Last access: 5 Jul 2023
- Rossignol P, Silva-Cardoso J, Kosiborod MN, et al. Pragmatic diagnostic and therapeutic algorithms to optimize new potassium binder use in cardiorenal disease. Pharmacol Res. 2022;182:106277. doi: 10.1016/j.phrs.2022.106277
- Kovesdy CP, Appel LJ, Grams ME, et al. Potassium homeostasis in health and disease: a scientific workshop cosponsored by the National kidney Foundation and the American Society of Hypertension. J Am Soc Hypertens. 2017;11(12):783–800. Epub 2017 Oct 10. PMID: 29030153. doi: 10.1016/j.jash.2017.09.011
- Kovesdy CP, Matsushita K, Sang Y, et al. CKD prognosis Consortium. Serum potassium and adverse outcomes across the range of kidney function: a CKD prognosis Consortium meta-analysis. Eur Heart J. 2018;39(17):1535–1542. PMID: 29554312; PMCID: PMC5930249. doi: 10.1093/eurheartj/ehy100
- Collins AJ, Pitt B, Reaven N, et al. Association of serum potassium with all-cause mortality in patients with and without heart failure, chronic kidney disease, and/or diabetes. Am J Nephrol. 2017;46(3):213–221. doi: 10.1159/000479802
- Bandak G, Sang Y, Gasparini A, et al. Hyperkalemia after initiating renin-angiotensin system blockade: the Stockholm Creatinine Measurements (SCREAM) project. J Am Heart Assoc. 2017;6(7):e005428. PMID: 28724651; PMCID: PMC5586281. doi: 10.1161/JAHA.116.005428
- Khosla N, Kalaitzidis R, Bakris GL. Predictors of hyperkalemia risk following hypertension control with aldosterone blockade. Epub 2009 Sep 9. PMID: 19738369. Am J Nephrol. 2009;30(5):418–424. doi: 10.1159/000237742
- Noori N, Kalantar-Zadeh K, Kovesdy CP, et al. Dietary potassium intake and mortality in long-term hemodialysis patients. Am J Kidney Dis. 2010;56(2):338–347. Epub 2010 Jun 30. PMID: 20580474; PMCID: PMC2910783. doi: 10.1053/j.ajkd.2010.03.022
- Smyth A, Dunkler D, Gao P, et al. ONTARGET and TRANSCEND investigators. The relationship between estimated sodium and potassium excretion and subsequent renal outcomes. Kidney Int. 2014;86(6):1205–1212. Epub 2014 Jun 11. PMID: 24918156. doi: 10.1038/ki.2014.214
- Patel S, Lam PH, Kanonidis EI, et al. Renin-angiotensin inhibition and outcomes in HFrEF and advanced kidney disease. Am j med. 2023;136(7):677–686. doi: 10.1016/j.amjmed.2023.03.017
- Ku E, Inker LA, Tighiouart H, et al. Initiation of ACE inhibitor and ARBs in patients with advanced chronic kidney disease. 2023. Submitted. (not yet published)
- Lazich I, Bakris GL. Prediction and management of hyperkalemia across the spectrum of chronic kidney disease. Epub 2014 Apr 18. PMID: 25016403. Semin Nephrol. 2014;34(3):333–339. doi: 10.1016/j.semnephrol.2014.04.008
- Jun M, Jardine MJ, Perkovic V, et al. Hyperkalemia and renin-angiotensin aldosterone system inhibitor therapy in chronic kidney disease: a general practice-based, observational study. PLoS One. 2019 [Published 2019 Mar 7];14(3):e0213192. doi: 10.1371/journal.pone.0213192
- Epstein M, Reaven NL, Funk SE, et al. Evaluation of the treatment gap between clinical guidelines and the utilization of renin-angiotensin-aldosterone system inhibitors. Am J Manag Care. 2015;21(11 Suppl):S212–S220.
- Leon SJ, Whitlock R, Rigatto C, et al. Hyperkalemia-related discontinuation of renin-angiotensin-aldosterone system inhibitors and clinical outcomes in CKD: a population-based cohort study. Am J Kidney Dis. 2022;80(2):164–173.e1. doi: 10.1053/j.ajkd.2022.01.002
- Fu EL, Evans M, Clase CM, et al. Stopping renin-angiotensin system inhibitors in patients with advanced CKD and risk of adverse outcomes: a nationwide study. J Am Soc Nephrol. 2021;32(2):424–435. doi: 10.1681/ASN.2020050682
- Nakayama T, Morimoto K, Uchiyama K, et al. Effects of renin-angiotensin system inhibitors on the incidence of unplanned dialysis. Hypertens Res. 2022;45(6):1018–1027. doi: 10.1038/s41440-022-00877-5
- Bhandari S, Mehta S, Khwaja A, et al. Renin-angiotensin system inhibition in advanced chronic kidney disease. N Engl J Med. 2022;387(22):2021–2032. doi: 10.1056/NEJMoa2210639
- Sterns RH, Rojas M, Bernstein P, et al. Ion-exchange resins for the treatment of hyperkalemia: are they safe and effective? J Am Soc Nephrol. 2010;21(5):733–735. doi: 10.1681/ASN.2010010079
- Pitt B, Bakris GL. New potassium binders for the treatment of hyperkalemia: current data and opportunities for the future. Hypertension. 2015;66(4):731–738. doi: 10.1161/HYPERTENSIONAHA.115.04889
- Weinstein J, Girard LP, Lepage S, et al. Prevention and management of hyperkalemia in patients treated with renin-angiotensin-aldosterone system inhibitors. CMAJ. 2021;193(48):E1836–E1841. doi: 10.1503/cmaj.210831
- Rossignol P, Moulin B, Halimi JM, et al. État des lieux sur l’hyperkaliémie chronique persistante en France : consensus d’experts par une approche Delphi [Current status of persistent chronic hyperkalaemia in France: An expert consensus based on a Delphi approach]. Nephrol Ther. 2022;18(4):278–286. French Epub 2022 Jun 30. PMID: 35781425. doi: 10.1016/j.nephro.2021.10.008
- Neuen BL, Oshima M, Agarwal R, et al. Sodium-glucose cotransporter 2 inhibitors and risk of hyperkalemia in people with type 2 diabetes: a meta-analysis of individual participant data from randomized, controlled trials. Circulation. 2022;145(19):1460–1470. 39.918 Q1. Epub 2022 Apr 8. PMID: 35394821. doi: 10.1161/CIRCULATIONAHA.121.057736
- Pagidipati NJ, Nelson AJ, Kaltenbach LA, et al. COORDINATE–diabetes site investigators. Coordinated care to optimize cardiovascular preventive therapies in type 2 diabetes: a randomized clinical trial. JAMA. 2023;329(15):1261–1270. PMID: 36877177; PMCID: PMC9989955. doi: 10.1001/jama.2023.2854
- Martínez-González NA, Djalali S, Tandjung R, et al. Substitution of physicians by nurses in primary care: a systematic review and meta-analysis. BMC Health Serv Res. 2014 [Published 2014 May 12];14(1):214. doi: 10.1186/1472-6963-14-214
- Bryant-Lukosius D, Spichiger E, Martin J, et al. Framework for evaluating the impact of advanced practice nursing roles. J Nurs Scholarsh. 2016;48(2):201–209. doi: 10.1111/jnu.12199
- Donald F, Kilpatrick K, Reid K, et al. Hospital to community transitional care by nurse practitioners: a systematic review of cost-effectiveness. Int J Nurs Stud. 2015;52(1):436–451. doi: 10.1016/j.ijnurstu.2014.07.011
