ABSTRACT
Objectives
To understand the role of primary care physicians (PCPs) in the recognition, diagnosis, and management of Crohn’s perianal fistulas (CPF) and their referral patterns and treatment expectations.
Methods
This survey-based study was conducted between September 2020 and October 2020. US-based PCPs managing at least one patient with Crohn’s disease per week were included. Participants were presented with two case vignettes relevant to primary care practice; Case Vignette 1 comprised three parts and focused on initial CPF presentation and progression to partial response; Case Vignette 2 focused on recurrent CPF. Survey questions elicited the physician’s clinical approach to each case. Data were presented as descriptive statistics.
Results
Overall, 151 PCPs (median 23 years in practice) who saw about three patients per month with new/existing CPF responded. For Case Vignette 1, upon identification of a fistulous tract, 89% of respondents would refer the patient, mostly to a colorectal surgeon or gastroenterologist. Most PCPs (69%) would begin the patient on medication; 46% would conduct a diagnostic/imaging study. Treatment expectations after referral varied: 55% of respondents believed surgeons would place a seton or use one prior to surgery; 23% expected medical management only; 23% were unsure. Case Vignette 2 revealed that 98% of PCPs preferred to be involved in patient care after referral; however, only 49% were. Of these, 76% considered reinforcing patient treatment adherence as their primary role. While 80% of PCPs were at least moderately satisfied with communication and care coordination with multidisciplinary teams, 52% considered lack of access to specialists as at least a moderate barrier to multidisciplinary team management.
Conclusion
PCPs want more involvement in multidisciplinary management of patients with CPF. Continuing education providing PCPs with up-to-date information on diagnostic modalities, treatment options, early diagnosis, the role of PCPs within a multidisciplinary team, and effective initial CPF care is required.
Plain Language Summary
What were the study’s aims?
To understand how primary care physicians recognize, treat, and monitor patients with Crohn’s disease-related perianal fistulas (small tunnels between the bowel and skin near the anus).
How was the study done?
US-based primary care physicians, including internists, were included if they had experience in treating patients with Crohn’s disease. Descriptions of the history and symptoms of two hypothetical patients were provided: one patient who may have Crohn’s perianal fistulas and another patient whose Crohn’s perianal fistulas had returned after being treated. After reading these descriptions, the physicians completed a questionnaire designed to show how they would help each patient.
What did the study find out?
Not all physicians treat patients with Crohn’s perianal fistulas in the same way in terms of diagnostic tests and medical treatments, although most said they would refer them to a specialist if a fistula was identified. Many wanted to be involved in patient care after referral to a specialist but only half were. Of those, most thought their main role was to ensure patients followed the treatments given by specialists. More than half of primary care physicians thought a lack of access to specialists could be a barrier to care.
How does this impact care?
The physicians surveyed want more involvement in multidisciplinary teams who look after patients with Crohn’s perianal fistulas. To do this, they need more education about the diagnosis and treatment of Crohn’s perianal fistulas, and clarity around their role within multidisciplinary teams who manage these patients.
1. Introduction
Crohn’s disease (CD), a relapsing inflammatory bowel disease (IBD), has an estimated prevalence of over 300 per 100,000 people in North America and Europe [Citation1]. Crohn’s Perianal fistulas (CPF) are a disabling manifestation of CD, forming a narrow tract with an internal opening in the anal canal or rectum and an external opening in the skin around the anus [Citation2,Citation3]. CPF can impact the physical, social, and emotional well-being of patients, as well as their work or school life, by causing pain and drainage of pus, stool, and/or blood from the fistula opening and occur in 20–40% of patients with CD [Citation3–9]. In addition, costs and healthcare resource utilization are significantly higher for patients with CPF versus those with CD [Citation10]. Management of CPF requires both medical and surgical interventions and a multidisciplinary approach to limit the acute infection risk, treat underlying inflammation, and ultimately achieve fistula closure, however many patients experience fistula recurrence or lack of response over time [Citation11–13]. While gastroenterologists and their nurse practitioners are most frequently associated with the treatment of IBDs, including CD, a recent study identified primary care physicians (PCPs) as being uniquely placed to facilitate and deliver fully integrated multidisciplinary care to these patients, which is critical to the delivery of optimal care [Citation11,Citation12,Citation14].
Several specialty society position statements and guidelines are available on how best to manage patients with CPF [Citation4,Citation13,Citation15–22], which often state that guidelines apply to all clinicians who treat IBD or perianal fistula but rarely mention the role of PCPs in the multidisciplinary management of CPF [Citation4,Citation13,Citation15–17,Citation19–22]. Large proportions of patients with IBD are managed in the primary care setting and potential knowledge gaps and a lack of confidence among PCPs in recognizing key symptoms associated with IBD have been previously reported [Citation23]. Healthcare issues relating to IBD are seemingly widespread and delayed diagnosis, particularly with respect to CD, can increase the likelihood of poor clinical outcomes, including fistula development [Citation24–26]. Therefore, understanding the educational needs of PCPs with respect to this patient population is critical. In conjunction with investigations into gastroenterologists, colorectal surgeons’ and their nurse practitioner/physician assistants’ perceptions of CPF, we developed a case vignette-based study to understand primary care recognition and diagnosis of CPF, patient referral patterns, and the role of the PCP in CPF management and their treatment expectations.
2. Methods
2.1. Development of the PCP survey
A survey was developed using case vignettes to investigate practicing PCPs’ current decisions and attitudes related to the management of patients with CPF. The survey included two patient case vignettes; initial presentation and progression to partial response (Case Vignette 1) and recurrent CPF (Case Vignette 2), with associated questions to understand the PCP’s evaluation, referral, and treatment expectations. The clinical vignettes were presented to respondents in a stepwise fashion with relevant questions presented at each stage. To ensure that responses were not influenced by case progression within each vignette, respondents were unable to modify their answers as they progressed through the survey. Additional questions were used to understand barriers to and satisfaction with current multidisciplinary management of patients with CPF. The survey was first piloted in cognitive interviews with PCPs with and without fistula management experience to determine areas of ambiguity and to ensure that all questions were being interpreted as intended. The full details of each case vignette are provided below, and questions asked of survey respondents at each stage are provided in the supplementary data.
2.1.1. Case Vignette 1: CPF initial case presentation and progression to partial response (supplementary data: questions 1–10)
Part 1 (presentation):
A 38-year-old man complains of 3 weeks of discomfort near his anus, especially while sitting, as well as frequent malodorous discharge on his underwear. He reports being diagnosed with CD 5 years ago, at which time he took mesalamine; however, he stopped taking it after about a year because he felt that his symptoms were controlled through diet modification. He has not had a follow-up with a gastroenterologist or a repeat colonoscopy since that time. He admits to mild abdominal pain and diarrhea over the past several months. He denies any fevers.
Visual inspection of the perianal area is notable for erythema and the opening of a fistulous tract. Digital rectal exam is notable for tenderness but is otherwise unremarkable. There is no fluctuation. You are unable to appreciate the internal opening of the fistulous tract. His CD activity is mild, without symptoms of systemic disease, such as fever, abdominal tenderness, or signs of obstruction.
Part 2 (referral):
The patient is referred to gastroenterology and colorectal surgery. He is started on metronidazole. Pelvic magnetic resonance imaging (MRI) reveals a trans-sphincteric fistula arising from the anal canal and penetrating the internal and external anal sphincters. He undergoes exam under anesthesia with seton placement, and findings on exam are consistent with those identified on MRI. There is no evidence of abscess. Colonoscopy reveals CD involvement of the distal ilium and proximal colon. No proctitis is present.
Part 3 (treatment):
Treatment with infliximab is started. Six months later, the patient’s symptoms have markedly improved after seton placement, and he no longer notices any drainage from the fistula. He reports intermittent perianal pain and being somewhat bothered by the seton. He denies any ongoing abdominal pain, diarrhea, or other CD symptoms. On exam, the external opening of the fistula tract has decreased in size. Gentle pressure on the area of the fistula tract does not express any fluid. Pelvic MRI demonstrates persistent fistula and inflammatory activity, without evidence of abscess. Infliximab trough level is 3.5 μg/mL. Anti-infliximab antibody testing is negative. He is restarted on an antibiotic.
2.1.2. Case Vignette 2: management of recurrent CPFs (supplementary data: questions 11–13)
A 35-year-old woman with an 8-year history of CD presents to her gastroenterologist with perianal pain, swelling, and fever. Pelvic MRI reveals a supralevator fistula arising from the anal canal, penetrating both the internal and external anal sphincters and forming a 1-cm abscess in the ischioanal space before coursing to the skin. She undergoes an urgent exam under anesthesia with abscess drainage and seton placement. She is started on infliximab and antibiotics.
Within the next year, she has two episodes of abscess recurrence for which she returns to the operating room for drainage and has adjustments made to her medication regimen. At follow-up, she reports ongoing pain and drainage that interfere with her daily activities. Pressure on the area of the fistula causes discharge of purulent material, although there is no evidence of abscess. Repeat MRI shows ongoing signs of inflammation and a persistent fistula, now with branching and a second internal opening. Endoscopy and MR enterography are consistent with mildly active CD without proctitis. She follows up with her gastroenterologist and colorectal surgeon, who offer additional medical and surgical management options.
2.2. Survey distribution and data collection
Emails of US-practicing clinicians were randomly selected from a proprietary database and purchased mailing lists. PCPs from urban, suburban, and rural practices were included. A quota method of sample collection was utilized; on the basis of power calculations to generalize results to a national audience, at least 150 PCPs were needed for analysis. The surveys were distributed in September 2020 and October 2020. The primary inclusion criteria were that PCPs must be practicing in the USA and managing at least one patient with CD per week. The survey was expected to take approximately 20–25 min to complete, and a monetary incentive (equivalent to US$50) was offered to physicians for their participation.
2.3. Survey analysis
Descriptive statistics were conducted on key items of the surveys.
2.4. Ethical considerations
The protocol was determined to be exempt from Institutional Review Board review by Western Institutional Review Board (Puyallup, WA) under 45 Code of Federal Regulations § 46.104(d)(2), because the research only includes interactions involving educational tests, survey procedures, interview procedures, or observations of public behavior. Identifying information, used only to provide honoraria to the respondents, was removed prior to analysis.
3. Results
3.1. Respondent demographics
Overall, 151 PCPs completed the survey, of whom 54% self-selected as family medicine physicians and 46% as general internists. PCPs self-reported as predominately in a community-based setting and the majority (58%) practiced in a suburban location. PCP respondents had a median of 23 (interquartile range: 18–28) years in practice (YIP). On average, the respondents saw 116 patients per week and 14 patients with CD each month, with about three of these patients having new or existing CPF ().
Table 1. Demographics of PCP survey respondents (N = 151).
3.2. Case Vignette 1: CPF initial case presentation and progression to partial response
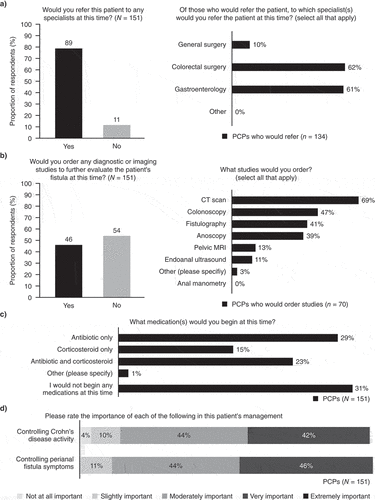
Overall, 85% of PCPs would include perianal fistula in their top three differential diagnoses for this patient. After visual inspection revealed the opening of a fistulous tract, most of the respondents (n = 134, 89%) would refer the patient to a specialist, with the majority choosing to refer to a colorectal surgeon and/or gastroenterologist (). Fewer than half (n = 70, 46%) of all PCP respondents would conduct a diagnostic/imaging study themselves prior to a referral (), and of these, 69% (n = 48) would order a computed tomography (CT) scan. Other tests chosen include a colonoscopy, fistulography, anoscopy, and, to a lesser degree, pelvic MRI, and endoscopic ultrasound. Prior to the referral, 69% (n = 104) of PCPs would begin with the patient on medication, of whom 98% (n = 102) would use an antibiotic and/or corticosteroid (). While there were no notable differences in referral patterns based on YIP, PCPs with fewer YIP indicated they were more likely to use corticosteroids and antibiotics when compared with PCPs with a higher number of YIP (34% of those with fewer than 18 years versus 21% with 19–25 YIP and 15% of those with more than 26 YIP). Overall, PCPs rated controlling both CD activity and perianal fistula symptoms as important ().
Figure 1. PCP respondents' insights regarding the initial referral and evaluation of the patient with CPF presented in Case Vignette 1.
3.2.1. Expectations upon specialist management
Case Vignette 1 continues as the patient is referred to gastroenterology and colorectal surgery, in which it is found that the patient has CD involvement of the distal ileum and proximal colon, but no proctitis. PCPs were asked what they expect the specialists to do at this point, but there was little consensus on the expected approach (). Just over half (55%) of PCPs expected that surgeons would place a seton or use one as a bridge to surgery, 23% expected medical management only, and 23% were unsure. Of the 82 PCPs (54%) expecting the gastroenterologist to begin medication, the most expected medications were 5-aminosalicylates (n = 50, 61%), corticosteroids (n = 49, 60%), or tumor necrosis factor inhibitors (n = 33, 40%). PCPs with fewer YIPs were more likely to select tumor necrosis factor inhibitors compared with those with a higher number of YIP (54% of those with fewer than 18 years versus 41% and 23% of those with 19–25 and more than 26 YIP, respectively). Other medications selected included other monoclonal antibodies (e.g. vedolizumab, n = 11, 13%), anti-metabolites (e.g. methotrexate; n = 9, 11%), thiopurines (e.g. azathioprine; n = 9, 11%), calcineurin inhibitors (e.g. tacrolimus; n = 2, 2%), and other (vitamins/probiotics; n = 1, 1%); only 2% (n = 2) were unsure about what medications would be offered by the gastroenterologist. After initial specialist management, PCPs indicated they were most likely to review the consultation notes and ask the patient about their CD symptoms and fistula drainage. At this point, PCPs indicated a moderate likelihood of conducting a rectal exam (3.1 on a 5-point likelihood scale).
Table 2. Expectations of specialist management among PCP survey respondents (N = 151, Case Vignette 1).
Case Vignette 1 progresses to indicate that a loose seton was placed and treatment with infliximab was started. This treatment was moderately successful, given that the fistula tract decreased in size, but pelvic MRI demonstrated persistent fistula and inflammatory activity. At this point, 51% (n = 77) of the PCPs indicated that they expected the gastroenterologist to continue current medical therapy and 43% (n = 65) expected the surgeon to proceed to surgery ().
3.3. Case Vignette 2: goal setting and the PCP's role in recurrent CPF management
When asked about long-term treatment goals for this patient, PCPs were most likely to select improving quality of life (QoL) followed by achieving fistula healing and resolving fistula symptoms (). Goals prioritized by fewer PCPs were avoiding major surgery/preserving continence and minimizing medication risks/side effects. PCPs also indicated a higher likelihood of discussing QoL issues with the patient compared with other options (medical and surgical management options, medication side effects, and general surgical complications). PCPs were not likely to discuss investigational treatment options with the patient. When asked how they would assist the patient in decision-making regarding fistula treatment, most PCPs indicated that they would discuss treatment goals with both the patient and the specialists and seek out further education themselves. Respectively, 42% (n = 63) and 24% (n = 36) of PCPs would provide their own treatment recommendations or refer the patient for a second opinion; responses to whether PCPs would make their own treatment recommendations differed according to practice setting (community setting: 39%, academic setting: 71%) and location (urban: 56%, suburban: 39%, rural: 21%). Overall, only 9% (n = 13) would search for potential clinical trials.
Table 3. PCP survey respondents' perspectives on CPF treatment goals and their role in the management of recurrent CPF (N = 151, Case Vignette 2).
3.4. The PCP's role in the management of patients with CPF
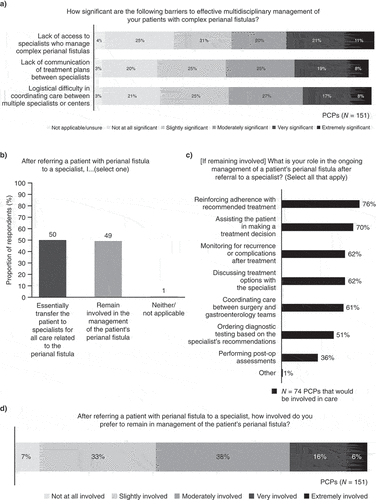
More than half of PCPs found lack of access to specialists who manage complex CPF, lack of communication of treatment plans between specialists, and logistical difficulty in coordinating care between multiple specialists or centers to be at least a moderately significant barrier to care (). After referring a patient with CPF to a specialist, half of PCPs remain involved in patient management, while the other half transfer the patient for all CPF care (). Of those who remain involved in patient management, reinforcing adherence and assisting the patient in making treatment decisions were considered the primary roles for PCPs (). Notably, when asked whether they prefer to stay involved in patient management post-referral, 93% of all PCPs wanted to maintain at least some involvement in the management of the patient’s CPF ().
3.5. Communicating and coordinating care with a multidisciplinary team
Overall, PCPs most commonly identified gastroenterologists (87%) and colorectal surgeons (78%) as members of the most recent multidisciplinary team involved in the care of their patients with complex CPF; however, the reported composition of multidisciplinary teams with respect to other specialisms (e.g. general surgeons, gastroenterology nurses, nurse practitioners, or physician’s assistants) was more varied (). The primary methods used to communicate and coordinate care with others in the multidisciplinary team were phone calls (68% of PCPs) and review of notes in a shared electronic medical record (EMR) system (63%). There was substantial variation in how PCPs were notified of imaging results; 47% (n = 71) expected to see the results in a shared EMR system, others were mixed between reviewing the results directly with a specialist, expecting to be notified, or requesting the results from the specialist. Despite this, about 80% (n = 120) of PCPs were at least moderately satisfied with the current processes and methods of communication and coordination of care in their patients with CPF.
Table 4. PCP respondents' (N = 151) insights into multidisciplinary team composition and the coordination of care in patients with CPF.
Given that these practice patterns could have been affected by the early phase of the COVID-19 pandemic coinciding with survey data collection (September 2020–October 2020), respondents were also asked if COVID-19 was affecting the management of their patients with anorectal disease. Overall, 28% indicated yes, with 50% responding no, and 22% unsure. Of those who responded in the affirmative, several reasons for disruption were indicated including the use of telemedicine visits, lack of face-to-face patient appointments, patient hesitation/delay of surgery, lack of specialist access, and limited available surgery options.
4. Discussion
This case vignette-based study surveyed 151 PCPs with the aim of understanding the recognition, diagnosis, and referral patterns of patients with CPF seen in a primary care setting. The survey also explored the role of PCPs in CPF management and PCPs’ treatment expectations after referral of a patient to a gastroenterologist or colorectal surgeon. Gastroenterologists, colorectal surgeons, and their nurse practitioners/physician’s assistants were also included and responded to an adapted version of this survey; the results of these surveys have been published separately [Citation27,Citation28].
Most PCPs would refer a patient with CPF to a specialist, although there was no agreement on whether referral should be to a gastroenterologist or colorectal surgeon, even though most society guidelines, including surgical guidelines, recommend that the medical management of CPF should be directed by a gastroenterologist [Citation29]. The choice of specialist may be guided by the availability or relationship with specialists local to the PCP, rather than expectations of care. Fewer than half of PCPs would order diagnostic or imaging studies of a patient’s fistula prior to the patient being seen by a specialist, and nearly a third would not start the patient on any medications. In patients with CPF, corticosteroid use is not recommended because it can increase fistula-related complications along with the need for surgery, and may decrease the likelihood of fistula healing [Citation30,Citation31]. Nevertheless, over a third of the PCPs who responded in this study would prescribe them to the patient described in Case Vignette 1. Only half of PCPs included in this study would start the same patient on an antibiotic. This may reflect the varied recommendations provided in guidelines regarding antibiotic use for CPF treatment [Citation13,Citation16,Citation17]. For example, while the American Gastroenterological Association guidelines state that antibiotics should not be used as a monotherapy for CPF but can act as an effective adjuvant therapy when combined with a biologic, guidelines from the American Society of Colon and Rectal Surgeons (ASCRS) recommend reserving antibiotic use for treating anorectal abscesses associated with fistulas in patients with CD [Citation16,Citation17]. In addition, biologic therapy is considered the mainstay of CPF medical therapy for induction and maintenance of fistula remission [Citation16]. The findings demonstrate that an education gap clearly exists among PCPs with respect to optimal treatment pathways for patients with CD and/or CPF. Few clinical tools exist to enhance the knowledge of PCPs in the treatment of patients with CD [Citation23], therefore development of a clinical educational tool for PCPs is warranted.
Given that many PCPs indicated that lack of access to specialists was a barrier to care, acquiring initial diagnostic information and establishing early care may enhance patient outcomes. The ASCRS guidelines state that imaging may be considered for recurrent or complex anal fistula or anorectal CD [Citation16]. Because most PCPs included perianal fistula as a differential diagnosis for the patient described in Case Vignette 1, ordering initial imaging studies or onward referral to a specialist should be the next step; however, as previously discussed, choosing an initial treatment option may be challenging because guidelines vary [Citation13,Citation16,Citation17,Citation19–22]. Thus, PCP education is needed on the initial evaluations and treatments required for a patient with suspected CPF while they wait for an appointment with a specialist, which should be centered on guideline-based evidence.
Overall, PCP respondents reported that controlling fistula symptoms was slightly more important than controlling the patient’s CD activity; but both were important. This may imply that PCPs are linking the patient’s perianal fistula symptoms with overall control of CD activity as some studies have identified a correlation between luminal disease and perianal disease activity, while others have reported that active proctitis decreases the chance of CPF healing [Citation11,Citation15,Citation32].
Regarding the role of PCPs in CPF management, 49% of the respondents indicated that they remain involved in the management of a patient’s CPF after referral, while most noted a preference for continued involvement in the patient’s care. With respect to the case vignettes, PCPs may not be aware of the types of medical and surgical treatments chosen by specialists, but over 75% indicated that they would contact the specialists to discuss potential treatment options. Many PCPs involved in fistula treatment see their role as reinforcing adherence and assisting the patient in making a treatment decision, both of which would require knowledge of treatment options. While some of this information could be acquired from discussions with specialists, PCPs also indicated that a key barrier to effective multidisciplinary management was a lack of access to specialists who manage the condition. PCPs may see themselves as a coordinator between specialists and a mediator/translator of the different options presented to patients by colorectal surgeons and gastroenterologists. PCPs require future continuing education initiatives that better inform them on how to have these discussions with their patients. The initiatives could target both primary care and specialist clinicians to aid understanding of how patients with CPF should be managed, determine respective roles, and establish relationships between different clinical specialties and PCPs. While PCPs may not find education on how to use the latest treatments useful to their practice, raising awareness of their existence and providing resources on how to find further educational materials may be welcomed. Additionally, future education for specialists on how PCPs could improve patient outcomes via reinforcement of adherence, symptom monitoring, and care coordination may be useful.
Besides gastroenterologists and colorectal surgeons, PCPs reported varied compositions with respect to specialists involved in management teams caring for their patients with complex CPF. About one-fifth of PCPs reported the involvement of advanced practice providers (e.g. nurse practitioners and physician assistants) or radiologists, while about a quarter noted the involvement of nurses, general surgeons, or dietitians in their most recent patient’s management team. When establishing the role of the PCP, education is needed on the roles of other members of a CPF multidisciplinary team. Establishing the roles of all clinicians may give PCPs better insights into the types of contributions that would be useful in improving patient care.
PCPs had varied practice settings, and the results from this study show that PCP–specialist communication varies as well. While phone calls and notes from a shared EMR were the predominant method of communicating with specialists, many PCPs may not have this type of data access or direct connection with a surgeon/gastroenterologist. Informal in-person discussions with specialists to consult on CPF cases were used by less than a third of PCPs; this has likely decreased in frequency owing to the COVID-19 pandemic. Considerations for communication between PCPs, their nurse practitioners/physician assistants, and specialists should be included in future education, because educators should be aware of the preferred methods of their learners to establish connections that are beneficial to both parties. While only 28% of PCPs indicated that the COVID-19 pandemic affected the care of their patients with CPF at the time of the survey, innovations in communication such as telemedicine, which allow PCPs to track and monitor patient symptoms as well as patient QoL, are likely to become more common for patient care and should also be optimized for CPF through educational efforts.
4.1. Limitations
The generalizability of these results is limited. First, case vignettes were used as a proxy for clinical practice and the survey was limited in the number of cases that could be presented. While case vignettes are a valid method of evaluating certain aspects of clinical practice [Citation33], the case presented may not be representative of all patients seen in a particular PCP’s practice. Additionally, the responses may have been subject to a social-desirability bias, in which respondents could have assumed what the researchers are seeking and gave what they believed ‘should’ be the appropriate response. Moreover, the provision of a monetary incentive to physicians for completing the survey introduced the potential for incentive bias in the results. Further, because these data were captured for US-practicing PCPs, their applicability to global practice may be limited. As with most surveys, selection bias was possible because recipients from a particular demographic group and/or who possess a specific set of characteristics may be more likely to respond to the survey. Only PCPs who see at least one patient with CD per week were included and therefore PCPs with less experience in treating patients with CD were excluded and further research may be required into their understanding of CPF. Finally, the overall prevalence of CD and perianal disease reported in this study may be higher than expected. This is likely due to the study inclusion criteria and self-reporting of patient numbers by PCPs.
5. Conclusions
PCPs want to be more involved in multidisciplinary management of their patients with CPF but do not have up-to-date knowledge of diagnostic modalities and treatment options. Owing to limited specialist access, PCPs need additional information on early diagnosis and establishing safe and effective initial care. To be included in multidisciplinary teams, increased continuing education is needed for PCPs to become aware of CPF guidelines and treatment protocols, and for specialists to understand how primary care can be important in maintaining patient adherence and shared decision-making.
Declaration of financial/other relationships
GD Salinas, E Belcher, and S Stacy are employees of CE Outcomes, LLC, which was contracted by Takeda Pharmaceuticals USA, Inc. to conduct the study. PP Nazarey and SE Cazzetta are employees of Takeda Pharmaceuticals USA, Inc. and receive stock/stock options from Takeda Pharmaceutical Company Limited. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
A reviewer on this manuscript has disclosed receiving research funding from Janssen Research & Development LLC. Peer reviewers on this manuscript have no other relevant financial relationships or otherwise to disclose.
Author contributions statement
Greg Salinas, Sylvie Stacy, Pradeep Nazarey, and Susan Cazzetta contributed to the conception/design of the study. Greg Salinas and Emily Belcher contributed to data acquisition. Greg Salinas, Emily Belcher, Sylvie Stacy, Pradeep Nazarey, and Susan Cazzetta contributed to data analysis and interpretation. All authors contributed to the drafting of the manuscript and approved the final version of the manuscript for publication.
Supplemental Material
Download MS Word (59.8 KB)Acknowledgments
Medical writing support was provided by Luke Humphreys of Oxford PharmaGenesis, Oxford, UK, and was funded by Takeda Pharmaceuticals USA, Inc.
Data availability statement
The data supporting the results reported in this article will be made available within 3 months from initial request to researchers who provide a methodologically sound proposal. The data set will be provided after its deidentification, in compliance with applicable privacy laws, data protection, and requirements for consent and anonymization.
SUPPLEMENTARY MATERIAL
Supplemental data for this article can be accessed online at https://doi.org/10.1080/00325481.2023.2277146
Additional information
Funding
References
- Molodecky NA, Soon IS, Rabi DM, et al. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology. 2012;142(1):46–54 e42. quiz e30. doi: 10.1053/j.gastro.2011.10.001
- Wlodarczyk M, Wlodarczyk J, Sobolewska-Wlodarczyk A, et al. Current concepts in the pathogenesis of cryptoglandular perianal fistula. J Int Med Res. 2021;49(2):300060520986669. doi: 10.1177/0300060520986669
- Marzo M, Felice C, Pugliese D, et al. Management of perianal fistulas in Crohn’s disease: an up-to-date review. World J Gastroenterol. 2015;21(5):1394–1403. doi: 10.3748/wjg.v21.i5.1394
- Gecse KB, Bemelman W, Kamm MA, et al. A global consensus on the classification, diagnosis and multidisciplinary treatment of perianal fistulising Crohn’s disease. Gut. 2014;63(9):1381–1392. doi: 10.1136/gutjnl-2013-306709
- Schwartz DA, Loftus EV Jr., Tremaine WJ, et al. The natural history of fistulizing Crohn’s disease in Olmsted county, Minnesota. Gastroenterology. 2002;122(4):875–880. doi: 10.1053/gast.2002.32362
- Adler J, Dong S, Eder SJ, et al. Perianal Crohn disease in a large multicenter pediatric collaborative. J Pediatr Gastroenterol Nutr. 2017;64(5):e117–e124. doi: 10.1097/MPG.0000000000001447
- Spinelli A, Yanai H, Girardi P, et al. The impact of Crohn’s perianal fistula on quality of life: results of an international patient survey. Crohns Colitis. 2023;55(3):otad036. doi: 10.1093/crocol/otad036
- Adegbola SO, Dibley L, Sahnan K, et al. Burden of disease and adaptation to life in patients with Crohn’s perianal fistula: a qualitative exploration. Health Qual Life Outcomes. 2020;18(1):370. doi: 10.1186/s12955-020-01622-7
- Jiang J, Cazzetta SE, Athavale A, et al. Observational burden of illness study in patients with Crohn’s disease with and without perianal fistulas in the United States. Gastro Hep Advances. 2023;2(8):1066–1076. doi: 10.1016/j.gastha.2023.08.011
- Chen G, Pedarla V, Null KD, et al. Health care costs and resource utilization among patients with Crohn’s disease with and without perianal fistula. Inflamm Bowel Dis. 2022;28(6):870–877. doi: 10.1093/ibd/izab198
- Wetwittayakhlang P, Al Khoury A, Hahn GD, et al. The optimal management of fistulizing Crohn’s disease: evidence beyond randomized clinical trials. J Clin Med. 2022;11(11):3045. doi: 10.3390/jcm11113045
- Panes J, Reinisch W, Rupniewska E, et al. Burden and outcomes for complex perianal fistulas in Crohn’s disease: systematic review. World J Gastroenterol. 2018;24(42):4821–4834. doi: 10.3748/wjg.v24.i42.4821
- Lichtenstein GR, Loftus EV, Isaacs KL, et al. Acg clinical guideline: management of Crohn’s disease in adults. Am J Gastroenterol. 2018;113(4):481–517. doi: 10.1038/ajg.2018.27
- Prasad SS, Potter M, Keely S, et al. Roles of healthcare professionals in the management of chronic gastrointestinal diseases with a focus on primary care: a systematic review. JGH Open. 2020;4(2):221–229. doi: 10.1002/jgh3.12235
- de Zoeten EF, Pasternak BA, Mattei P, et al. Diagnosis and treatment of perianal Crohn disease: NASPGHAN clinical report and consensus statement. J Pediatr Gastroenterol Nutr. 2013;57(3):401–412. doi: 10.1097/MPG.0b013e3182a025ee
- Gaertner WB, Burgess PL, Davids JS, et al. The American Society of Colon and Rectal Surgeons clinical practice guidelines for the management of anorectal abscess, fistula-in-ano, and rectovaginal fistula. Dis Colon Rectum. 2022;65(8):964–985. doi: 10.1097/DCR.0000000000002473
- Feuerstein JD, Ho EY, Shmidt E, et al. AGA clinical practice guidelines on the medical management of moderate to severe luminal and perianal fistulizing Crohn’s disease. Gastroenterology. 2021;160(7):2496–2508. doi: 10.1053/j.gastro.2021.04.022
- Lamb CA, Kennedy NA, Raine T, et al. British Society of Gastroenterology consensus guidelines on the management of inflammatory bowel disease in adults. Gut. 2019;68(Suppl 3):s1–s106. doi: 10.1136/gutjnl-2019-318484
- Torres J, Bonovas S, Doherty G, et al. ECCO guidelines on therapeutics in Crohn’s disease: medical treatment. J Crohns Colitis. 2020;14(1):4–22. doi: 10.1093/ecco-jcc/jjz180
- Adamina M, Bonovas S, Raine T, et al. ECCO guidelines on therapeutics in crohn’s disease: surgical treatment. J Crohns Colitis. 2020;14(2):155–168. doi: 10.1093/ecco-jcc/jjz187
- Gionchetti P, Dignass A, Danese S, et al. 3rd European evidence-based consensus on the diagnosis and management of Crohn’s disease 2016: part 2: surgical management and special situations. J Crohns Colitis. 2017;11(2):135–149. doi: 10.1093/ecco-jcc/jjw169
- Gomollon F, Dignass A, Annese V, et al. 3rd European evidence-based consensus on the diagnosis and management of Crohn’s disease 2016: part 1: diagnosis and medical management. J Crohns Colitis. 2017;11(1):3–25. doi: 10.1093/ecco-jcc/jjw168
- Bennett AL, Munkholm P, Andrews JM. Tools for primary care management of inflammatory bowel disease: do they exist? World J Gastroenterol. 2015;21(15):4457–4465. doi: 10.3748/wjg.v21.i15.4457
- Tan M, Holloway RH, Lange K, et al. General practitioners’ knowledge of and attitudes to inflammatory bowel disease. Intern Med J. 2012;42(7):801–807. doi: 10.1111/j.1445-5994.2011.02586.x
- Rubin GP, Hungin AP, Kelly PJ, et al. Inflammatory bowel disease: epidemiology and management in an English general practice population. Aliment Pharmacol Ther. 2000;14(12):1553–1559. doi: 10.1046/j.1365-2036.2000.00886.x
- Lee DW, Koo JS, Choe JW, et al. Diagnostic delay in inflammatory bowel disease increases the risk of intestinal surgery. World J Gastroenterol. 2017;23(35):6474–6481. doi: 10.3748/wjg.v23.i35.6474
- Salinas G, Belcher E, Cazzetta S, et al. Surgical management of patients with complex perianal fistula: results of a US national case-based survey to determine future educational needs. J Am Coll Surg. 2021;233(5):e27. doi: 10.1016/j.jamcollsurg.2021.08.076
- Salinas GD, Belcher ED, Cazzetta SE, et al. S818 medical management of patients with complex perianal fistula: results of a US national case-based survey to determine future educational needs. Official J Am Coll Gastroenterol ACG. 2021;116(1):S379. doi: 10.14309/01.ajg.0000776804.12123.ab
- Lee MJ, Heywood N, Sagar PM, et al. Association of Coloproctology of Great Britain and Ireland consensus exercise on surgical management of fistulating perianal Crohn’s disease. Colorectal Dis. 2017;19(5):418–429. doi: 10.1111/codi.13672
- Adler J, Lin CC, Gadepalli SK, et al. Association between steroid-sparing therapy and the risk of perianal fistulizing complications among young patients with Crohn disease. JAMA Netw Open. 2020;3(6):e207378. doi: 10.1001/jamanetworkopen.2020.7378
- Irving PM, Gearry RB, Sparrow MP, et al. Review article: appropriate use of corticosteroids in Crohn’s disease. Aliment Pharmacol Ther. 2007;26(3):313–329. doi: 10.1111/j.1365-2036.2007.03379.x
- Gold SL, Cohen-Mekelburg S, Schneider Y, et al. Perianal fistulas in patients with Crohn’s disease, part 1: current medical management. Gastroenterol Hepatol (N Y). 2018;14(8):470–481.
- Peabody JW, Luck J, Glassman P, et al. Measuring the quality of physician practice by using clinical vignettes: a prospective validation study. Ann Intern Med. 2004;141(10):771–780. doi: 10.7326/0003-4819-141-10-200411160-00008