ABSTRACT
Background
The prevalence of metabolic dysfunction-associated steatotic liver disease (MASLD) is increasing worldwide. Primary care providers play a critical role in the screening, diagnosis, and management of MASLD and/or metabolic dysfunction-associated steatohepatitis (MASH), though they can face challenges in this setting, particularly where healthcare resources are limited and barriers to care exist. To address these challenges, several guidelines have been developed to provide evidence-based recommendations for the clinical assessment and management of patients with MASLD/MASH.
Aims
To provide a unified, simple-to-understand, practical guide for MASLD screening, diagnosis, and management based on current guideline recommendations, for use by primary care providers in daily practice.
Methods
Evidence-based recommendations from several international guidelines were summarized, focusing on the similarities and differences between them.
Results
Recommendations are broadly aligned across the guidelines, but several key differences are evident. Practical guidance is provided on screening, identifying target populations for risk stratification, initial evaluation of individuals with suspected MASLD, surveillance, risk stratification and referral, as well as approaches to the management of MASLD and associated comorbidities, with specific considerations for the primary care setting.
Conclusions
Primary care providers are ideally placed to identify at-risk individuals, implement evidence-based interventions to prevent the development of fibrosis and cirrhosis, and effectively manage comorbidities. Equipping primary care providers with the necessary knowledge and tools to effectively manage MASLD/MASH may help to improve patient outcomes and reduce the burden of liver disease.
1. Introduction
Metabolic dysfunction-associated steatotic liver disease (MASLD) is an important driver of chronic liver disease worldwide [Citation1], and is associated with a considerable healthcare and socioeconomic burden and reduced health-related quality of life [Citation2,Citation3]. MASLD is defined by the presence of hepatic steatosis (i.e. accumulation of fat in the liver), identified by imaging or biopsy in the presence of ≥1 metabolic risk factor () and the absence of moderate or excessive alcohol consumption and other causes of hepatic steatosis [Citation4]. A new category, metabolic dysfunction and alcohol associated steatotic liver disease (MetALD), has been defined for patients with MASLD who consume alcohol in quantities greater than 140–350 g/week (females) and 210–420 g/week (males) [Citation4].
![Figure 1. Progression of MASLD to MASH and other liver-related outcomes [Citation4,Citation7].](/cms/asset/a67297a7-04af-4c81-abbc-ef6db3716608/ipgm_a_2325332_f0001_oc.jpg)
MASLD represents a spectrum of disease that ranges from isolated steatosis to the more severe metabolic dysfunction-associated steatohepatitis (MASH) [Citation4,Citation5]. MASH is characterized by the presence of active hepatocyte injury (ballooning, i.e. hepatocyte swelling and rounding) and lobular and portal inflammation (i.e. infiltration of inflammatory cells in the lobules or portal tracts of the liver) in addition to steatosis, and is associated with an increased risk of progression to adverse liver-related outcomes and mortality [Citation4–7] (; ). In the United States (US), liver disease secondary to MASH has become the most common indication for liver transplantation among women and the second most common among men [Citation9].
Worldwide, the burden of MASLD/MASH is growing, along with associated metabolic diseases such as obesity [Citation10,Citation11]. Between 2016 and 2019, an estimated 38.2% of the global population had MASLD, while the global prevalence of MASH was estimated at 5.3% [Citation10]. In the US, the prevalence of MASH was found to be much higher (14%) among middle-aged adults [Citation12]. MASLD is considered the hepatic manifestation of metabolic syndrome and is associated with metabolic and cardiovascular conditions, including obesity, insulin resistance, type 2 diabetes (T2D), and atherogenic dyslipidemia [Citation4,Citation5,Citation13]. Accordingly, the estimated global prevalence of MASLD and MASH, respectively, is considerably higher among individuals with overweight (70.0% and 33.5%) or obesity (75.3% and 33.7%) versus people with a body mass index (BMI) within the normal range [Citation10,Citation11]. Although MASLD is most commonly present alongside obesity and/or T2D, an estimated 7%–20% of affected individuals have a BMI <25 kg/m2 [Citation14]. Other key risk factors for MASLD include age, male sex, and Hispanic ethnicity [Citation5,Citation15–17]. In addition to demographic and metabolic risk factors, underlying genetic factors have been identified that confer susceptibility to MASLD/MASH and/or affect risk of poor outcomes, including variants of the genes encoding patatin-like phospholipase domain-containing protein 3 (PNPLA3), and transmembrane 6 superfamily member 2 (TM6SF2) [Citation18–20]. However, variants of 17B-hydroxysteroid dehydrogenase 13 (HSD17B13) have been shown to have a protective effect against the most severe outcomes in MASLD/MASH [Citation21].
Early diagnosis and staging of MASLD (and appropriate action thereafter) help to minimize the risk of progression to MASH and its associated serious long-term outcomes. However, the condition is often asymptomatic, or presents with nonspecific symptoms. Consequently, the disease may go unrecognized or undiagnosed until it has progressed to more advanced stages [Citation5,Citation22–24]. Indeed, a US population-based study reported that <5% of adults with MASLD were aware of their disease [Citation24], and most individuals with MASH and clinically significant fibrosis (stages F2–F4) remained undiagnosed in primary care and endocrinology clinics [Citation5].
More than 95% of individuals at high risk of having or developing MASH are treated in primary care [Citation25]. Therefore, primary care providers are in an ideal position to make timely diagnoses, identify individuals at high risk of adverse outcomes, and plan patient management accordingly [Citation5]. Several clinical guidelines have been recently published to assist in this process, including recommendations from the American Association for the Study of Liver Diseases (AASLD); the American Association of Clinical Endocrinology (AACE) cosponsored by the AASLD; the American Gastroenterological Association (AGA); and cross-specialty recommendations from the European Association for the Study of the Liver (EASL), European Association for the Study of Diabetes (EASD), and European Association for the Study of Obesity (EASO) [Citation5,Citation6,Citation26,Citation27]. The AGA have also recently published a clinical update on the diagnosis and management of MASLD in lean individuals [Citation14], and in 2021, the EASL published clinical practice guidelines on noninvasive tests for evaluation of liver disease severity and prognosis [Citation28]. Additionally, in 2022, the American Diabetes Association (ADA) and EASD emphasized the role of diabetes medications in the management of MASLD [Citation29], while in January 2024, the ADA updated their guidance on the management of MASLD to include similar clinical practice recommendations to those from the AGA and AACE [Citation30].
In this review, we use updated MASLD/MASH nomenclature in place of the previous nonalcoholic fatty liver disease (NAFLD)/nonalcoholic steatohepatitis (NASH) terminology. The updated MASLD term encompasses patients with hepatic steatosis and at least one of five cardiometabolic risk factors [Citation31]. It has been concluded that it is reasonable to consider that findings from studies using NAFLD/NASH terminology are still valid and applicable under the new MASLD definitions. This is based on an investigation where results showed that among 261 patients with NAFLD, only 2.3% did not meet the metabolic criteria for MASLD [Citation31].
In this article, we review recommendations for MASLD screening, diagnosis, and management in current guidelines, focusing on similarities and highlighting key differences. The aim is to provide a unified, simple-to-understand practical guide that primary care providers can utilize in their clinical practice.
2. Materials and methods
Evidence-based recommendations from the AASLD, AACE-AASLD, AGA, ADA, EASL, and EASL-EASD-EASO [Citation5,Citation6,Citation26–28,Citation30] were reviewed and summarized.
3. Results
3.1. Risk stratification and referral
3.1.1. Screening
As MASLD is often asymptomatic, screening in the primary care setting is critical [Citation26]. Recommendations on screening of key populations have been incorporated into current clinical practice guidelines following the development and validation of noninvasive tests (e.g. the Fibrosis-4 index [FIB-4]) [Citation5,Citation26,Citation27,Citation30], alongside evidence that screening of high-risk patients can be cost-effective for healthcare providers [Citation32].
3.1.2. Target populations for risk stratification
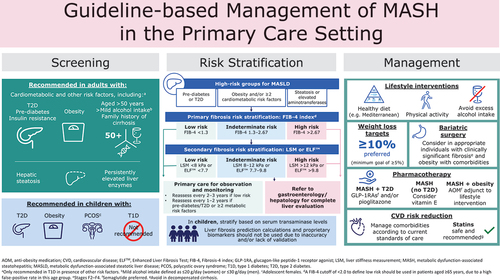
Available guidelines are broadly aligned on the target populations for screening (). High-risk groups for MASH with hepatic fibrosis who should be considered for screening include individuals who are aged ≥50 years, have obesity (including those scheduled for bariatric surgery) have evidence of hepatic steatosis on imaging or elevated aminotransferases, have pre-diabetes, T2D and/or metabolic risk factors, or have a family history of cirrhosis [Citation5,Citation6,Citation26,Citation27,Citation30]. There is evidence to support the screening of patients with T2D in primary care for MASLD and it has been suggested that screening for MASLD-induced fibrosis should become part of the holistic assessment in these patients [Citation32]. Clinical guidance for steatotic liver disease in lean individuals proposes an age threshold of 40 years for the screening of individuals with T2D [Citation14], which has been shown to be cost-effective in individuals with T2D [Citation33].
Table 2. Guideline recommendations on target populations for screening of advanced fibrosis associated with MASLD in high-risk populations.
3.1.3. Practical considerations in the identification of at-risk individuals
General population-based screening for advanced fibrosis associated with MASLD is not currently recommended [Citation27]. Furthermore, limited treatment and management options for MASH have historically led to concerns regarding the long-term benefits and cost-effectiveness of large-scale screening [Citation34]. However, the treatment paradigm for MASH is likely to change, with various agents in Phase 3 clinical development, including resmetirom, for which positive pivotal Phase 3 trial data have been reported and regulatory evaluation is pending [Citation35]. This potential change in the treatment landscape, alongside the increased use of simple, low-cost, noninvasive tools for risk stratification in recent years, suggests that identification of individuals at risk of fibrosis and disease progression is warranted to prompt early intervention with targeted lifestyle modification, while approved pharmacotherapy awaits. As such, it is essential that primary care providers are aware of the wide range of at-risk groups and conduct targeted screening accordingly. Although elevated liver chemistry tests are often associated with MASLD, it is important to note that as many as 50% of affected individuals can have normal alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels [Citation36], and this alone should not be relied upon to guide patient management and referral to specialist clinicians. Primary care providers should be aware that MASH is more prevalent among Hispanic individuals compared with Whites, Asians, or African Americans [Citation12,Citation37], likely due, in part, to increased prevalence of the PNPLA3 I148M polymorphism among Hispanics versus other ethnicities [Citation19]. As such, providers may wish to tailor screening recommendations accordingly, based on ethnicity.
3.1.4. Recommendations for initial evaluation of individuals with suspected MASLD
Initial evaluation should include medical history, alcohol intake history, and routine laboratory tests [Citation5,Citation26,Citation27]. These enable clinicians to rule out other causes of hepatic steatosis and/or elevated liver enzymes, and also provide measurements for calculation of simple fibrosis scores (such as FIB-4) for risk stratification [Citation5,Citation26,Citation27]. EASL-EASD-EASO guidelines emphasize the importance of a comprehensive assessment of dietary and physical activity habits as part of initial evaluations [Citation6], and AASLD recommends a physical examination to examine body fat distribution, features of insulin resistance, and features of advanced liver disease [Citation27]. Recent and current medication should also be assessed for drugs that may lead to, or exacerbate, steatohepatitis (e.g. corticosteroids, methotrexate, tamoxifen, irinotecan, amiodarone, and 5-fluorouracil) [Citation27].
Historically, imaging techniques such as ultrasound were recommended as part of an initial evaluation of high-risk individuals to diagnose hepatic steatosis [Citation6]; however, ultrasound is only accurate in identification when the total area of steatosis exceeds 20%, and has suboptimal sensitivity for lesser steatosis [Citation26,Citation38]. Current guidance from AACE-AASLD and the AGA notes that it is reasonable to proceed directly to fibrosis risk stratification without the need for ultrasound in high-risk groups [Citation5,Citation26]. Genetic testing and gene-based risk stratification are not currently recommended in a clinical setting [Citation5,Citation27].
3.1.5. Recommendations for risk stratification and referral
Key recommendations for risk stratification and referral across the clinical practice guidelines are summarized in and . Recommendations for risk stratification are generally consistent across the most recent clinical guidance from the AASLD, AACE, AGA, ADA, and the 2021 EASL update (including guidance for steatotic liver disease in lean individuals), which incorporate evolving evidence supporting the application of noninvasive tools [Citation5,Citation14,Citation26–28,Citation30]. In brief, at-risk individuals should undergo primary risk assessment with nonproprietary liver scores [Citation5,Citation14,Citation26–28,Citation30]. FIB-4 is the most validated noninvasive test, is simple to calculate with minimal cost, and has greater diagnostic accuracy for excluding advanced fibrosis compared with many other noninvasive tests [Citation26,Citation27,Citation39]. FIB-4 is also associated with risk of subsequent liver-related morbidity and mortality, and all-cause mortality [Citation40,Citation41]. As such, recent clinical practice guidelines universally recommend FIB-4 for the first-line assessment of liver fibrosis, although other tests, such as the AST to platelet ratio index (APRI) or NAFLD fibrosis score (NFS), can be used in place of FIB-4, if necessary [Citation5,Citation14,Citation26–28,Citation30].
Figure 2. Risk stratification for advanced fibrosis related to MASLD/MASH [Citation5,Citation6,Citation26–30].
![Figure 2. Risk stratification for advanced fibrosis related to MASLD/MASH [Citation5,Citation6,Citation26–30].](/cms/asset/a56abefe-8857-4478-9355-10b90ee23af4/ipgm_a_2325332_f0002_oc.jpg)
Table 3. Summary of guideline recommendations for risk stratification and referral.
Guidelines concur that individuals with a low FIB-4 score (<1.3) do not need further evaluation at this time and can continue management in primary care, while referral to a hepatologist or gastroenterologist should be considered for those with a high FIB-4 score (>2.67) [Citation5,Citation14,Citation26–28]. EASL Citation2021 guidelines recommend a single cutoff for FIB-4 (>1.3), as an indication for secondary risk stratification [Citation28]. Individuals with an indeterminate/intermediate FIB-4 score (1.3–2.67) are recommended to undergo secondary risk stratification using vibration-controlled transient elastography (VCTE) with FibroScan® to obtain a liver stiffness measurement (LSM) [Citation5,Citation14,Citation26–28]. However, since the availability of elastography may be limited in some clinical settings [Citation27], guidelines also recommend the use of the commercial blood fibrosis biomarker, Enhanced Liver Fibrosis test (ELFTM), for secondary risk stratification [Citation5,Citation14,Citation26,Citation27,Citation30]. Individuals at low risk of clinically significant fibrosis by VCTE (<8.0 kPa) or ELFTM (<7.7) can remain in primary care, while those at indeterminate/intermediate or high risk (VCTE ≥8.0 kPa or ELFTM ≥7.7) should be referred to gastroenterology or hepatology for a thorough evaluation of liver health that may include liver biopsy or magnetic resonance elastography (MRE) [Citation5,Citation14,Citation26,Citation27,Citation30]. Up to 10% of at-risk patients with MASH will be mis-stratified by low FIB-4 cutoffs [Citation26]. Additional screening should be considered for patients with thrombocytopenia, alcohol use, or comorbidities such as growth hormone deficiency [Citation26,Citation42], and those with a family member who have at-risk MASH.
It should be noted that age has a considerable effect on the performance of the FIB-4 test, with low specificity in individuals aged ≥65 years, which results in a high false positive rate [Citation43]. As a result, it is proposed that a higher FIB-4 cutoff of <2.0 is used in elderly patients, and this has demonstrated improved specificity without adversely affecting sensitivity [Citation43]. The FIB-4 test also performs poorly in patients aged <35 years, and an alternative method of fibrosis assessment should be used in this population [Citation43]. Recommendations for children with suspected MASLD/MASH differ from those for adults. The AACE-AASLD joint guidelines note that liver fibrosis prediction calculations and proprietary biomarkers should not be used in children as they are either inaccurate or require further validation, and as such, advocate the use of serum aminotransferases for primary risk stratification [Citation5]. Although current AASLD guidelines do not include recommendations for pediatric MASLD, the previous AASLD guidelines published in 2018 highlighted that VCTE (FibroScan®) is approved by the US Food and Drug Administration (FDA) for use in children [Citation34].
3.1.6. Recommendations for surveillance in primary care
Among individuals considered at low risk of clinically significant fibrosis following risk stratification, whose care remains with primary care providers, guidelines recommend repeat testing with FIB-4 and/or VCTE/ELFTM to refine stratification of advanced fibrosis and risk of liver-related events [Citation5,Citation14,Citation26,Citation27,Citation30] (. There is limited evidence regarding the optimal frequency of retesting, and, as such, there is some minor variance between guidelines. Generally, it is advised to retest every 2–3 years for low-risk individuals, with more frequent retesting (every 1–2 years) in those at higher risk, such as those with T2D or ≥2 cardiometabolic risk factors [Citation5,Citation26,Citation27,Citation30]. The AGA guidelines for steatotic liver disease in lean individuals recommend slightly more frequent retesting (every 6–12 months for those with stage ≥F2 fibrosis and every 1–2 years for those with stage F0/F1 fibrosis) [Citation14].
In addition to monitoring for risk of clinically relevant fibrosis, primary care providers should assess alcohol intake on a regular basis [Citation27]. Individuals with MASLD should be screened for the presence of T2D, metabolic syndrome, and cardiovascular disease (CVD), as well as obstructive sleep apnea in individuals with MASLD with overweight or obesity [Citation5,Citation6,Citation27]. Adherence to age-appropriate cancer screening is also recommended [Citation27].
3.1.7. Practical considerations for risk stratification and referral
While histological examination by liver biopsy remains the ‘gold standard’ procedure for the diagnosis of MASH and staging of fibrosis severity, its invasive nature alongside limitations due to intra-observer and inter-observer variabilities, sampling variability, patient discomfort, and cost, make it unsuitable as a screening tool [Citation5]. As such, liver biopsy should be reserved for specific clinical scenarios following referral to gastroenterology or hepatology, e.g. in cases of diagnostic uncertainty or persistently elevated liver enzymes [Citation5,Citation26–28,Citation30].
In primary care settings, where the prevalence of individuals with severe disease is lower than in tertiary care centers, noninvasive tests with a high negative predictive value are therefore useful to rule out advanced fibrosis without the need for a biopsy, and can also be used to monitor the evolving risk of adverse outcomes [Citation28,Citation44]. The introduction of two-tier, noninvasive risk stratification into primary screening has greatly reduced the number of unnecessary referrals to specialist clinicians [Citation5]. Indeed, one study that evaluated FIB-4 followed by ELFTM or VCTE, respectively, in 1000 individuals with MASLD and indeterminate/intermediate FIB-4 reported 114% and 118% improvement in the detection of advanced fibrosis, and an 85% and 78% reduction in unnecessary referrals, compared with historical standard care (i.e. medical history, full blood count, liver function tests, and abdominal ultrasound) [Citation45]. Primary care providers may wish to consider harnessing the potential of electronic medical records for automatic calculation of FIB-4 in individuals at high risk of advanced fibrosis, a practice which has been implemented successfully in many settings. In primary care, assessment using simple 12-lead electrocardiography may also be valuable, since evidence has shown that abnormal ECG findings may be independently associated with advanced fibrosis in patients with MASLD [Citation46].
Primary care providers should be aware of the limited access to consultants in managed care settings and the limited availability of confirmatory imaging tools, such as VCTE (FibroScan®) in some areas. Alternative imaging techniques include shear wave elastography (SWE) and MRE; however, there are limited data on the performance characteristics of ultrasound elastography techniques such as SWE, while MRE is highly accurate in identifying patients with advanced fibrosis, it is expensive if performed with full abdominal magnetic resonance imaging, and has limited availability in some settings [Citation5,Citation27].
Finally, primary care providers should recognize that the histopathological features of MASLD/MASH can differ in children compared with adults [Citation34], and risk assessment and referral should be tailored accordingly. Guideline recommendations are currently not as comprehensive for children as they are for adults. Indeed, of guidelines published in the previous 2 years, only AACE-AASLD guidance for the primary care setting includes specific recommendations for pediatric populations [Citation5], although publication of the AASLD Pediatric MASLD Guidance is expected soon [Citation27].
3.2. Treatment and management of MASLD
3.2.1. Goals of management
For individuals with MASLD, the aims of treatment and management are to decrease MASLD-related morbidity and mortality, and to reverse steatohepatitis and fibrosis, or at least halt fibrosis progression and reduce progression to cirrhosis or hepatocellular carcinoma [Citation6,Citation26]. Management should also reduce cardiovascular risk and promote cardiometabolic health [Citation5].
3.2.2. MASLD management approaches
Guideline-recommended approaches to the treatment and management of MASLD include lifestyle intervention, pharmacotherapy, vitamin E, and bariatric surgery, and are summarized in [Citation5,Citation6,Citation26,Citation27,Citation30].
Table 4. Summary of recommendations on the treatment and management of MASLD/MASH.
For individuals who require weight loss, a goal of ≥5%, and preferably ≥10%, loss of total body weight is recommended based on evidence demonstrating that greater weight loss is often associated with greater benefits on liver histology and cardiometabolic parameters [Citation5,Citation27,Citation30].
3.2.2.1. Lifestyle intervention
Lifestyle modifications consisting of healthy nutrition and exercise are recommended as first-line therapy for individuals with MASLD [Citation5,Citation6,Citation26,Citation27,Citation30] ().
Studies of lifestyle interventions have demonstrated benefits in individuals with MASLD, but are often limited by their small sample sizes, heterogeneous populations, and short durations of ≤12 months [Citation5]. A small, randomized controlled trial (N = 31) found that among individuals with biopsy-proven MASH who received lifestyle intervention (dietary counseling, exercise, and behavioral modification), those who achieved ≥7% body weight loss had significant improvements in steatosis, lobular inflammation, ballooning injury, and overall NAFLD disease activity score (NAS) compared with those who lost <7% of their body weight [Citation47]. In a prospective cohort study of individuals (N = 261) who participated in a 52-week lifestyle intervention program, a higher proportion of those with ≥5% body weight loss had NAS reductions and MASH resolution versus those who lost <5% of their weight. Additionally, all participants who lost ≥10% body weight had NAS reductions, while 90% had MASH resolution and 45% had fibrosis regression [Citation48]. In recent years, there has been a growing interest in the association between MASLD and sarcopenia [Citation49]. Patients with MASLD have an increased risk of sarcopenia compared with patients without MASLD [Citation50,Citation51], and there is also a higher prevalence of sarcopenia in patients with MASH compared with MASLD [Citation52]. In addition, sarcopenia is a strong predictor of mortality and morbidity in patients with MASLD/MASH [Citation49]. Weight loss interventions without exercise may exacerbate loss of skeletal muscle mass; therefore, it is important that these interventions are carried out with consideration of muscle health [Citation49]. A cross-sectional study found that higher muscle mass was negatively associated with risk of MASLD, and lower muscle mass was inversely associated with MASLD severity [Citation53].
Structured weight loss and exercise programs tailored to individual lifestyles and personal preferences are recommended, as they offer greater benefit than standard counseling [Citation5,Citation6,Citation30]. Dietary recommendations include a reduction in macronutrient content according to the Mediterranean diet, which is linked to a decrease in hepatic steatosis, improved insulin sensitivity, and reduced mortality [Citation5,Citation6,Citation26,Citation27,Citation30]. This is based on the daily consumption of vegetables and fresh fruit, unsweetened fiber-rich cereals, nuts, fish, white meat, and olive oil, with limited intake of saturated fat, processed food, and simple sugars [Citation26].
Physical activity can be tracked by wearable devices. A study using electronic health records data found that higher daily step counts above 8200 were associated with a reduced risk of obesity and diabetes [Citation54]. There may also be a corresponding positive impact on MASLD, given the close relationship between these metabolic conditions.
Of note, the likelihood of achieving sustained weight loss with lifestyle modification alone is low. Individuals typically regain one-third of the weight lost in the year following intervention and continue to gain weight thereafter without further therapy [Citation55]. Recommendations for long-term maintenance of weight loss include monthly or more frequent in-person or virtual sessions with a trained healthcare provider; a reduced-calorie diet based on individual preferences and health status; 200–300 minutes/week of aerobic activity (e.g. brisk walking); regular monitoring of food intake and physical activity; and daily/weekly monitoring of weight [Citation55].
Adults with MASLD should restrict alcohol consumption based on evidence that suggests that heavy consumption accelerates liver injury and fibrosis progression [Citation27], and even low levels of alcohol intake are associated with increased risks for advanced liver disease [Citation26]. Clinical trials including patients with MASLD typically define excessive alcohol intake as >21 standard drinks per week in men and >14 drinks per week for women over a 2-year period [Citation56]. In a retrospective study of 8345 individuals with MASLD, 10–19 g (equivalent to approximately 1–2 units) of daily general alcohol use or 0–9 g (approximately 0–1 unit) of daily non-wine alcohol use doubled the risk for adverse liver-related disease compared with individuals who were abstinent throughout their lifetime [Citation57]. There is no safe threshold for alcohol intake in patients with advanced fibrosis [Citation26].
Lifestyle intervention, including dietary modification and exercise, is recommended for lean individuals with steatotic liver disease [Citation14,Citation27]. AGA guidance suggests targeting a modest weight loss of 3%–5% in lean individuals, though AASLD highlights that weight loss recommendations may not be appropriate in this population [Citation14,Citation27]. In pediatric MASLD, lifestyle interventions should be conducted as a first-line approach. Dietary changes and increased physical activity have been shown to improve steatosis and hepatic inflammation in children with MASLD, but there is very limited evidence for benefits of other interventions [Citation5,Citation6].
3.2.2.2. Pharmacological interventions
Although no pharmacological treatments are currently approved for patients with MASLD/MASH, medications approved for other indications have demonstrated benefits in this population [Citation27]. Some minor differences exist between guidelines with regard to which patients are candidates for available therapies. However, there is a general consensus that treatment should be reserved for patients with progressive MASH at a higher risk of complications. Key recommendations for pharmacotherapy are summarized in .
AGA guidelines recommend pharmacotherapy for MASLD in individuals at indeterminate/intermediate or high risk of advanced fibrosis [Citation26]. EASL-EASD-EASO guidance recommends the use of pharmacotherapy in patients with clinically significant fibrosis (stage F2 and higher). Pharmacotherapy may also be considered for prevention in patients with less severe disease who are at high risk of disease progression [Citation6].
Pharmacotherapy in individuals with T2D and MASLD aims to treat both hyperglycemia and obesity, taking into consideration the high prevalence of significant fibrosis and increased risk of disease progression and liver-related mortality in this population [Citation30].
3.2.2.3. Glucagon-like peptide-1 receptor agonists
Glucagon-like peptide-1 receptor agonists (GLP-1RAs) are recommended for the treatment of individuals with T2D or obesity and MASH [Citation5,Citation26,Citation27]. The benefits of GLP‐1RAs on MASH are thought to be due to their effect on insulin resistance and weight loss, thereby affecting multiple components of metabolic syndrome [Citation58,Citation59]. Exenatide was shown to improve liver enzymes in individuals with MASLD, elevated liver enzymes, obesity, and T2D [Citation60]. Studies have demonstrated histological resolution of MASLD with liraglutide and semaglutide treatment [Citation61,Citation62]. A recent investigation also showed that semaglutide improved noninvasive markers of liver injury associated with fibrosis progression [Citation63]. GLP-1RAs may be considered for the treatment of obesity and T2D in children, which may offer benefits for MASLD [Citation5].
3.2.2.4. Pioglitazone
Pioglitazone improves liver histology and may improve fibrosis in patients with MASLD, with or without T2D [Citation26,Citation30]. It should be noted that pioglitazone is contraindicated in patients with decompensated cirrhosis [Citation26].
3.2.2.5. Sodium-glucose cotransporter-2 inhibitors
Sodium-glucose cotransporter-2 inhibitors (SGLT2i) have been shown to reduce plasma aminotransferase levels and reduce liver fat in patients with T2D [Citation64,Citation65]. Data from several small studies also suggest that SGLT2i may improve hepatic steatosis and decrease the risk of MASLD, as well as offering potential cardiometabolic, renoprotective, and weight loss benefits; however, due to a lack of histological outcomes in clinical studies, the therapeutic impact of SGLT2i on liver histology in individuals with MASLD/MASH is currently unclear [Citation5,Citation27,Citation65–68]. The AACE-AASLD guidelines recommend SGLT2is, along with GLP-1RAs or pioglitazone, to offer cardiometabolic benefit but not as a treatment for MASLD or MASH, highlighting that there is no evidence of benefit from the treatment of steatohepatitis with SGLT2i [Citation5].
3.2.2.6. Vitamin E
All guidelines recommend vitamin E as an option for the treatment of MASLD in individuals without diabetes, based on evidence demonstrating an improvement in steatohepatitis in this population [Citation5,Citation6,Citation26,Citation27,Citation30]. Vitamin E is not currently recommended for individuals with T2D or advanced fibrosis due to insufficient evidence [Citation5].
3.2.2.7. Other agents
Metformin, acarbose, dipeptidyl peptidase IV inhibitors, insulin, ursodeoxycholic acid, statins, and silymarin should not be used as treatment for MASLD, as evidence has not demonstrated a meaningful histological benefit [Citation5,Citation27].
3.2.2.8. Investigational agents
In the SURPASS-3 MRI substudy, the dual glucose-dependent insulinotropic polypeptide (GIP)/GLP-1RA tirzepatide significantly reduced liver fat content after 1 year of treatment, compared with insulin degludec, in patients with T2D without a history of significant alcohol consumption [Citation69]. Ongoing Phase 3 clinical trials are exploring multiple drug candidates targeting energy intake, energy disposal, lipotoxic liver injury, inflammation, and fibrosis in patients with MASLD [Citation44] (). These include GLP-1RAs (semaglutide) and other medications for T2D (SGLT2i; pioglitazone); resmetirom, a liver-directed, orally active, selective thyroid hormone receptor-β agonist; and lanifibranor, a pan-peroxisome proliferator-activated receptor agonist.
Table 5. Ongoing and recently completed Phase 3 clinical trials in patients with MASLD/MASH (Clinicaltrials.gov entries).
In the REGENERATE trial, obeticholic acid significantly improved fibrosis and key components of disease activity in adults with MASH [Citation70]; however, the drug recently failed to obtain FDA approval in the US due to concerns over an unfavorable risk/benefit profile, and, as such, its development in MASH has been discontinued [Citation71]. Topline results from the MAESTRO-NASH Phase 3 clinical trial have demonstrated significant improvements in MASH resolution and liver fibrosis with resmetirom [Citation35]. Another study is investigating the effect of estradiol administration on MASH in postmenopausal women (), and combination therapies, which may allow targeting of both metabolic dysfunction and liver damage, are also under evaluation [Citation44].
3.2.2.9. Bariatric surgery
Bariatric surgery can reduce liver fat, resolve MASLD, and improve hepatic fibrosis [Citation6,Citation27]. Recommendations for bariatric surgery differ between guidelines. AACE-AASLD guidance states that bariatric surgery may be considered as an option in individuals with a BMI of >35 kg/m2 (≥32.5 kg/m2 in Asian populations), particularly if T2D is present [Citation5]. AASLD guidelines recommend considering bariatric surgery in individuals who meet criteria for metabolic weight loss surgery [Citation27], while AGA guidance states that bariatric surgery should be considered in individuals with clinically significant fibrosis and obesity with comorbidities [Citation26]. For individuals with MASLD and compensated cirrhosis, caution is required before recommending bariatric surgery, and it is not recommended in individuals with decompensated cirrhosis due to limited evidence and a high risk of postoperative liver-related complications [Citation5,Citation27,Citation30]. Of note, there is a lack of large, long-term controlled studies to establish the best surgical approach in patients with MASLD undergoing bariatric surgery [Citation5].
3.2.2.10. Referral for liver transplantation
Referral for liver transplantation evaluation should be considered in patients with cirrhosis complicated by medically refractory hepatic decompensation (e.g. ascites, hepatic encephalopathy, variceal hemorrhage), synthetic dysfunction (i.e. Model for End-stage Liver Disease score ≥15), or hepatocellular carcinoma without metastasis [Citation72].
3.2.2.11. Management of comorbidities
Comorbidities, such as obesity and components of metabolic syndrome, including hypertension, hyperglycemia, and dyslipidemia, should be treated aggressively to decrease CVD risk [Citation5,Citation26,Citation27] (). The potential liver-related benefits of any medication should also be considered when managing comorbidities, following current standards of care [Citation27].
In patients with early-stage fibrosis, CVD is the main driver of morbidity and mortality, therefore highlighting the need for CVD risk reduction strategies in all patients with MASLD [Citation26]. Statins are generally well tolerated, with a low frequency of drug-induced liver injury, and are recommended for CVD risk reduction [Citation6,Citation26,Citation27,Citation73]. In individuals with decompensated cirrhosis and high CVD risk, AASLD guidelines state that statins may be considered [Citation27], while AGA guidance recommends that statins are avoided due to limited data in this population [Citation26].
AACE-AASLD guidance recommends obesity pharmacotherapy ‒ preferably semaglutide 2.4 mg/week or liraglutide 3 mg/day ‒ as adjunctive therapy to lifestyle modification in individuals with obesity and MASLD to treat or prevent T2D, CVD, and other end-stage manifestations of obesity [Citation5]. Semaglutide 2.4 mg/week and liraglutide 3 mg/day (adjunct to lifestyle modification) are also the preferred agents for chronic weight management in individuals with a BMI of ≥27 kg/m2 and MASLD/MASH [Citation5].
3.2.2.12. Practical considerations for treatment and management
A multidisciplinary approach to the care of individuals with MASLD is critical, as management of comorbidities and achieving sustained weight loss may require involvement from other healthcare providers, such as dieticians, obesity specialists, endocrinologists, and cardiologists. As a pivotal member of the team, primary care providers should focus on weight loss and managing metabolic conditions [Citation5,Citation26,Citation27].
The lack of approved pharmacotherapies for MASLD places emphasis on the importance of lifestyle measures to manage the disease [Citation44]. However, providing patients with the education and support needed to make long-term changes remains a challenge, particularly in the primary care setting where time is limited [Citation74–76]. Few patients achieve clinically meaningful weight loss, and less than half maintain weight loss after 5 years [Citation27]. Accordingly, it is clear that there are various barriers to effective weight loss. For example, many patients have only limited access to weight loss medications due to the lack of recognition of obesity as a disease [Citation77], while patients without T2D may lack access to a dietician. Among the barriers experienced by patients are lack of weight loss knowledge and lack of awareness of their weight, and the seriousness of MASLD [Citation78]. Equipping patients with the appropriate knowledge and skills is essential to successfully achieve and maintain meaningful weight loss [Citation78]. Setting realistic expectations of how much weight loss can be expected with a particular intervention is also important to avoid disappointment, which could lead to discontinuation and feelings of low self-esteem [Citation79].
An understanding of the broader consequences of MASLD is also important. Patients may face stigma either because of the condition itself (in part due to inclusion of the term ‘nonalcoholic’ in the previous name), or because of associated obesity, which can result in discrimination, feelings of blame, social isolation, and impaired health-related quality of life [Citation80,Citation81]. Obesity-related stigma impacts self-esteem and body image, and can increase stress and worsen metabolic and sleep disorders, leading to weight gain and progression of MASLD [Citation80]. Perceived stigma can also negatively impact overall mental health and is associated with reduced engagement in care and lifestyle modification programs [Citation80].
Identifying which patients are candidates for available pharmacological options can be challenging due to gaps in knowledge, even among specialist physicians [Citation74]. Treatment decisions should take into consideration anti-obesity and diabetes medications, particularly GLP-1RAs with proven benefits for steatohepatitis. Providers should be aware that pioglitazone and GLP-1RAs, the preferred pharmacotherapeutic options for individuals with T2D and MASLD, can be combined to limit pioglitazone-associated weight gain [Citation26]. A greater awareness of the potential benefits of such medications is likely to change the treatment paradigm for MASLD in the coming years [Citation5].
Evidence suggests that the use of pharmacotherapy is low among both primary care providers and other specialists treating MASLD [Citation82,Citation83]. A survey of US primary care providers found that 58% would recommend weight loss and calorie restriction as a first step in the management of MASLD, while 8% would recommend vitamin E [Citation82]. The major barrier to evaluation and treatment was a lack of confidence in knowledge of the disease and its management (reported by 58% of primary care providers). Other barriers included time constraints, cost of evaluation and treatment, a lack of comfort discussing obesity, and a perceived lack of patient compliance [Citation82]. A study of gastroenterologists showed that 72% of their patients were treated with lifestyle modification, while 28% received pharmacological therapy [Citation83].
Pharmacotherapy for CVD is also underprescribed in MASLD, despite the increased risk of CVD in this population [Citation84,Citation85]. Currently 40%–50% of individuals in whom treatment is indicated do not receive statin therapy due to concerns over hepatotoxicity [Citation84,Citation85]. Primary care providers should be reassured that statins have demonstrated safety across the MASLD disease spectrum, including individuals with dyslipidemia [Citation27,Citation30,Citation85].
Despite several guidelines being available, greater awareness of the recommendations is required among primary care providers treating patients with MASLD/MASH. Educational initiatives are needed to address existing gaps in knowledge and clinical skills which, in turn, will help to encourage greater implementation of the recommendations in daily practice.
4. Conclusions
Several new therapies for MASH are in late-stage clinical development and may be approved for use in the upcoming years, emphasizing the urgent need to identify individuals at risk of developing this disease who are eligible for early intervention. New evidence from ongoing studies will provide a greater understanding of the natural history of MASLD/MASH, and will help to define the optimal diagnostic and treatment pathways for this patient population [Citation5]. Various blood and imaging noninvasive tests are now available; however, their place in the primary care setting is still unclear. Pediatric MASLD is a growing area of interest, but awareness among primary care providers is limited [Citation5]. New guidance on pediatric MASLD will soon be available, but further research is needed to guide treatment decisions.
In conclusion, MASH is a major global health concern and its prevalence is expected to increase in the coming years. Early diagnosis and management of MASH are crucial for improving patient outcomes, and primary care providers are ideally placed to identify at-risk individuals and manage comorbidities. Evidence-based consensus guidelines are available for the screening, diagnosis, and treatment of patients with MASH. However, greater awareness and implementation of these guidelines are required among primary care providers, and ongoing research will help to define new diagnostic and treatment pathways for this patient population.
List of abbreviations
AACE, American Association of Clinical Endocrinology; AASLD, American Association for the Study of Liver Diseases; ADA, American Diabetes Association; AGA, American Gastroenterological Association; ALT, alanine aminotransferase; AOM, anti-obesity medication; APRI, aspartate aminotransferase to platelet ratio index; ARFI, acoustic radiation force impulse; AST, aspartate aminotransferase; BMI, body mass index; CVD, cardiovascular disease; DPP-4, dipeptidyl peptidase-4; EASD, European Association for the Study of Diabetes; EASL, European Association for the Study of the Liver; EASO, European Association for the Study of Obesity; ECG, electrocardiography; ELFTM, Enhanced Liver Fibrosis test; FIB-4, Fibrosis-4 index; GIP, glucose-dependent insulinotropic polypeptide; GLP-1RA, glucagon-like peptide-1 receptor agonist; HbA1c, glycated hemoglobin; HCC, hepatocellular carcinoma; HDL, high-density lipoprotein; HSD17B13, 17B-hydroxysteroid dehydrogenase 13; LSM, liver stiffness measurement; MASH, metabolic dysfunction-associated steatohepatitis; MASLD, metabolic dysfunction-associated steatotic liver disease; MetALD, metabolic dysfunction and alcohol associated steatotic liver disease; MRE, magnetic resonance elastography; NAFLD, nonalcoholic fatty liver disease; NAS, NAFLD activity score; NFS, nonalcoholic fatty liver disease fibrosis score; NS, not specified; PCOS, polycystic ovary syndrome; PNPLA3, patatin-like phospholipase domain-containing protein 3; SGLT2i, sodium-glucose cotransporter-2 inhibitor; SWE, shear wave elastography; T1D, type 1 diabetes; T2D, type 2 diabetes; TM6SF2, transmembrane 6 superfamily member 2; UDCA, ursodeoxycholic acid; US, United States; VCTE, vibration-controlled transient elastography.
Declaration of financial/other relationships
Alina M. Allen has received research funding to her institution from Novo Nordisk, Pfizer, and Target Pharma, and has served as an advisory board member for Novo Nordisk. Michael Charlton has served as a consultant for Bristol Myers Squibb, Celgene, Histoindex, Intercept, Madrigal, NGM, Novo Nordisk, Pfizer, Terns, and Theratechnologies. He has received research grants from Gilead Sciences. Kenneth Cusi has received research support (University of Florida; Principal Investigator) from Boehringer Ingelheim, Echosens, Inventiva, Labcorp, and Perspectum. He has served as a consultant for 89bio, Aligos Therapeutics, Arrowhead, AstraZeneca, Bristol Myers Squibb, Eli Lilly & Co., Merck, Novo Nordisk, ProSciento, Sagimet Biosciences, Siemens, and Terns Pharma. Stephen A. Harrison consults, advises, is involved with trials, has received grants from, and owns stock in Akero, Galectin, GENFIT, Hepion, and NGM Bio. He consults, advises, is involved with trials, and has received grants from Axcella, Gilead, Intercept, Madrigal, and Poxel. He consults, advises, has received grants from, and owns stock in NorthSea Therapeutics. He consults, advises, and is involved with trials for Terns. He consults, advises, and has received grants from HighTide, Novartis, Novo Nordisk, and Sagimet. He consults, advises, and owns stock in HistoIndex, Metacrine, and Sonic Incytes. He consults, has received grants from, and owns stock in Cirius. He consults, is involved with trials for, and has received grants from ENYO and Viking. He is involved with trials for and has received grants from Genentech. He consults and is involved with trials for Ionis. He consults for and has received grants from CiVi, CymaBay, Galmed, and Pfizer. He consults for and owns stock in Hepta Bio. He consults and advises for Altimmune, Echosens North America, Foresite Labs, and Medpace. He advises and owns stock in ChronWell. He consults for AgomAb, Alentis, Aligos Therapeutics, Alimentiv, Blade, Bluejay, Boston Pharmaceuticals, Boxer Capital, Can-Fite BioPharma, the Chronic Liver Disease Foundation (CLDF), CohBar, Corcept, Fibronostics, Fortress Biotech, Galecto, Gelesis, GlaxoSmithKline, GNS Healthcare, GRI Bio, Hepagene, Indalo, Inipharm, Innovate Biopharmaceuticals, Kowa Research Institute, Merck, MGGM, NeuroBo, Nutrasource, Perspectum, Piper Sandler, Prometic (now Liminal BioSciences), Ridgeline Therapeutics, Silverback, and Zafgen (now Larimar). He advises for Arrowhead BVF Partners, Humana, and PathAI. He received grants from Bristol Myers Squibb, Conatus, Immuron, and Second Genome. Kris V. Kowdley advises, is on the speakers’ bureau for, and has received grants from Gilead and Intercept. He advises, has received grants for, and owns stock in Inipharm. He advises and has received grants from 89bio, CymaBay, GENFIT, Ipsen, Madrigal, Mirum, NGM Bio, Pfizer, Pliant, and Zed. He advises Enact, HighTide, and Protagonist. He is on the speakers’ bureau for AbbVie. He has received grants from Boston Pharmaceuticals, Corcept, GlaxoSmithKline, Hanmi, Janssen, Novo Nordisk, Terns, and Viking. Mazen Noureddin consults for 89bio, Altimmune, Boehringer Ingelheim, Cytodyn, Echosens, GlaxoSmithKline, Madrigal, Merck, Novo Nordisk, Prespecturm, Roche Diagnostics, Siemens, Takeda, and Terns. He has received speaker fees from Echosens and Novo Nordisk, and served on an advisory board or data safety monitoring board for 89bio, Altimmune, Boehringer Ingelheim, CytoDyn, Echosens, GlaxoSmithKline, Madrigal, Merck, Novo Nordisk, Prespecturm, Roche Diagnostic, and Siemens. He owns stock/stock options in ChronWell, CIMA, and Rivus Pharma. Jay H. Shubrook reports serving as a consultant to Abbott, Bayer, and Novo Nordisk. He has served on advisory boards for Abbott, AstraZeneca, Bayer, Eli Lilly, Nevro, and Novo Nordisk. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed. Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author contributions
Alina M. Allen is the guarantor of the article. All authors approved the final version of the article, including the authorship list, have reviewed and edited the manuscript outline, reviewed and edited all drafts of the manuscript, and supplied reference material.
Acknowledgments
Medical writing and editorial support were provided by Bakhouche Bakhouche, of OPEN Health Group, and Ruth Brown and Sally Humphries, contract writers working on behalf of OPEN Health Group (and were funded by Novo Nordisk Inc.) under the direction of the authors in accordance with Good Publication Practice 2022 guidelines (http://www.ismpp.org/gpp-2022). Novo Nordisk Inc. also performed a medical accuracy review. Stephen Harrison contributed to this article as per the ICMJE authorship criteria, however, he passed away during the publication process. We thank him for his valuable contribution to this work and dedicate this article to his memory.
Additional information
Funding
References
- Chen H, Zhan Y, Zhang J, et al. The global, regional, and national burden and trends of NAFLD in 204 countries and territories: an analysis from global burden of disease 2019. JMIR Public Health Surveill. 2022;8(12):e34809. doi: 10.2196/34809
- Allen AM, Lazarus JV, Younossi ZM. Healthcare and socioeconomic costs of NAFLD: a global framework to navigate the uncertainties. J Hepatol. 2023;79(1):209–217. doi: 10.1016/j.jhep.2023.01.026
- Lazarus JV, Mark HE, Anstee QM, et al. Advancing the global public health agenda for NAFLD: a consensus statement. Nat Rev Gastroenterol Hepatol. 2022;19:60–78. doi: 10.1038/s41575-021-00523-4
- Rinella ME, Lazarus JV, Ratziu V, et al. A multi-society Delphi consensus statement on new fatty liver disease nomenclature. Hepatology. 2024;29:101133. doi: 10.1016/j.aohep.2023.101133
- Cusi K, Isaacs S, Barb D, et al. American Association of Clinical Endocrinology clinical practice guideline for the diagnosis and management of nonalcoholic fatty liver disease in primary care and endocrinology settings: co-sponsored by the American Association for the Study of Liver Diseases (AASLD). Endocr Pract. 2022;28(5):528–562. doi: 10.1016/j.eprac.2022.03.010
- European Association for the Study of the Liver (EASL). European Association for the Study of Diabetes (EASD), European Association for the Study of Obesity (EASO). EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J Hepatol. 2016;64(6):1388–1402. doi: 10.1016/j.jhep.2015.11.004
- Calzadilla Bertot L, Adams LA. The natural course of non-alcoholic fatty liver disease. Int J Mol Sci. 2016;17(5):774. doi: 10.3390/ijms17050774
- Kleiner DE, Brunt EM, Van Natta M, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41(6):1313–1321. doi: 10.1002/hep.20701
- Noureddin M, Vipani A, Bresee C, et al. NASH leading cause of liver transplant in women: updated analysis of indications for liver transplant and ethnic gender variances. Am J Gastroenterol. 2018;113(11):1649–1659. doi: 10.1038/s41395-018-0088-6
- Younossi ZM, Golabi P, Paik JM, et al. The global epidemiology of nonalcoholic fatty liver disease (NAFLD) and nonalcoholic steatohepatitis (NASH): a systematic review. Hepatology. 2023;77(4):1335–1347. doi: 10.1097/HEP.0000000000000004
- Quek J, Chan KE, Wong ZY, et al. Global prevalence of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in the overweight and obese population: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2023;8(1):20–30. doi: 10.1016/S2468-1253(22)00317-X
- Harrison SA, Gawrieh S, Roberts K, et al. Prospective evaluation of the prevalence of non-alcoholic fatty liver disease and steatohepatitis in a large middle-aged US cohort. J Hepatol. 2021;75(2):284‒291. doi: 10.1016/j.jhep.2021.02.034
- Kim CH, Younossi ZM. Nonalcoholic fatty liver disease: a manifestation of the metabolic syndrome. Cleve Clin J Med. 2008;75(10):721–728. doi: 10.3949/ccjm.75.10.721
- Long MT, Noureddin M, Lim JK. AGA clinical practice update: diagnosis and management of nonalcoholic fatty liver disease in lean individuals: expert review. Gastroenterology. 2022;163(3):764–774.e1. doi: 10.1053/j.gastro.2022.06.023
- Estes C, Razavi H, Loomba R, et al. Modeling the epidemic of nonalcoholic fatty liver disease demonstrates an exponential increase in burden of disease. Hepatology. 2018;67(1):123–133. doi: 10.1002/hep.29466
- Rich NE, Noureddin M, Kanwal F, et al. Racial and ethnic disparities in non-alcoholic fatty liver disease in the USA. Lancet Gastroenterol Hepatol. 2021;6(6):422–424. doi: 10.1016/S2468-1253(21)00100-X
- Setiawan VW, Stram DO, Porcel J, et al. Prevalence of chronic liver disease and cirrhosis by underlying cause in understudied ethnic groups: the multiethnic cohort. Hepatology. 2016;64(6):1969–1977. doi: 10.1002/hep.28677
- Eslam M, Valenti L, Romeo S. Genetics and epigenetics of NAFLD and NASH: clinical impact. J Hepatol. 2018;68(2):268–279. doi: 10.1016/j.jhep.2017.09.003
- Romeo S, Kozlitina J, Xing C, et al. Genetic variation in PNPLA3 confers susceptibility to nonalcoholic fatty liver disease. Nat Genet. 2008;40:1461–1465. doi: 10.1038/ng.257
- Kozlitina J, Smagris E, Stender S, et al. Exome-wide association study identifies a TM6SF2 variant that confers susceptibility to nonalcoholic fatty liver disease. Nat Genet. 2014;46:352–356. doi: 10.1038/ng.2901
- Pirola CJ, Garaycoechea M, Flichman D, et al. Splice variant rs72613567 prevents worst histologic outcomes in patients with nonalcoholic fatty liver disease. J Lipid Res. 2019;60(1):176–185. doi: 10.1194/jlr.P089953
- LaBrecque D, Abbas Z, Anania F, et al. World Gastroenterology Organisation global guidelines [Internet]. Nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. World Gastroenterology Organisation. 2012 [cited 2023 Aug 23]. Available from: https://www.worldgastroenterology.org/UserFiles/file/guidelines/nafld-nash-english-2012.pdf
- Cook N, Geier A, Schmid A, et al. The patient perspectives on future therapeutic options in NASH and patient needs. Front Med. 2019;6:61. doi: 10.3389/fmed.2019.00061
- Alqahtani SA, Paik JM, Biswas R, et al. Poor awareness of liver disease among adults with NAFLD in the United States. Hepatol Commun. 2021;5(11):1833–1847. doi: 10.1002/hep4.1765
- DeLegge MH. Recruitment and retention of patients for nonalcoholic steatohepatitis clinical trials. Gastroenterol Clin North Am. 2020;49(1):123–140. doi: 10.1016/j.gtc.2019.09.006
- Kanwal F, Shubrook JH, Adams LA, et al. Clinical care pathway for the risk stratification and management of patients with nonalcoholic fatty liver disease. Gastroenterology. 2021;161(5):1657–1669. doi: 10.1053/j.gastro.2021.07.049
- Rinella ME, Neuschwander-Tetri BA, Siddiqui MS, et al. AASLD Practice Guidance on the clinical assessment and management of nonalcoholic fatty liver disease. Hepatology. 2023;77(5):1797–1835. doi: 10.1097/HEP.0000000000000323
- European Association for the Study of the Liver (EASL). EASL Clinical Practice Guidelines on non-invasive tests for evaluation of liver disease severity and prognosis – 2021 update. J Hepatol. 2021;75(3):659–689. doi: 10.1016/j.jhep.2021.05.025
- Davies MJ, Aroda VR, Collins BS, et al. Management of hyperglycemia in type 2 diabetes, 2022. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2022;45(11):2753‒2786. doi: 10.2337/dci22-0034
- ElSayed NA, Aleppo G, Aroda VR, et al. American Diabetes Association Professional Practice Committee. 4. Comprehensive medical evaluation and assessment of comorbidities: standards of care in diabetes—2024. Diabetes Care. 2024;47(Suppl. 1):S52–S76. doi: 10.2337/dc24-S004
- Song SJ, Lai JC, Wong GL, et al. Can we use old NAFLD data under the new MASLD definition? J Hepatol. 2024;80(2):e54–e56. doi: 10.1016/j.jhep.2023.07.021
- Forlando R, Stanic T, Jayawardana S, et al. A prospective study on the prevalence of MASLD in people with type-2 diabetes in the community. Cost effectiveness of screening strategies. Liver Int. 2024;44(1):61–71. doi: 10.1111/liv.15730
- Noureddin M, Jones C, Alkhouri N, et al. Screening for nonalcoholic fatty liver disease in persons with type 2 diabetes in the United States is cost-effective: a comprehensive cost-utility analysis. Gastroenterology. 2020;159(5):1985–1987.e4. doi: 10.1053/j.gastro.2020.07.050
- Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018;67(1):328–357. doi:10.1002/hep.29367
- Madrigal Pharmaceuticals [Internet]. Madrigal announces positive topline results from the pivotal phase 3 MAESTRO-NASH clinical trial of resmetirom for the treatment of NASH and liver fibrosis. 2022 [cited 2023 Aug 23]. Avaialble from: https://ir.madrigalpharma.com/news-releases/news-release-details/madrigal-announces-positive-topline-results-pivotal-phase-3
- Noureddin M, Loomba R. Nonalcoholic fatty liver disease: indications for liver biopsy and noninvasive biomarkers. Clin Liver Dis. 2012;1(4):104–107. doi: 10.1002/cld.65
- Rich NE, Oji S, Mufti AR, et al. Racial and ethnic disparities in nonalcoholic fatty liver disease prevalence, severity, and outcomes in the United States: a systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2018;16(2):198–210.e2. doi: 10.1016/j.cgh.2017.09.041
- Dasarathy S, Dasarathy J, Khiyami A, et al. Validity of real time ultrasound in the diagnosis of hepatic steatosis: a prospective study. J Hepatol. 2009;51(6):1061–1067. doi: 10.1016/j.jhep.2009.09.001
- Siddiqui MS, Yamada G, Vuppalanchi R, et al. Diagnostic accuracy of noninvasive fibrosis models to detect change in fibrosis stage. Clin Gastroenterol Hepatol. 2019;17(9):1877–1885.e5. doi: 10.1016/j.cgh.2018.12.031
- Unalp-Arida A, Ruhl CE. Liver fibrosis scores predict liver disease mortality in the United States population. Hepatology. 2017;66(1):84–95. doi: 10.1002/hep.29113
- Lee J, Vali Y, Boursier J, et al. Prognostic accuracy of FIB-4, NAFLD fibrosis score and APRI for NAFLD-related events: a systematic review. Liver Int. 2021;41(2):261–270. doi: 10.1111/liv.14669
- Doycheva I, Erickson D, Watt KD. Growth hormone deficiency and NAFLD: an overlooked and underrecognized link. Hepatol Commun. 2022;6(9):2227–2237. doi: 10.1002/hep4.1953
- McPherson S, Hardy T, Dufour J-F, et al. Age as a confounding factor for the accurate non-invasive diagnosis of advanced NAFLD fibrosis. Am J Gastroenterol. 2017;112(5):740–751. doi: 10.1038/ajg.2016.453
- Dufour J-F, Antsee QM, Bugianesi E, et al. Current therapies and new developments in NASH. Gut. 2022;71(10):2123–2134. doi: 10.1136/gutjnl-2021-326874
- Srivastava A, Jong S, Gola A, et al. Cost-comparison analysis of FIB-4, ELF and fibroscan in community pathways for non-alcoholic fatty liver disease. BMC Gastroenterol. 2019;19:122. doi: 10.1186/s12876-019-1039-4
- Kang MK, Kim MC. Clinical implications of cardiac symptoms and electrocardiographic abnormalities for advanced liver fibrosis in patients with nonalcoholic fatty liver disease. Medicina (Kaunas). 2023;59(2):375. doi: 10.3390/medicina59020375
- Promrat K, Kleiner DE, Niemeier HM, et al. Randomized controlled trial testing the effects of weight loss on nonalcoholic steatohepatitis. Hepatology. 2010;51(1):121‒129. doi: 10.1002/hep.23276
- Vilar-Gomez E, Martinez-Perez Y, Calzadilla-Bertot L, et al. Weight loss through lifestyle modification significantly reduces features of nonalcoholic steatohepatitis. Gastroenterology. 2015;149(2):367–378. doi: 10.1053/j.gastro.2015.04.005
- Chan W-K, Chuah K-H, Rajaram B, et al. Metabolic dysfunction-associated steatotic liver disease (MASLD): a state-of-the-art review. J Obes Metab Syndr. 2023;32(3):197–213. doi: 10.7570/jomes23052
- Kim HK, Bae SJ, Lee MJ, et al. Association of visceral fat obesity, sarcopenia, and myosteatosis with non-alcoholic fatty liver disease without obesity. Clin Mol Hepatol. 2023;29(4):987–1001. doi: 10.3350/cmh.2023.0035
- Sinn DH, Kang D, Kang M, et al. Nonalcoholic fatty liver disease and accelerated loss of skeletal muscle mass: a longitudinal cohort study. Hepatology. 2022;76(6):1746–1754. doi: 10.1002/hep.32578
- Koo BK, Kim D, Joo SK, et al. Sarcopenia is an independent risk factor for non-alcoholic steatohepatitis and significant fibrosis. J Hepatol. 2017;66(1):123–131. doi: 10.1016/j.jhep.2016.08.019
- Du J, Ma S, Fang L, et al. Association between regional body muscle mass and non-alcoholic fatty liver disease: an observational study using data from the REACTION study. J Pers Med. 2023;13(2):209. doi: 10.3390/jpm13020209
- Master H, Annis J, Huang S, et al. Association of step counts over time with the risk of chronic disease in the All of Us Research Program. Nat Med. 2022;28:2301‒2308. doi: 10.1038/s41591-022-02012-w
- Wadden TA, Tronieri JS, Butryn ML. Lifestyle modification approaches for the treatment of obesity in adults. Am Psychol. 2020;75(2):235–251. doi: 10.1037/amp0000517
- Sanyal AJ, Brunt EM, Kleiner DE, et al. Endpoints and clinical trial design for nonalcoholic steatohepatitis. Hepatology. 2011;54(1):344–353. doi: 10.1002/hep.24376
- Åberg F, Puukka P, Salomaa V, et al. Risks of light and moderate alcohol use in fatty liver disease: follow-up of population cohorts. Hepatology. 2020;71(3):835–848. doi: 10.1002/hep.30864
- Mantovani A, Petracca G, Beatrice G, et al. Glucagon‐like peptide‐1 receptor agonists for treatment of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis: an updated meta‐analysis of randomized controlled trials. Metabolites. 2021;11(2):73. doi: 10.3390/metabo11020073
- Barritt AS 4th, Marshman E, Noureddin M. Review article: role of glucagon‐like peptide‐1 receptor agonists in non‐alcoholic steatohepatitis, obesity and diabetes—what hepatologists need to know. Aliment Pharmacol Ther. 2022;55(8):944–959. doi: 10.1111/apt.16794
- Shao N, Kuang HY, Hao M, et al. Benefits of exenatide on obesity and non-alcoholic fatty liver disease with elevated liver enzymes in patients with type 2 diabetes. Diabetes Metab Res Rev. 2014;30(6):521–529. doi: 10.1002/dmrr.2561
- Armstrong MJ, Gaunt P, Aithal GP, et al. Liraglutide safety and efficacy in patients with non-alcoholic steatohepatitis (LEAN): a multicentre, double-blind, randomised, placebo-controlled phase 2 study. Lancet. 2016;387(10019):679–690. doi: 10.1016/S0140-6736(15)00803-X
- Newsome PN, Buchholtz K, Cusi K, et al. A placebo-controlled trial of subcutaneous semaglutide in nonalcoholic steatohepatitis. N Engl J Med. 2021;384:1113–1124. doi: 10.1056/NEJMoa2028395
- Loomba R, Abdelmalek MF, Armstrong M, et al. Semaglutide 2.4 mg once weekly improved liver and metabolic parameters, and was well tolerated, in patients with non-alcoholic steatohepatitis-related cirrhosis: a randomised, placebo-controlled phase 2 trial [oral presentation]. J Hepatol. 2022;77(Suppl 1):S10.LB001. doi: 10.1016/S0168-8278(22)00440-8
- Cusi K. Time to include nonalcoholic steatohepatitis in the management of patients with type 2 diabetes. Diabetes Care. 2020;43(2):275–279. doi: 10.2337/dci19-0064
- Gastaldelli A, Cusi K. From NASH to diabetes and from diabetes to NASH: mechanisms and treatment options. JHEP Rep. 2019;1(4):312–328. doi: 10.1016/j.jhepr.2019.07.002
- Pradhan R, Yin H, Yu O, et al. Glucagon-like peptide 1 receptor agonists and sodium–glucose cotransporter 2 inhibitors and risk of nonalcoholic fatty liver disease among patients with type 2 diabetes. Diabetes Care. 2022;45(4):819–829. doi: 10.2337/dc21-1953
- Genua I, Cusi K. Pharmacological approaches to NAFLD: current and future therapies. Diabetes Spectr. 2024;37(1):48–58. Epub ahead of print. doi: 10.2337/dsi23-0012
- Cusi K, Bril F, Barb D, et al. Effect of canagliflozin treatment on hepatic triglyceride content and glucose metabolism in patients with type 2 diabetes. Diab Obes Metab. 2019;21(4):812–821. doi: 10.1111/dom.13584
- Gastaldelli A, Cusi K, Landó LF, et al. Effect of tirzepatide versus insulin degludec on liver fat content and abdominal adipose tissue in people with type 2 diabetes (SURPASS-3 MRI): a substudy of the randomised, open-label, parallel-group, phase 3 SURPASS-3 trial. Lancet Diabetes Endocrinol. 2022;10(6):393–406. doi: 10.1016/S2213-8587(22)00070-5
- Younossi ZM, Ratziu V, Loomba R, et al. Obeticholic acid for the treatment of non-alcoholic steatohepatitis: interim analysis from a multicentre, randomised, placebo-controlled phase 3 trial. Lancet. 2019;394(10215):2184–2196. doi: 10.1016/S0140-6736(19)33041-7
- GlobalNewswire [Internet]. Intercept receives complete response letter from FDA for obeticholic acid as a treatment for pre-cirrhotic fibrosis due to NASH. 2023 [cited 2023 Aug 23]. Available from: https://www.globenewswire.com/news-release/2023/06/22/2693358/0/en/Intercept-Receives-Complete-Response-Letter-from-FDA-for-Obeticholic-Acid-as-a-Treatment-for-Pre-Cirrhotic-Fibrosis-due-to-NASH.html
- Esteban JPG, Asgharpour A. Evaluation of liver transplant candidates with non-alcoholic steatohepatitis. Transl Gastroenterol Hepatol. 2022;7:24. doi: 10.21037/tgh.2020.03.04
- Thapar M, Russo MW, Bonkovsky HL. Statins and liver injury. Gastroenterol Hepatol. 2013;9(9):605–606.
- Lazure P, Tomlinson JW, Kowdley KV, et al. Clinical practice gaps and challenges in non-alcoholic steatohepatitis care: an international physician needs assessment. Liver Int. 2022;42(8):1772‒1782. doi: 10.1111/liv.15324
- von dem Knesebeck O, Koens S, Marx G, et al. Perceptions of time constraints among primary care physicians in Germany. BMC Fam Pract. 2019;20:142. doi: 10.1186/s12875-019-1033-5
- Linzer M, Konrad TR, Douglas J, et al. Managed care, time pressure, and physician job satisfaction: results from the physician worklife study. J Gen Intern Med. 2000;15(7):441–450. doi: 10.1046/j.1525-1497.2000.05239.x
- Roser P, Bajaj SS, Stanford FC. International lack of equity in modern obesity therapy: the critical need for change in health policy. Int J Obes. 2022;46:1571–1572. doi: 10.1038/s41366-022-01176-2
- Gu Y, Zhou R, Kong T, et al. Barriers and enabling factors in weight management of patients with nonalcoholic fatty liver disease: a qualitative study using the COM-B model of behaviour. Health Expect. 2023;26(1):355‒365. doi: 10.1111/hex.13665
- Do A, Kuszewski EJ, Langberg KA, et al. Incorporating weight loss medications into hepatology practice for nonalcoholic steatohepatitis. Hepatology. 2019;70(4):1443‒1456. doi: 10.1002/hep.30658
- Lazarus JV, Kakalou C, Palayew A, et al. A Twitter discourse analysis of negative feelings and stigma related to NAFLD, NASH and obesity. Liver Int. 2021;41(10):2295‒2307. doi: 10.1111/liv.14969
- Carol M, Pérez-Guasch M, Solà E, et al. Stigmatization is common in patients with non-alcoholic fatty liver disease and correlates with quality of life. PLoS One. 2022;17(4):e0265153. doi: 10.1371/journal.pone.0265153
- Said A, Gagovic V, Malecki K, et al. Primary care practitioners survey of non-alcoholic fatty liver disease. Ann Hepatol. 2013;12(5):758‒765. doi: 10.1016/S1665-2681(19)31317-1
- Ratziu V, Cadranel J-F, Serfaty L, et al. A survey of patterns of practice and perception of NAFLD in a large sample of practicing gastroenterologists in France. J Hepatol. 2012;57(2):376‒383. doi: 10.1016/j.jhep.2012.03.019
- Ayada I, van Kleef LA, Zhang H, et al. Dissecting the multifaceted impact of statin use on fatty liver disease: a multidimensional study. EBioMedicine. 2023;87:104392. doi: 10.1016/j.ebiom.2022.104392
- Kothari S, Dhami-Shah H, Shah SR. Antidiabetic drugs and statins in nonalcoholic fatty liver disease. J Clin Exp Hepatol. 2019;9(6):723–730. doi: 10.1016/j.jceh.2019.06.003