ABSTRACT
Objective
Despite the availability of a wide range of oral nutritional supplements (ONS) offerings, individuals with malnutrition are still struggling to meet their nutritional targets. A new concentrated and high-protein energy-dense ONS (≥2.1 kcal/mL;32 g protein/200 mL) with high-quality protein (60% whey protein) has emerged as a pivotal formula to reach the patient’s energy-protein requirements, enhance compliance, and maximize stimulation of muscle protein synthesis, key factors driving better nutritional, functional, and clinical outcomes. The purpose of this article is to provide our clinical experience using this new nutritionally concentrated ONS as a therapeutic strategy for patients with DRM.
Methods
Three clinical cases have been examined using new assessment procedures and a new form of nutritional therapy, and their impact on the nutritional and functional outcomes in patients with moderate-to-severe DRM.
Results
A tailored individualized nutritional interventions improved anthropometric, biochemical, and functional outcomes (Case 1,2, and 3) assessed using hand grip strength, bioimpedance and muscle ultrasound, and as well as good gastrointestinal tolerance (Case 1) and compliance to the ONS in patients with DRM (Case 1,2,3).
Conclusion
The use of this novel high-protein energy-dense formula with high-quality protein source (≥2.1 kcal/mL; 32 g protein/200 mL; 60% whey protein) overcome common practical challenges in the medical nutrition therapy of patients with DRM, either because these patients require a highly concentrated formulation to meet nutritional requirements due to loss of appetite, lack of interest in food, and high caloric-protein needs due to disease, and a large quantity and quality of protein to optimize muscle recovery due to sarcopenia, common in patients with moderate-severe malnutrition.
1. Introduction
Disease-related malnutrition (DRM) has been widely recognized as a prevalent and impactful condition across all health and social care settings [Citation1]. The condition is typically characterized by alterations in body composition, including muscle mass catabolism and loss of lean body mass, functional impairment, and adverse clinical outcomes in patients with chronic illness or acute disease episodes [Citation2–4]. The prevalence of DRM is alarmingly high in various clinical settings. Previous reports estimated that up to 34% of patients are malnourished or at risk of malnutrition upon hospital admission in Europe [Citation5–7], which increases to 39% in older adults [Citation8]. Meanwhile, DRM prevalence ranges from 21% to 69% in outpatient settings, depending on the underlying condition and assessment methods [Citation9–11].
DRM is associated with a myriad of detrimental health and economic outcomes. Patients with DRM typically present with impaired functional status, including reduced muscle strength, diminished physical performance, and substantial impairments in basic daily activities [Citation12–14]. DRM significantly increases the risk of morbidity in both inpatient and outpatient settings, including infection, pressure ulcers, impaired wound healing, bone disorders, frequent hospital admissions, and prolonged hospitalization [Citation4,Citation15,Citation16]. Furthermore, DRM may exacerbate the primary pathology, leading to unfavorable disease outcomes [Citation17–19]. Several reports demonstrated that hospitalized patients with DRM had 1.5–2 times mortality risk compared to well-nourished patients [Citation20,Citation21]. Patients with DRM usually exhibit impaired quality of life (QoL) and increased risk of depression and anxiety due to social isolation, impaired activities, and lack of independence [Citation13]. From an economic standpoint, the burden of DRM on healthcare systems is significant. Malnourished patients typically necessitate extended hospital stays, more frequent readmissions, and increased healthcare interventions, contributing to increased medical costs [Citation22,Citation23].
Therefore, scientific societies have widely recognized DRM as an important condition that needs prompt evaluation and management in hospital and outpatient settings. Given the impact of adequate protein intake on muscle synthesis and body mass [Citation24], the European Society for Clinical Nutrition and Metabolism (ESPEN) recommended an energy intake of 30 kcal/kg body weight (BW)/day and a higher protein intake in older adults and patients with DRM [Citation3,Citation25]. In these groups, a protein intake of at least 1.1 g/kg BW/day, covering high protein quality, is recommended to meet nutritional requirements and optimize muscle synthesis [Citation26,Citation27]. The current body of evidence demonstrates that the provision of ONS in patients with DRM significantly improved the nutritional status and clinical outcomes [Citation3]. Hence, nutritional guidelines have recommended oral nutritional supplements (ONS) with diet for patients with DRM [Citation3,Citation25]. Despite the well-established clinical and economic benefits of ONS [Citation28–30], many patients with DRM were found to be non-compliant with ONS, leading to insufficient energy and protein intake [Citation31]. Several ONS-related factors can contribute to the high noncompliance rate in DRM patients, including loss of appetite, large volume/portion, treatment complexity, non-tolerance of ONS, long duration of treatment and monotonous or unsatisfactory taste or texture [Citation32,Citation33].
High-protein energy-dense ONS has emerged as a pivotal formula to reach the patient’s energy-protein requirements and enhance compliance, key factors driving better nutritional, functional, and clinical outcomes. High protein-energy dense ONS (≥2 kcal/mL; >20% energy from protein) and low-volume significantly improved compliance of patients with malnutrition [Citation34] and improved palatability [Citation35]. Nonetheless, the current evidence still indicates suboptimal nutritional and functional gains in patients with DRM receiving ONS. Recent studies showed that only 4% of older hospitalized adults received the recommended daily protein intake [Citation36] and that nearly 83% of hospitalized patients at risk of malnutrition do not meet the recommended protein intake [Citation37]. Moreover, up to 32% of ONS are wasted due to noncompliance [Citation36]. These figures reflect a suboptimal utilization of energy-dense ONS with high protein content in clinical practice, which can negatively affect the nutritional and clinical outcomes of DRM.
The aims of this manuscript are to provide our clinical experience using a novel nutritionally high-concentrated ONS as a therapeutic strategy for patients with DRM due to several underlying causes and explore the clinical benefits regarding nutritional status, functional and clinical outcomes.
2. Case description
Written informed consent was obtained from all four participants for the publication of this manuscript. The study protocol was approved by the local ethics committee of East Health Area of Valladolid, Spain (Ref No. PI 22–783).
2.1. Case 1: managing malnutrition using a novel complete high-protein energy-dense ONS in a polymorbid patient
An 84-year-old female was referred to the Endocrinology and Nutrition Unit with a diagnosis of short bowel syndrome for nutritional consultation. Her medical history revealed a polymorbid condition encompassing hyperuricemia, polymyalgia rheumatica on corticosteroid therapy, osteopenia, primary hypothyroidism under replacement therapy, and mesenteric thrombosis.
Three years ago, she underwent surgery for mesenteric ischemia and subsequently re-operated due to suture dehiscence and received parenteral nutrition, which was transitioned to oral tolerance during her hospitalization. She received protein energy ONS (1.5kcal/mL;15 g of protein/serving of 200 mL), one serving daily, which she continued post-discharge and for three years until she arrived at our medical office.
Upon arrival at our unit, we carried out a nutritional assessment. The anthropometric evaluation yielded the following data: weight of 56 kg, resulting in a 16.42% decrease from her usual weight of 67 kg; body mass index (BMI) of 21.88 kg/m2.; calf circumference (CC) of 31 cm; hand grip dynamometry of 10 kg (EWGSOP2 cutoff point for low strength: <16 kg). Additional assessment with bioimpedance (BIA) (Akern EFG BIA 101 system, Italy) and muscle ultrasound (PIIXMED™ MSK iSarc, Dawako Medtech, S.L., Valencia, Spain) were assessed too (). Concerning her dietary intake, she consumed five meals daily, though her intake was only 50% of her usual diet due to diminished appetite. She reported no symptoms of nausea or vomiting, but she had several daily bowel movements (10/day). The biochemical analysis revealed a low serum albumin level of 3.40 g/dl (reference range: 3.97–4.94) and decreased total protein of 5.6 g/dl (reference range: 6.4–8.3).
Table 1. Muscle ultrasound findings of the anterior rectum before (v1) and after the nutritional intervention (v3). The figure shows the (a) longitudinal and (b) transversal basal section before nutritional intervention and after it (c and d, respectively).
Therefore, a diagnosis of severe protein-calorie malnutrition and sarcopenia was established, and a comprehensive nutritional intervention was started with specific recommendations for short bowel syndrome and physical activity. We maintained the protein-energy ONS and improved dietary advice. A subsequent visit at one month indicated the patient´s weight was 47.3 kg and the BMI 18.48 kg/m2. The patients continued to show frequent bowel movements (five/day). Thus, loperamide was prescribed three times a day. To address severe protein and calorie malnutrition and maximize muscle protein synthesis, the ONS regimen was changed to two servings daily (2 servings of 200 mL) with a new novel formula, a complete high-protein energy-dense ONS (≥2.1 kcal/mL;32 g protein/200 mL) with high-quality protein (60% whey protein) (Nestlé Health Science, Switzerland), for up to three months.
At three months, during outpatient follow-up, improvements in nutritional, functional and clinical outcomes were observed. The patient showed weight gain, reaching 49.70 kg (5.1% body weight increase), with a BMI of 19.41 kg/m2, AC of 27, CC of 32 cm, and a right dynamometry of 12 kg, and improved her functional status as demonstrated in the morphofunctional outcomes (; ). Additionally, she experienced an improvement in the number of bowel movements (two/day).
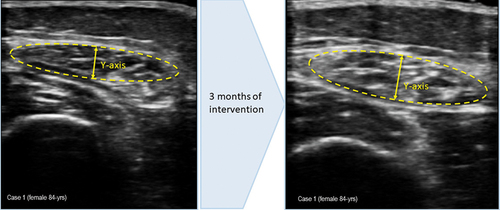
Figure 1. (a) measurement of muscle ultrasound images upon arrival at the endocrinology and nutrition unit. Muscle thickness (Y-axis): 0.73 cm; muscle is in cm2: 2.11 cm2, (b) measurement of muscle ultrasound images at 3 months of intervention, during outpatient follow-up. Muscle thickness (Y-axis): 0.74 cm; muscle is in cm2: 2.11 cm2.
In the last follow-up (four months after the start of the intervention), the patient continued to gain weight. Her appetite improved, and her daily bowel movements decreased to one daily. Due to the effectiveness of the intervention, the patient continued consuming the ONS until reaching the nutritional targets, which were achieved after seven months of the nutritional intervention.
2.2. Case 2: managing malnutrition using a novel complete high-protein energy-dense ONS in a patient with respiratory disease
A 61-year-old female, ex-smoker (30 cigarettes per day), with a previously resolved tonsil cancer treated by chemoradiotherapy and subclinical hypothyroidism, was admitted to the hospital due to community-acquired bilateral pneumonia, which was subsequently complicated by the secondary inappropriate secretion of antidiuretic hormone (SIADH). This episode led to the diagnosis of chronic obstructive pulmonary disease (COPD). Because she lost weight during her hospitalization stay, she was referred to the Endocrinology and Nutrition Unit for a nutrition consultation.
Upon initial assessment in our unit, the anthropometric evaluation yielded the following data: weight of 35.6 kg, resulting in a 28.8% decrease from her usual weight of 50 kg; BMI of 15.40 kg/m2; mid-upper AC of 20 cm; CC of 31.5 cm; hand grip dynamometry of 20 kg (EWGSOP2 cutoff point for low strength: <16 kg). The biochemical analysis reveals a normal serum albumin level of 4.70 g/dl (reference range: 3.97–4.94) and a normal total protein level of 7 g/dl (reference range: 6.4–8.3). Concerning her dietary intake and physical activity habits, the patient consumed small quantities in five meals daily with an inadequate caloric and protein intake, maintained her low appetite, and performed minimal physical activity.
Therefore, the patient was diagnosed with severe calorie malnutrition and a high risk of protein malnutrition. A tailored nutritional intervention was prescribed to improve body weight, avoid muscle atrophy, maintain lung strength, and correct protein deficiency. Due to the difficulty of achieving the energy and protein requirements through the diet, a high-protein energy-dense oral nutritional supplement (≥2.1 kcal/ml; 32 g/bottle of 200 mL; Nestlé Health Science, Switzerland) with a high amount of whey protein (60%) was prescribed, twice a day.
During a 3-month nutritional intervention, the patient reported significant improvements in their anthropometric measurements, including favorable gastrointestinal tolerance and adherence to the ONS. She gained 5.2 kg (40.8 kg weight; 14.6% body weight increase; BMI: 17.64 kg/m2), increased her CC to 32.5 cm (3.2% CC increase) and an improvement in hand grip strength to 22 kg (10% strength increase). The progression of parameters from the first visit assessed via bioelectrical impedance analysis (BIA; Akern EFG BIA 101 system, Italy) and muscle ultrasound (PIIXMED™ MSK iSarc, Dawako Medtech, S.L., Spain) revealed an improvement as well (; ) due to the effectiveness of the treatment we recommended to continue with the ONS therapy to reach the nutrition targets.
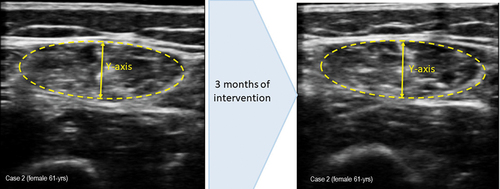
Figure 2. (a) measurement of muscle ultrasound images before intervention. Muscle thickness (Y-axis): 1.01 cm; muscle is in cm2: 2.72. (b) measurement of muscle ultrasound images at 3 month of intervention. Muscle thickness (Y-axis): 0.94 cm; muscle is in cm2: 2.78.
After some weeks, following the nutritional intervention, the patient presented exertional dyspnea, and inhaled corticosteroids were prescribed to her. The checkup revealed a slight decline in her anthropometric parameters (weight from 40.8 to 39.5 kg and CC from 32.5 to 31 cm) but with a stable hand grip strength (22 kg). To enhance her nutritional status and prevent any potential decline, the ONS regimen was increased to 3 bottles per day.
During her subsequent nutrition consultation, a 6-month review from the baseline intervention, the patient increased 12% of her weight and consequent BMI (from 15.40 kg/m2 to 17.31 kg/m2) and her grip strength (10%). As a result of these findings, the number of ONS servings was decreased to two servings daily, with instructions to escalate to three servings on days of diminished intake. The patient continued to exhibit stable nutritional and clinical status on the twice-daily ONS regimen.
2.3. Case 3: managing malnutrition using a novel complete high-protein energy-dense ONS in a patient with colon adenocarcinoma with pulmonary metastases
A 78-year-old man diagnosed two years ago with stage IV colon adenocarcinoma with pulmonary metastases, supra and infra diaphragmatic adenopathies, and peritoneal implants was referred to the Endocrinology and Nutrition Unit due to a significant decline in his nutritional status and hyporexia with a loss of 20 kg in less than a year.
When he arrived at our unit for nutritional consultation, the patient was receiving palliative chemotherapy on an outpatient basis with good tolerance.
On his initial nutritional assessment in our unit, the anthropometric evaluation yielded the following data: significant weight loss (23.6%) over the last three months, going from his usual 90 kg to 68.7 kg; BMI of 24.6 kg/m2; mid-upper AC of 24 cm; CC of 32 cm; and hand grip dynamometry of 17 kg (EWGSOP2 cutoff point for low strength: <16 kg). The biochemical analysis revealed low albumin level of 2.9 g/dl (reference range: 3.97–4.94), low total protein at 5.3 g/dl (reference range: 6.4–8.3), low iron levels at 47 µg/dl (reference range: 59–158), leukopenia with a count of 2180 µl (reference range: 4000–10000), neutropenia with 770 µl (reference range: 1800–8000), and anemia with hemoglobin at 10.5 g/dl (reference range: 12–18). Due to his hyporexia, he drastically reduced his food intake. The dietary assessment revealed a 25% reduction in food intake compared to his usual intake. As for its functional capacity, the patient reported very little exercise and the need to use a walking stick due to profound/deep fatigue and significant loss of muscle strength.
Thus, a diagnosis of severe protein-calorie malnutrition with sarcopenia was established. An early nutritional plan with two bottles a day (400 mL) of a high-protein energy-dense oral nutritional supplement with a high amount of whey protein (≥2.1 kcal/ml; 32 g of protein/bottle of 200 mL; 60% Whey protein, Nestlé Health Science, Switzerland), combined with a physical exercise were prescribed. The aim of prescribing this highly concentrated ONS was to improve the patient’s nutritional intake and weight, stimulate muscle protein synthesis, and enhance muscle strength.
The three-month follow-up review revealed an effective intervention with good tolerance to nutritional supplements. The patient weighed 69.8 kg (1.6% body weight increase), improved his BMI (24.99 kg/m2), increased his AC of 25 cm (4.2% increase), CC of 33 cm (3.1% increase). From the first visit, he displayed an improvement in his function and muscle mass measured by hand grip dynamometry (20 kg; 17.6% strength increase), BIA (Akern EFG BIA 101 system, Italy) and muscle ultrasound (PIIXMED™ MSK iSarc, Dawako Medtech, S.L., Valencia, Spain; ; ). He continued to receive ONS two servings daily, alongside his oral chemotherapy and recommended physical activity.
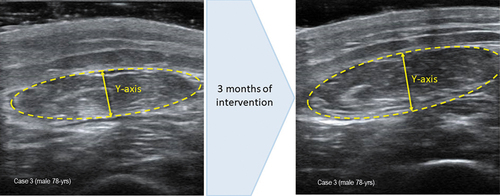
Figure 3. (a) measurement of muscle ultrasound images before intervention. Muscle thickness (Y-axis): 0.81 cm; muscle is in cm2: 2.47. (b) measurement of muscle ultrasound images at 3 month of intervention. Muscle thickness (Y-axis): 0.89 cm; muscle is in cm2: 2.85.
In the following outpatient checkups, the patient maintained an adequate intake and good adherence to the prescribed dose of two bottles of ONS per day.
3. Discussion
DRM is a multifaceted condition arising from several etiological factors [Citation11,Citation38]. This article focused on the utilization of a novel super high-protein energy-dense oral nutritional supplement with a high amount of whey protein in three cases with moderate-to-severe DRM: a polymorbid older adult, a patient with COPD and a patient with cancer.
Polymorbid older adults and patients with catabolic inflammatory responses, such as malignancy, COPD, and heart failure (HF), are at increased risk of DRM [Citation39,Citation40]. If not properly managed, DRM can adversely affect the outcomes of underlying pathologies and lead to declined functional status, increased morbidity and mortality, and impaired QoL [Citation16–18,Citation41]. The optimal management of DRM typically relies on personalized dietary plans tailored to individual energy, protein, and micronutrient needs while accounting for underlying disease conditions and patient preferences [Citation3].
Patients with COPD are at risk of malnutrition due to several factors, including increased energy expenditure and systemic inflammation [Citation42]. Malnourished patients with COPD usually exhibit a loss of appetite or an early feeling of satiety, requiring small energy-dense nutritional servings to improve tolerance [Citation43]. Hence, energy-dense ONS (>2 kcal/mL) with low-volume formats and improved palatability is recommended for malnourished patients with COPD to improve their compliance and nutritional outcomes [Citation44]. Recent trials showed that high-protein energy-dense ONS led to satisfactory tolerance, high acceptability, and improved exercise capacity in malnourished patients with COPD [Citation45,Citation46]. Our clinical experience demonstrated that a novel, highly concentrated formula with high-protein energy-dense and whey protein is associated with good tolerance and better nutritional outcomes in malnourished patients with COPD (Case 2). As previously mentioned, high-protein formulas are recommended for patients with DRM to compensate for high catabolic status and optimize skeletal muscle recovery [Citation44,Citation47]. However, the quality of the protein source is equally vital to meet nutritional demands, particularly in the DRM population. Protein quality is determined by the amino acid profile and the digestibility of the protein, both of which significantly influence the protein requirements [Citation26]. Previous reports showed that blends of high-quality proteins, such as whey or casein, provide a high content of essential (EAAs) and branched-chain amino acids (BCAAs) [Citation48,Citation49], which are vital for protein synthesis [Citation50]. In particular, whey protein, which is rich in branched amino acids such as leucine, has been recognized as the most pivotal BCAA in stimulating muscle protein synthesis. Not only does leucine serve as a building block for protein synthesis, but it also acts as a key signaling molecule, triggering the activation of the mammalian target of rapamycin (mTOR) pathway, a crucial regulator of muscle protein synthesis [Citation51]. In return, scientific societies recommend high quantities of leucine (2.5–2.8 g per meal) for the aging population and patients at risk of malnutrition [Citation26]. In this clinical experience, high-protein ONS, with high whey protein (60%) and leucine quantities, improved muscle recovery and skeletal muscle strength in DRM patients with sarcopenia. Interestingly, Case 1 had comprised the intestine due to severe mesenteric ischemia, yet the patient showed good tolerance to the ONS, which can be attributed to the high whey content; whey protein is a fast-acting protein that has previously shown high digestibility and rapid bioaccessibility and intestinal absorption [Citation52]. It is worth noting that the patient received an anti-diarrheal medication, which might have contributed to the good tolerance to ONS.
Severe skeletal muscle wasting is prevalent among cancer patients and a leading cause of cancer-related morbidity, mortality, and low adherence to chemotherapeutic regimens [Citation53]. Previous reports showed that cancer patients usually exhibit severe impairment in the skeletal muscle energy metabolism in the form of cyclic anabolic stimulation and suppressed basal protein synthesis [Citation54,Citation55]. However, several studies indicated that myofibrillar protein synthesis is normal in patients with upper gastrointestinal cancer-related cachexia and remains responsive to the dietary supply of amino acids [Citation56]. Therefore, international guidelines emphasize the need for protein intake in cancer patients, which should be above 1 g/kg/day and, if possible, up to 1.5 g/kg/day [Citation57]. In our experience, high-protein energy-dense ONS was associated with good tolerance and better nutritional outcomes in a case with advanced colon cancer (Case 3).
Despite the evident benefits of this novel ONS in patients with DRM, significant gaps in knowledge and understanding persist in the literature. Firstly, the long-term effects of such supplementation and adherence need to be studied, as studies addressing adherence are scarce. Another area that warrants further investigation is the formulation of ONS, specifically, the ideal composition and ratio of various micronutrients that would be most beneficial for different subsets of DRM patients and its effect in correcting micronutrient malnutrition. A recent publication that studied the four-week administration of a high-protein energy-dense ONS demonstrated to improve micronutrient concentrations but did not completely correct deficiencies [Citation58]. Our research focused on a small, specific sample of three DRM patients to provide an in-depth analysis of their responses to the nutritional intervention. Therefore, this lack of knowledge encourages us to continue researching the long-term use of ONS and the sustainability in DRM populations. Future research with a larger sample size is needed to validate and extend our findings.
4. Conclusion
The implementation of high-protein energy-dense ONS (≥2.1 kcal/mL;32 g protein/200 mL) with high-quality protein (60% whey protein) during tailored individualized nutritional interventions resulted in adequate nutritional and functional outcomes, as well as good gastrointestinal tolerance and compliance, in patients with moderate-severe disease-related malnutrition.
Our clinical experience demonstrates that the use of this novel high-protein and high energy-dense ONS overcomes common practical challenges in the management of patients with DRM, either because: 1) these patients require a highly concentrated formulation to meet nutritional requirements due to loss of appetite, lack of interest in food, and high caloric-protein needs due to disease, and 2) a large quantity and quality of protein to compensate for protein breakdown and optimize muscle recovery due to sarcopenia, common in patients with moderate-severe malnutrition. Further research with a larger sample size is needed to validate and extend our findings.
Declaration of financial/other relationships
JJ López-Gómez has received payment or honoraria for lectures, presentations, participation in speakers’ bureaus, manuscript writing, or educational events from Nestlé Health Science, Vegenat Healthcare, Abbott Nutrition, Persan Pharma, Adventia Pharma, and Nutricia. Additionally, JJ López-Gómez has been supported for attending meetings and/or travel by Adventia Pharma, Nutricia, and Persan Pharma. Furthermore, JJ López-Gómez has participated in Data Safety Monitoring Boards or Advisory Boards for Abbott Nutrition, Nutricia, Persan Pharma, Vegenat Healthcare, and Nestlé Health Science. BR Bachiller and D de Luis Roman received honoraria from Nestlé Health Science for the development of the present manuscript. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed. Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author contributions
All authors were involved in the writing of clinical cases, discussion, and conclusion. They also contributed to patient care. All the authors reviewed and approved the final article.
Acknowledgments
The authors acknowledge the patients and their families and the healthcare professionals at the affiliation center. Medical writing and editorial support in preparing this paper were provided by Content Ed Net Switzerland.
Data availability statement
All relevant data are included in the article. Further inquiries can be directed to the corresponding authors.
Additional information
Funding
References
- Burgos R, Joaquín C, Blay C, et al. Disease-related malnutrition in hospitalized chronic patients with complex needs. Clin Nutr. 2020;39(5):1447–1453. doi: 10.1016/j.clnu.2019.06.006
- Cederholm T, Barazzoni R, Austin P, et al. ESPEN guidelines on definitions and terminology of clinical nutrition. Clin Nutr. 2017;36(1):49–64. doi: 10.1016/J.CLNU.2016.09.004
- Gomes F, Schuetz P, Bounoure L, et al. ESPEN guidelines on nutritional support for polymorbid internal medicine patients. Clin Nutr. 2018;37(1):336–353. doi: 10.1016/j.clnu.2017.06.025
- Meyer F, Valentini L. Disease-related malnutrition and sarcopenia as determinants of clinical outcome. Visc Med. 2019;35(5):282–291. doi: 10.1159/000502867
- Russell CA, Elia M. Nutrition screening survey in the UK and Republic of Ireland in 2010. Br Assoc Parenter Enter Nutr. 2012;76. https://www.bapen.org.uk/pdfs/nsw/nsw-2011-report.pdf
- Schindler K, Pernicka E, Laviano A, et al. How nutritional risk is assessed and managed in European hospitals: a survey of 21,007 patients findings from the 2007–2008 cross-sectional nutritionDay survey. Clin Nutr. 2010;29(5):552–559. doi: 10.1016/j.clnu.2010.04.001
- de Luis D, Lopez Guzman A. Nutritional status of adult patients admitted to internal medicine departments in public hospitals in Castilla y leon, Spain — a multi-center study. Eur J Intern Med. 2006;17(8):556–560. doi: 10.1016/j.ejim.2006.02.030
- Kaiser MJ, Bauer JM, Rämsch C, et al. Frequency of malnutrition in older adults: a multinational perspective using the mini nutritional assessment. J Am Geriatr Soc. 2010;58(9):1734–1738. doi: 10.1111/j.1532-5415.2010.03016.x
- Collins PF, Stratton RJ, Kurukulaaratchy R, et al. Prevalence of malnutrition in outpatients with chronic obstructive pulmonary disease. Proc Nutr Soc. 2010;69(OCE2). doi: 10.1017/s0029665109993430
- Vermeeren MAP, Creutzberg EC, Schols AMWJ, et al. Prevalence of nutritional depletion in a large outpatient population of patients with COPD. Respir Med. 2006;100(8):1349–1355. doi: 10.1016/j.rmed.2005.11.023
- Sze S, Pellicori P, Kazmi S, et al. Prevalence and prognostic significance of malnutrition using 3 scoring systems among outpatients with heart failure: a comparison with body mass index. JACC Hear Fail. 2018;6(6):476–486. doi: 10.1016/j.jchf.2018.02.018
- Felder S, Lechtenboehmer C, Bally M, et al. Association of nutritional risk and adverse medical outcomes across different medical inpatient populations. Nutrition. 2015;31(11–12):1385–1393. doi: 10.1016/j.nut.2015.06.007
- D’Amelio P, Rosso B, Fornelli G, et al. Malnutrition reduces quality of life and performance in hospitalized elderly. Open J Endocr Metab Dis. 2014;4(6):147–157. doi: 10.4236/ojemd.2014.46015
- Holst M, Beck AM, Rasmussen HH, et al. Insufficient intake of energy and protein is related to physical functional capacity among COPD patients referred to municipality based pulmonary rehabilitation. Clin Nutr ESPEN. 2019;30:35–41. doi: 10.1016/j.clnesp.2019.02.009
- Ishida Y, Maeda K, Nonogaki T, et al. Malnutrition at admission predicts in-hospital falls in hospitalized older adults. Nutrients. 2020;12(2):541. doi: 10.3390/nu12020541
- Agarwal E, Ferguson M, Banks M, et al. Malnutrition and poor food intake are associated with prolonged hospital stay, frequent readmissions, and greater in-hospital mortality: results from the nutrition care day survey 2010. Clin Nutr. 2013;32(5):737–745. doi: 10.1016/j.clnu.2012.11.021
- Shepherd AB, Bowell K. ‘Mind the gap’: the importance of managing malnutrition in chronic obstructive pulmonary disease. Br J Nurs. 2019;28(22):1442–1449. doi: 10.12968/bjon.2019.28.22.1442
- Sze S, Pellicori P, Zhang J, et al. The impact of malnutrition on short-term morbidity and mortality in ambulatory patients with heart failure. Am J Clin Nutr. 2021;113(3):695–705. doi: 10.1093/ajcn/nqaa311
- Riesgo H, Castro A, Del Amo S, et al. Prevalence of risk of malnutrition and risk of Sarcopenia in a reference hospital for COVID-19: relationship with mortality. Ann Nutr Metab. 2021;77(6):324–329. doi: 10.1159/000519485
- De Luis DA, Terroba MC, Cuellar L, et al. Association of anthropometric and biochemical markers with length of stay and mortality in the hospital. Eur Rev Med Pharmacol Sci. 2013;17:1321–1325.
- López-Gómez JJ, Cerezo-Martín JM, Gómez-Hoyos E, et al. Diagnóstico de desnutrición y su relación con el pronóstico en el paciente hospitalizado con enfermedad oncológica. Endocrinol Diabetes Nutr. 2023;70(5):304–312. doi: 10.1016/j.endinu.2023.02.010
- Ljungqvist O, De Man F. Under nutrition: a major health problem in Europe. Nutr Hosp. 2009;24(3):369–370.
- Morán López JM, Enciso Izquierdo FJ, Luengo Pérez LM, et al. Impacto económico de la desnutrición relacionada con la enfermedad en el hospital San Pedro de Alcántara. Estimación del ahorro asociado a una atención nutricional especializada de calidad. Endocrinol Diabetes Nutr. 2017;64(8):446–450. doi: 10.1016/j.endinu.2017.05.004
- Deutz NEP, Ashurst I, Ballesteros MD, et al. The underappreciated role of low muscle mass in the management of malnutrition. J Am Med Dir Assoc. 2019;20(1):22–27. doi: 10.1016/j.jamda.2018.11.021
- Volkert D, Beck AM, Cederholm T, et al. ESPEN practical guideline: clinical nutrition and hydration in geriatrics. Clin Nutr. 2022;41(4):958–989. doi: 10.1016/j.clnu.2022.01.024
- Bauer J, Biolo G, Cederholm T, et al. Evidence-based recommendations for optimal dietary protein intake in older people: a position paper from the prot-age study group. J Am Med Dir Assoc. 2013;14(8):542–559. doi: 10.1016/j.jamda.2013.05.021
- Kuehneman T, Gregory M, de Waal D, et al. Academy of nutrition and dietetics evidence-based practice guideline for the management of heart failure in adults. J Acad Nutr Diet. 2018;118(12):2331–2345. doi: 10.1016/j.jand.2018.03.004
- Gressies C, Kaegi-Braun N, Gomes F, et al. Letter to the editor: is nutritional support effective in malnourished polymorbid medical inpatients? Clin Nutr. 2023;42(1):45–52. doi: 10.1016/j.clnu.2022.11.007
- Philipson TJ, Snider JT, Lakdawalla DN, et al. Impact of oral nutritional supplementation on hospital outcomes. Am J Manag Care. 2013;19(2):121–128. doi: 10.1016/s0261-5614(13)60017-5
- Snider JT, Jena AB, Linthicum MT, et al. Effect of hospital use of oral nutritional supplementation on length of stay, hospital cost, and 30-day readmissions among medicare patients with COPD. Chest. 2015;147(6):1477–1484. doi: 10.1378/chest.14-1368
- Hubbard GP, Elia M, Holdoway A, et al. A systematic review of compliance to oral nutritional supplements. Clin Nutr. 2012;31(3):293–312. doi: 10.1016/j.clnu.2011.11.020
- Liljeberg E, Andersson A, Blom Malmberg K, et al. High adherence to oral nutrition supplements prescribed by dietitians: a cross-sectional study on hospital outpatients. Nutr Clin Pract. 2019;34(6):887–898. doi: 10.1002/ncp.10243
- De Luis DA, Izaola O, Lopez JJ, et al. Oral nutritional supplements and taste adherence in malnourished adults inpatients, effect on adhesion during hospital stance. Ann Nutr Metab. 2015;67(4):205–209. doi: 10.1159/000440684
- Parsons EL, Stratton RJ, Cawood AL, et al. Oral nutritional supplements in a randomized trial are more effective than dietary advice at improving quality of life in malnourished care home residents. Clin Nutr. 2017;36(1):134–142. doi: 10.1016/j.clnu.2016.01.002
- Hubbard GP, Fry C, Sorensen K, et al. Energy-dense, low-volume paediatric oral nutritional supplements improve total nutrient intake and increase growth in paediatric patients requiring nutritional support: results of a randomized controlled pilot trial. Eur J Pediatr. 2020;179(9):1421–1430. doi: 10.1007/s00431-020-03620-9
- Weijzen MEG, Kouw IWK, Geerlings P, et al. During hospitalization, older patients at risk for malnutrition consume <0.65 grams of protein per kilogram body weight per day. Nutr Clin Pract. 2020;35(4):655–663. doi: 10.1002/ncp.10542
- Pullen K, Collins R, Stone T, et al. Are energy and protein requirements met in hospital? J Hum Nutr Diet. 2018;31(2):178–187. doi: 10.1111/jhn.12485
- Yazdanpanah L, Shidfar F, Moosavi AJ, et al. Energy and protein intake and its relationship with pulmonary function in chronic obstructive pulmonary disease (COPD) patients. Acta Med Iran. 2010;48:374–379.
- Koroušić Seljak B, Mlakar Mastnak D, Mrevlje Ž, et al. A multi-center survey on hospital malnutrition and cachexia in Slovenia. Eur J Clin Nutr. 2020;74:419–426. doi: 10.1038/s41430-019-0485-y
- Itoh M, Tsuji T, Nemoto K, et al. Undernutrition in patients with COPD and its treatment. Nutrients. 2013;5(4):1316–1335. doi: 10.3390/nu5041316
- Söderström L, Rosenblad A, Adolfsson ET, et al. Nutritional status predicts preterm death in older people: a prospective cohort study. Clin Nutr. 2014;33(2):354–359. doi: 10.1016/j.clnu.2013.06.004
- Nordén J, Grönberg AM, Bosaeus I, et al. Nutrition impact symptoms and body composition in patients with COPD. Eur J Clin Nutr. 2015;69(2):256–261. doi: 10.1038/ejcn.2014.76
- Hsieh MJ, Yang TM, Tsai YH. Nutritional supplementation in patients with chronic obstructive pulmonary disease. J Formos Med Assoc. 2016;115(8):595–601. doi: 10.1016/j.jfma.2015.10.008
- Banner J. Managing malnutrition in COPD. Including a pathway for the appropriate use of ONS to support community healthcare professionals. Br Assoc Parenter Enter Nutr (Bapen); British Diet Assoc Lung Found (Blf);Royal Coll Gen Pract 2020; 2016. Available form: www.malnutritionpathway.co.uk/copd/%0Ahttps://www.malnutritionpathway.co.uk/library/mm_copd.pdf
- Aldhahir AM, Aldabayan YS, Alqahtani JS, et al. A double-blind randomized controlled trial of protein supplementation to enhance exercise capacity in COPD during pulmonary rehabilitation: a pilot study. ERJ Open Res. 2021;7(1):00077–2021. doi: 10.1183/23120541.00077-2021
- Huang WJ, Fan XX, Yang YH, et al. A review on the role of oral nutritional supplements in chronic obstructive pulmonary disease. J Nutr Heal Aging. 2022;26(7):723–731. doi: 10.1007/s12603-022-1822-8
- Vest AR, Chan M, Deswal A, et al. Nutrition, obesity, and cachexia in patients with heart failure: a consensus statement from the heart failure Society of America Scientific Statements Committee. J Card Fail. 2019;25(5):380–400. doi: 10.1016/j.cardfail.2019.03.007
- Burd NA, Beals JW, Martinez IG, et al. Food-first approach to enhance the regulation of post-exercise skeletal muscle protein synthesis and remodeling. Sport Med. 2019;49(S1):59–68. doi: 10.1007/s40279-018-1009-y
- Reidy PT, Walker DK, Dickinson JM, et al. Protein blend ingestion following resistance exercise promotes human muscle protein synthesis. J Nutr. 2013;143(4):410–416. doi: 10.3945/jn.112.168021
- Church DD, Hirsch KR, Park S, et al. Essential amino acids and protein synthesis: insights into maximizing the muscle and whole-body response to feeding. Nutrients. 2020;12(12):1–14. doi: 10.3390/nu12123717
- Zhang S, Zeng X, Ren M, et al. Novel metabolic and physiological functions of branched chain amino acids: a review. J Anim Sci Biotechnol. 2017;8(1). doi: 10.1186/s40104-016-0139-z
- Mulet-Cabero AI, Torcello-Gómez A, Saha S, et al. Impact of caseins and whey proteins ratio and lipid content on in vitro digestion and ex vivo absorption. Food Chem. 2020;319:126514. doi: 10.1016/j.foodchem.2020.126514
- Hardee JP, Montalvo RN, Carson JA. Linking cancer cachexia-induced anabolic resistance to skeletal muscle oxidative metabolism. Oxid Med Cell Longev. 2017;2017:1–14. doi: 10.1155/2017/8018197
- Vanderveen BN, Fix DK, Carson JA. Disrupted skeletal muscle mitochondrial dynamics, mitophagy, and biogenesis during cancer cachexia: a role for inflammation. Oxid Med Cell Longev. 2017;2017:1–13. doi: 10.1155/2017/3292087
- Dworzak F, Ferrari P, Gavazzi C, et al. Effects of cachexia due to cancer on whole body and skeletal muscle protein turnover. Cancer. 1998;82:42–48. doi: 10.1002/(SICI)1097-0142(19980101)82:1<42:AID-CNCR5>3.0.CO;2-M
- MacDonald AJ, Johns N, Stephens N, et al. Habitual myofibrillar protein synthesis is normal in patients with upper GI cancer cachexia. Clin Cancer Res. 2015;21(7):1734–1740. doi: 10.1158/1078-0432.CCR-14-2004
- Muscaritoli M, Arends J, Bachmann P, et al. ESPEN practical guideline: Clinical Nutrition in cancer. Clin Nutr. 2021;40(5):2898–2913. doi: 10.1016/j.clnu.2021.02.005
- Sanchez M, Courtois-Amiot P, Capdepon A, et al. Four-week administration of an energy and protein dense oral nutritional supplement improves micronutrient concentrations but does not completely correct deficiencies in institutionalized malnourished older adults. Front Nutr. 2023;10. doi: 10.3389/fnut.2023.1249936
