ABSTRACT
Since its first use just over a century ago, insulin treatment has evolved dramatically, such that the molecules are physiologic in nature, and treatment can now closely resemble the natural hormone response over 24 hours. Newer, longer-acting basal insulin analogs have provided insulin therapies with improved characteristics and, therefore, ease of use, and can readily be incorporated as part of routine treatment for type 2 diabetes (T2D), but evidence suggests that insulin remains underused in people with T2D. We review the barriers to initiation of basal insulin and the education needed to address these barriers, and we provide practical pointers, supported by evidence, for primary care physicians and advanced practice providers to facilitate timely initiation of basal insulin in the people with T2D who will benefit from such treatment.
Plain Language Summary
Type 2 diabetes is a complex disease. It causes increased amounts of sugar in the blood, which can cause damage to the body. Medications are given to people with type 2 diabetes to keep their blood sugar at normal levels. Unfortunately, type 2 diabetes worsens over time, so regular adjustments to medications are needed to keep blood sugar levels controlled.
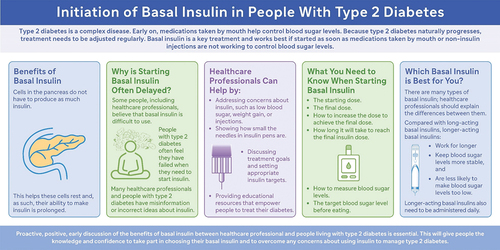
Basal insulin, which is a type of insulin that works over the entire day, is a key treatment for type 2 diabetes. It works best if it is started as soon as other medications (tablets or non-insulin injections) are not working to control blood sugar levels. Unfortunately, delays in starting basal insulin are common. Some healthcare professionals and people with type 2 diabetes believe insulin is difficult to use. False information on insulin is common; for example, some people with diabetes believe that their symptoms are caused by insulin treatment rather than high blood sugar.
This review summarizes key information to encourage effective conversations between healthcare professionals and people with type 2 diabetes about starting basal insulin. Proactive, positive, early discussion of the benefits of basal insulin can help to: 1) address concerns, 2) set appropriate, individual treatment targets, and 3) provide practical information and training to help with injecting insulin. This will give people living with type 2 diabetes the knowledge and confidence to take an active part in managing their diabetes and overcome any barriers to using basal insulin.
1. Introduction
The year 2021 marked the 100th year since the discovery of insulin, a treatment that would transform type 1 diabetes (T1D) from a severe illness into a manageable condition [Citation1]. Since its first therapeutic administration in 1922 [Citation2], insulin therapy has continued to evolve, with preparations becoming more physiologic over time [Citation3]. While it is well known that T1D is characterized by an autoimmune response that destroys pancreatic beta cells and mandates insulin replacement, about 90% of diabetes cases are type 2 diabetes (T2D) [Citation4], in which, over time, chronic hyperglycemia and elevated free fatty acids damage beta cells. As such, treatment for T2D often needs to be changed regularly to keep pace with the changing pathophysiology of the condition.
People with T2D may also have other comorbidities; the most prevalent coexisting chronic conditions are congestive heart failure, chronic obstructive pulmonary disease, and chronic kidney disease [Citation5], and it is well established that comorbidities affect treatment choice [Citation6]. The American Diabetes Association (ADA) Standards of Care in Diabetes 2024 guidelines [Citation7] recommend that individuals with T2D who have established atherosclerotic cardiovascular disease (CVD) or indicators of high cardiovascular risk, heart failure, or chronic kidney disease should receive a ‘sodium – glucose cotransporter 2 inhibitor (SGLT2i) and/or glucagon-like peptide 1 receptor agonists (GLP-1 RA) with demonstrated CVD benefit, independent of glycated hemoglobin A1C (A1C), independent of metformin use, and in consideration of person-specific factors.’ As such, GLP-1 RAs are often the first injectable therapy used for the treatment of T2D. An oral formulation of semaglutide is available [Citation8] and the recently approved dual glucagon-like peptide-1/glucose-dependent insulinotropic peptide receptor agonist, tirzepatide, significantly reduces glycemia and reduces body weight [Citation9]. However, T2D is a progressive and complex pathophysiological condition that affects multiple organs [Citation10], most notably a progressive decline in beta-cell function [Citation11], resulting in an inability to produce endogenous insulin. As such, medications do not ‘fail,’ and perhaps more importantly, neither do people with T2D; rather, as the disease progresses, different treatments are needed to address the increasing organ involvement, culminating in the requirement for insulin replacement therapy to counter declining beta-cell function. Indeed, results from several retrospective analyses suggest that when used over a prolonged period, there is a loss of glycemic control with some antihyperglycemic agents, and that persistence can be suboptimal. For example, the antihyperglycemic benefits of GLP-1 RA therapies have been reported to plateau after 1 year, being maintained at 4 years in only one-third of recipients [Citation12]. A United Kingdom study revealed GLP-1 RA discontinuation rates of 45.2% and 64.7% at 12 and 24 months, respectively [Citation13], with similar results (47.7% and 70.1%, respectively), with a median time to discontinuation of 13 months being reported in a United States study [Citation14].
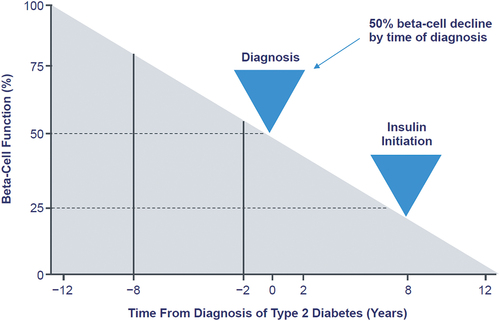
Reasons for GLP-1 RA discontinuation that were most frequently reported by physicians included inadequate blood glucose control (45.6%), nausea/vomiting (43.8%), and gastrointestinal side effects (36.8%), while the top reasons reported by those taking treatments were ‘Made me feel sick’ (64.4%) and ‘Made me throw up’ (45.4%) [Citation15]. For SGLT2i therapies, meta-analysis results suggest that efficacy is maintained over 24 months [Citation16], but other studies have shown loss of glycemic control over longer periods [Citation17,Citation18]. The suggestion that the decline over time in the efficacy of noninsulin agents occurs because of progressive beta-cell failure accords with estimations that for most oral agents to provide adequate glucose lowering, residual beta-cell function must be at least 15% to 20% [Citation19]. Further, when A1C increases, the contribution of fasting hyperglycemia to overall A1C increases [Citation20]. Estimates suggest that between 50% and 80% of beta-cell function has been lost by the time T2D is diagnosed [Citation21,Citation22], and modeling suggests beta-cell function has declined to the point that insulin replacement is necessary within 8 years () [Citation23]. It follows that approximately 20–30% of people with T2D eventually need insulin [Citation24,Citation25]. However, insulin therapy must be individualized, which is of particular importance for older adults with T2D [Citation26], and in addition must be appropriately initiated and intensified [Citation27]. A number of reports suggest insulin is underused [Citation28–30].
2. Objective
The introduction of first-generation (long-acting) basal insulin analogs in 2000 (insulin glargine 100 U/mL [Gla-100]; insulin detemir [IDet]), followed by second-generation (longer-acting) analogs (insulin glargine 300 U/mL [Gla-300]; insulin degludec [IDeg]) in 2015 [Citation31], means that there are now many options that can readily be integrated into the treatment armamentarium for T2D. The aim of this review is to provide the rationale and practical pointers, supported by evidence, for primary care physicians and advanced practice providers (jointly termed healthcare professionals [HCPs]) to facilitate timely initiation of basal insulin in people with T2D.
3. Why do we delay insulin therapy?
Despite the improvements in insulin preparations, a retrospective analysis of data from more than 80,000 people from the United Kingdom Clinical Practice Research Datalink (UK CPRD) [Citation32] published in 2013 revealed that the median time to intensification with insulin therapy after failure of oral therapy was approximately 7 years, regardless of A1C or number of oral antihyperglycemic therapies taken, with mean A1C at treatment intensification to insulin ranging from 9.4% to 9.8%.
3.1. Common barriers to initiation of basal insulin
Barriers to starting insulin therapy are multifactorial and can be specific to the person with T2D, the HCP, the healthcare system [Citation33], and/or the regimen itself. Many people have misconceptions or receive misinformation suggesting that prolonged use of insulin (rather than prolonged hyperglycemia) causes adverse outcomes [Citation34]. Psychological insulin resistance has been described by numerous groups [Citation35,Citation36] and ‘typically represents a complex of beliefs about the meaning of insulin therapy, poor self-efficacy concerning the skills needed for insulin therapy, and a lack of accurate information’ [Citation37], all of which can culminate in a reluctance to initiate insulin. Reports suggest that many HCPs wait to prescribe insulin until it is absolutely necessary, citing concerns over weight gain and hypoglycemia [Citation38]. In particular, a misplaced sense of having failed to control T2D with oral therapies can result in reluctance to start insulin. Perhaps one of the most concerning misconceptions, from both HCPs and people with T2D alike, is the belief that if noninsulin therapies are adhered to, insulin therapy can be delayed indefinitely. One survey showed that 40% of primary care physicians agreed or strongly agreed that most patients would not need to progress to insulin therapy if they followed physician recommendations, and 41% disagreed or strongly disagreed that most patients will eventually need to go on insulin regardless of how well they adhere to their treatment regimen [Citation39].
3.2. Cost as a barrier to initiation of insulin
Historically, cost and affordability have been significant barriers to initiation of insulin [Citation40]; however, with recent changes due to the Inflation Reduction Act in the United States, costs can be lowered and out-of-pocket costs capped for people aged ≥65 years enrolled in Medicare [Citation41]. Further, because of recent moves by manufacturers to significantly reduce the cost of insulin in the United States, improvements in insulin affordability are now set to be expanded to all people who require insulin, beginning in early 2024. It remains noteworthy that a real-world study confirming improved glucose control with the longer-acting basal insulin, Gla-300 versus long-acting basal insulins revealed that healthcare resource utilization, namely, all-cause and diabetes-related hospitalizations and all-cause, diabetes-related, and hypoglycemia-related emergency department visits, were all significantly lower for Gla-300 than for long-acting analogs [Citation42].
4. Spotlight on the benefits of timely insulin therapy
The two main advantages of the timely use of insulin are (1) the potent glucose-lowering efficacy provides beta-cell rest, and (2) preserved beta-cell function permits an insulin-sparing strategy that can lower the risk of hypoglycemia and weight gain by preventing the need for large insulin doses [Citation43]. The ORIGIN trial demonstrated that early use of insulin in people with impaired fasting glucose or impaired glucose response resulted in a 28% reduced risk of developing diabetes [Citation44]. Real-world data from the EARLY trial showed that initiating basal insulin in people with T2D uncontrolled on oral therapy at less versus more than 5 years since diagnosis was associated with a weight difference of −0.4 kg [Citation45], and also revealed that the insulin requirement was higher for individuals with higher body weight, longer duration of diabetes, higher baseline A1C, and microalbuminuria [Citation46]. In the LANMET trial, insulin was initiated at 9 years’ duration; although there was an A1C reduction of 2.4% (from 9.6% at baseline), participants experienced 5.4 hypoglycemic episodes per patient year and weight gain of 2.6 kg [Citation47]. Another study revealed that the increase in body weight associated with insulin therapy was highly dependent on baseline A1C and insulin dose [Citation48].
4.1. Insulin as initial therapy
When considering insulin as initial therapy for T2D, the ADA Standards of Care state that ‘it is common practice to initiate insulin therapy for patients who present with blood glucose levels ≥300 mg/dL (16.7 mmol/L) or A1C > 10% (86 mmol/mol) or if the individual has symptoms of hyperglycemia (i.e. polyuria or polydipsia) or evidence of catabolism (weight loss),’ adding that ‘as glucose toxicity resolves, simplifying the medication plan and/or changing to noninsulin agents is often possible.’ Newly added guidance states that in this population, initiation of insulin should be considered regardless of background glucose-lowering therapy or disease stage [Citation7]. The ADA/European Association for the Study of Diabetes (EASD) guidelines state that ‘in specific circumstances, insulin may be the preferred agent for glucose lowering, specifically in the setting of severe hyperglycemia (A1C > 10%), particularly when associated with weight loss or ketonuria/ketosis and with acute glycemic dysregulation (e.g. during hospitalization, surgery, or acute illness), in underweight people, or when the diagnosis of type 1 diabetes is suspected’ [Citation49].
4.2. Insulin in combination with other antihyperglycemic agents
In general, guidelines recommend that insulin should be added to existing antihyperglycemic therapy when glycemic targets are not met [Citation7,Citation41,Citation49–52]. Recognizing the balance of A1C control with safety, all guidelines recommend that A1C targets should be individualized according to patient characteristics, with the ADA noting that the guideline ‘proposes general targets appropriate for many people but emphasizes the importance of individualization based on key patient characteristics,’ and that ‘glycemic goals must be individualized in the context of shared decision-making to address individual needs and preferences and consider characteristics that influence risks and benefits of therapy’ [Citation50]. The 2024 ADA Standards of Care now state that ‘early combination therapy can be considered in adults with type 2 diabetes at treatment initiation to shorten time to attainment of individualized treatment goals’ [Citation7]. Although long considered the gold standard metric for glucose control, A1C can be affected by conditions such as iron deficiency [Citation53], hemoglobinopathies [Citation54], and pregnancy [Citation55]; and as such, values may not always truly reflect glycemic status, as mentioned in a new section of the 2024 ADA Standards of Care, entitled ‘Glycemic Assessment by A1C’ [Citation50]. The International Consensus on Use of Continuous Glucose Monitoring () recommends the use of time in range (TIR) as a key measure of short-term glycemic control [Citation56,Citation57]. A decrease in TIR has been shown to be associated with an increased risk for microvascular events [Citation58,Citation59], macrovascular events [Citation60], and mortality [Citation61]. The 2024 ADA Standards of Care state that glycemic status should be assessed ‘by A1C and/or appropriate continuous glucose monitoring (CGM) metrics at least two times a year and more frequently (e.g. every 3 months) for individuals not meeting treatment goals, with frequent or severe hypoglycemia or hyperglycemia, changing health status, or growth and development in youth’ [Citation50]. The SWITCH PRO study [Citation62] showed that IDeg U100 was associated with improved TIR compared with Gla-100 in people with T2D, and the InRange study [Citation63] has shown that TIR for IDeg U100 and Gla-300 are comparable in people with T1D.
Table 1. Recommendations for time in range [57].
4.3. Which individuals benefit from insulin therapy?
Another approach to assigning insulin therapy is to consider patient groups who would benefit from such treatment. The ‘Rationale for Timely Insulin Therapy in Type 2 Diabetes Within the Framework of Individualised Treatment: 2020 Update’ [Citation43] suggests that the following groups of people have ‘patient-centred indications of timely initiation of insulin treatment’: those with severe insulin-deficient diabetes; with severe autoimmune diabetes/latent autoimmune diabetes in adults with antibodies; with early manifestation of diabetes-related complications (retinopathy, nephropathy) and A1C out of target despite dual or triple therapy with oral agents/GLP-1 RAs; with A1C above target after 6 months of receiving dual and triple combination therapy; older adults with sarcopenia, cachexia, and chronic infections; and those with symptoms of ‘high sugar’ (weakness, infections, dermatological problems, erectile dysfunction, nocturia). In their review paper, Mehta and colleagues also provide practical advice on the initiation, titration, and switching of basal insulin therapy () [Citation64].
Figure 2. People with T2D who are candidates for basal insulin. HbA1c, glycated hemoglobin A1C; T2D, type 2 diabetes. ‘Practical guidance on the initiation, titration, and switching of basal insulins: a narrative review for primary care’ by Mehta R, Goldenberg R, Katselnik D, Kuritzky L. Ann Med. 2021;53(1):998–1009 [Citation64] is licensed under CC by 4.1.
![Figure 2. People with T2D who are candidates for basal insulin. HbA1c, glycated hemoglobin A1C; T2D, type 2 diabetes. ‘Practical guidance on the initiation, titration, and switching of basal insulins: a narrative review for primary care’ by Mehta R, Goldenberg R, Katselnik D, Kuritzky L. Ann Med. 2021;53(1):998–1009 [Citation64] is licensed under CC by 4.1.](/cms/asset/3e49a133-00a1-4e84-9305-a3f44f3b17fc/ipgm_a_2328511_f0002_b.gif)
5. Important topics to address when introducing basal insulin therapy
It is important to note that time spent introducing insulin early in the course of T2D in a positive manner can reduce delays in initiation as well as nonadherence and/or nonpersistence. An international study has shown that compared with those who were willing to start insulin therapy, people who were unwilling or ambivalent reported significantly more negative and fewer positive beliefs about starting insulin, more negative feelings about their current medications, and more diabetes-related distress [Citation65]. To accept the need for insulin therapy, people need to understand the pathophysiological defects associated with T2D, especially the unavoidable decline in beta-cell number and function.
5.1. Understanding patient preference
It is also important to assess patient preference and satisfaction, and to discuss these before starting basal insulin. It is well published that many people prefer oral to injectable therapy [Citation66,Citation67]; however, another study has shown that people using basal insulin report greater satisfaction than those using other medications [Citation68]. Age is an important factor; older people are a very heterogeneous group in terms of physical fitness, mental fitness, comorbidities, and social setting, so treatment must be tailored accordingly. Complexity and flexibility of treatment regimens also affect preferences, as can other treatments, overall health, and personal circumstances. The perceptions that HCPs have can sometimes be very different from actual patient preference [Citation69]; this disconnect needs to be rectified in order for shared decision-making to be successful.
5.2. Anticipating and addressing potential barriers to initiation of insulin
Before prescribing insulin, it is pertinent to anticipate potential barriers to initiation, for example fear of hypoglycemia, weight gain, or injections. This is best done by engaging with the patient and asking open-ended questions (see ). It may be helpful to use the insulin treatment appraisal scale (ITAS), which is a 20-statement questionnaire answered on a 5-point Likert scale [Citation70]. Ahead of the requirement for insulin therapy, it is important to provide education on home glucose/continuous glucose monitoring use and interpretation to empower people to interpret numbers, reach goals, recognize trends, and avoid hypoglycemia. Preconceived notions that insulin pen needles are the same size as those used for phlebotomy can be addressed through review of the injection pen, performing dummy injections, and doing the first injection in the office.
5.3. Providing education on key topics
While it is important to acknowledge that severe hypoglycemia [Citation71] and weight gain [Citation72] are significant harms, it should be emphasized that these risks are highest when insulin therapy is used incorrectly [Citation73]. The ADA Standards of Care recommend that ‘glucagon should be prescribed for all individuals taking insulin or at high risk for hypoglycemia. Family, caregivers, school personnel, and others providing support to these individuals should know its location and be educated on how to administer it’ [Citation50]. Education on hypoglycemia awareness is essential, as hypoglycemia unawareness is common, particularly as hypoglycemia symptoms can range vastly. Per the ADA, Level 1 hypoglycemia is typically defined as fasting plasma glucose (FPG) <70 mg/dL. While a definitive answer can be provided only by measuring blood glucose, if hypoglycemia symptoms are present, corrective treatment should not be delayed while waiting for a result. People with T2D need to know how to apply the ‘15–15 Rule,’ which simply means consuming 15 grams of carbohydrate to raise blood glucose and checking after 15 minutes, repeating if blood glucose remains below 70 mg/dL. Providing information on good carbohydrate sources, (such as glucose tablets or a gel tube [per label]; 4 ounces [1/2 cup] of juice or 6 oz. of regular [not diet] soda; 1 tablespoon of sugar or corn syrup, 1 teaspoon of honey or 2–3 hard candies, jellybeans, or gumdrops) is important; it is also imperative to advise that carbohydrates high in fiber, such as beans or lentils, or those that contain fat, such as chocolate, should be avoided when treating hypoglycemia because fiber and fat slow down absorption of sugar. The 2024 ADA Standards of Care state that ‘all individuals taking insulin or at risk for hypoglycemia should receive structured education for hypoglycemia prevention and treatment, with ongoing education for those who experience hypoglycemic events,’ adding that ‘one or more episodes of Level 2 or 3 hypoglycemia should prompt reevaluation of the treatment plan, including deintensifying or switching diabetes medications if appropriate.’ It also advises that use of CGM is beneficial and recommended for individuals at high risk for hypoglycemia [Citation50].
5.4. Other considerations
Insulin is rarely contraindicated, but because insulin preparations contain cresol, insulin should not be administered to individuals with cresol sensitivity. Caution should be taken when administering insulin to individuals experiencing vomiting and diarrhea because insulin could exacerbate hypokalemia; as such, when initiating insulin in people who are also taking therapies such as diuretics that also cause hypokalemia, potassium levels should be monitored. Concomitant antihyperglycemic therapies also require assessment. For people who are at risk of hypoglycemia and/or weight gain, basal insulin can be successfully used alongside metformin [Citation45], GLP-1 RAs [Citation74], or SGLT2 inhibitors [Citation75].
6. Preparing for long-term success: effective titration
Effective titration is key for the long-term successful use of basal insulin. Hypoglycemia risk is often highest during the titration phase. A retrospective study of over 55,000 insulin-naive people with T2D revealed that approximately 4.5% experienced hypoglycemia during the first month after initiation of basal insulin therapy [Citation76]. Furthermore, people who experienced hypoglycemia within 6 months were more likely to discontinue insulin within the first 12 months [Citation76]. As such, ensuring that there are well understood plans for titration of insulin and prevention of hypoglycemia is key to long-term treatment success.
6.1. Initial dose
Many people with T2D do not understand the connection between FPG and insulin dosing; therefore, clearly defining the starting dose, target FPG, expected final dose, and the expected time to reach this dose can help immensely. Basal insulin is routinely started at a dose of 10 U/day or 0.1–0.2 U/kg/day [Citation7,Citation64], but can be increased to 15 U/day for people who are overweight and/or have very high A1C. Target FPG is typically 120 or 130 mg/dL, although for older adults a less stringent goal of 130 or 140 mg/dL is often employed. Recommended starting doses vary for different insulins and individual product labels should be consulted.
6.2. Titration algorithms
It is important to educate that different titration approaches (HCP-led or self-titration) can be used depending on preference and ability. Standardized treat-to-target titration algorithms provide an easy approach. One such algorithm () was developed based on the AT.LANTUS trial [Citation77]. If people find algorithms difficult to adhere to, simplified algorithms or technology-assisted dose adjustments can be used. A simple approach is to increase dose by 1 U per day or by 2–4 U once or twice per week until FPG levels are within the target range or the dose exceeds 0.5 U/kg/day.
Table 2. AT.LANTUS treat-to-target algorithm for basal insulin titration [Citation77].
6.3. Adjustment of other antihyperglycemic medications
When starting basal insulin, doses of other antihyperglycemic medications may need to be adjusted as the insulin dose is increased; metformin, GLP-1 RAs, and SGLT2 inhibitors are usually continued, and sulfonylurea therapies are generally stopped to reduce hypoglycemia risk. The 2024 ADA Standards of Care specifically state that to minimize the risk of hypoglycemia and treatment burden when starting insulin therapy, ‘the need for and/or dose of glucose-lowering agents with higher hypoglycemia risk (i.e. sulfonylureas and meglitinides)’ should be reassessed [Citation7]. Consideration needs to be given to the possibility that discontinuing sulfonylureas may require an increase in basal insulin dose. Thiazolidinediones are generally stopped in people with heart failure because of the increased risk of edema in these individuals.
6.4. Avoiding overbasalization
After the successful initiation of basal insulin, HCPs need to be aware of overbasalization. For example, if the basal insulin dose exceeds 0.5 U/kg/day, if the bedtime–morning glucose differential is high (≥50 mg/dL), if glucose variability is high, or if hypoglycemia occurs, therapy should be reevaluated, and the use of additional agents to manage post-prandial glucose excursions considered [Citation7]. The concept of overbasalization should be explained in a simple and clear manner to people with T2D, paying particular attention to the relationship between FPG and required dose.
7. Which basal insulin should we choose?
It is important to be able to explain the differences between available basal insulin analogs. Other than neutral protamine hagedorn insulin (NPH; brand names: Humulin®, Novolin®), which is an intermediate-acting insulin administered once or twice daily, all other basal insulin analogs can be classed as long-acting (first-generation basal insulin analogs; insulin glargine 100 U/mL [Gla-100]/Lantus®; insulin determir [IDet]/Levemir®) or longer-acting (second-generation basal insulin analogs; insulin glargine 300 U/mL [Gla-300]/Toujeo®; insulin degludec [IDeg] U100/U200/Tresiba®). The choice of long-acting analogs has been increased by the recent approval of a number of Lantus biosimilars (Semglee®, Basaglar®, Rezvoglar®). However, it is important to note that biosimilars are not interchangeable with the brand product or with each other unless comparative studies have been conducted and interchangeable status granted.
7.1. Differences between long-acting and longer-acting basal insulin analogs
Studies have shown that the longer-acting basal insulin analogs have a longer duration of action (>24 versus ≤24 h) and more stable glucose profiles with less variability daily and between days than long-acting analogs [Citation78,Citation79], which means that the risk of hypoglycemia is much lower compared with long-acting analogs; however, they do have to be administered daily [Citation31]. Two of the longer-acting analogs — Gla-300 and IDeg U200—deliver the same dose of insulin in a smaller volume than Gla-100 or IDeg U100, with Gla-300 being delivered in a third of the volume of Gla-100 and IDeg U200 in half the volume of IDeg U100 [Citation80,Citation81]. Additionally, because of their use with high-capacity insulin pens that can deliver up to 160 U per injection, longer-acting analogs offer the potential to simplify delivery of insulin for individuals who require doses >80 U. However, the results of a recently published survey-based study have revealed underuse of high-capacity insulin pens, particularly in primary care [Citation82].
Further advances have culminated in the development of once-weekly insulin icodec, although it has not yet received regulatory approval. A phase 2 study showed comparable efficacy with Gla-100, although incidence of Level 1 hypoglycemia (blood glucose <70 mg/dL) was numerically higher for insulin icodec than for Gla-100 (53.6% versus 37.7%, respectively), as was clinically significant or severe hypoglycemia (blood glucose <54 mg/dL: 16.0% versus 9.8%, respectively) [Citation83]. The ONWARDS 4 phase 3a study compared insulin icodec with Gla-100 in people on a basal–bolus regimen. Improvements in glycemic control were similar between insulin icodec and Gla-100, as were combined rates of Level 2 (<54 mg/dL) and Level 3 hypoglycemia (hypoglycemia with severe cognitive impairment requiring external assistance for recovery) [Citation84]. Switching from a previous basal insulin to either insulin icodec or IDeg was compared in the ONWARDS 2 phase 3a study. The results indicated a superiority in A1C reduction after 26 weeks for insulin icodec versus IDeg (estimated treatment difference of − 0·22% [95% CI: −0·37 to − 0·08]; p = 0.0028), although this was associated with modest weight gain for insulin icodec versus IDeg (+1·40 kg versus − 0·30 kg, respectively), and numerically but not statistically significantly higher rates of Level 2 or Level 3 hypoglycemia (0.73 versus 0.27 events per person per year, respectively; estimated rate ratio 1.93 [95% CI: 0.93 to 4.02]) [Citation85].
8. Insulin initiation with one eye on the patient and the other on the clock: new ways of working
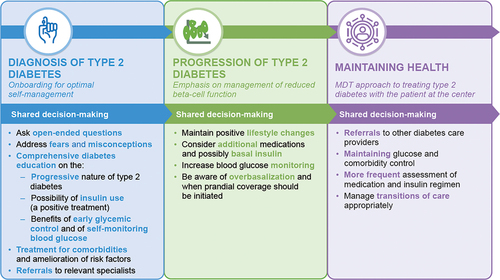
Given the daily time pressures, initiation of insulin therapy can feel like an overwhelming task for HCPs. However, timely planning and introducing the concept of insulin as a key treatment in advance of the point at which it becomes a necessity can facilitate timely initiation, and time spent early in the course of T2D improves outcomes and therefore reduces the demands of future care [Citation86]. Key pointers for education and priorities according to the stage of T2D are outlined in .
8.1. Benefits of shared decision-making
It is well known that people who play an active role in their healthcare have improved outcomes versus those who do not [Citation87]. The benefits of adopting a shared decision-making approach between people with T2D and HCPs are well reported [Citation88,Citation89], and such an approach is particularly useful with conditions such as T2D, for which treatment is associated with significant demands on daily life [Citation90]. However, HCPs often underestimate the emotional impact of living with T2D; although depression is prevalent in people with T2D, diabetes distress differs from depression or anxiety [Citation91]. The 2024 ADA Standards of Care guidelines recommend that both those with diabetes, as well as caregivers and family members are screened for diabetes distress at least annually, with more frequent monitoring being considered when treatment targets are not met, at transitional times, and/or in the presence of diabetes complications [Citation92].
Management should therefore not be considered the sole responsibility of the primary care physician or advanced practice provider, and help should be sought from the wider healthcare team. Recognizing that the provision of ‘patient-centered care’ can be challenging, allowing the patient to determine when they are ready to start insulin without asserting the dangers of delaying treatment, being cognizant of the benefits of early conversation about insulin, and introducing insulin in a positive manner (highlighting that it is a requirement of the natural progression of T2D rather than a failure on the part of medication or the person with T2D) can all help with acceptance of the need for insulin therapy and empower the well-informed patient to effectively manage their T2D.
8.2. Use of diabetes self-management education and support
Studies have shown that receiving diabetes education was the most important determinant for treatment satisfaction [Citation93], that a higher proportion of people who received diabetes education interventions achieved A1C targets compared with those who did not [Citation94], and also, that people who receive diabetes self-management education and support (DSMES) are more likely to receive care in accordance with guidelines, to adhere to treatment, and to have lower costs [Citation95]. However, despite this, referral rates are low [Citation96]. An algorithm for the identification and referral of patients with T2D to DSMES programs is provided by the “Consensus Report of the American Diabetes Association, the Association of Diabetes Care & Education Specialists, the Academy of Nutrition and Dietetics, the American Academy of Family Physicians, the American Academy of PAs, the American Association of Nurse Practitioners, and the American Pharmacists Association” [Citation97]. Briefly, the algorithm has five guiding principles: (1) patient engagement: care that reflects person’s life, preferences, priorities, culture, experiences, and capacity; (2) information sharing to determine what the patient needs to make decisions about daily self-management; (3) psychosocial and behavioral support, e.g. for diabetes-related distress and depression; (4) integration with other therapies (e.g. referrals for behavioral therapy, medication management, and physical therapy); and (5) coordinated care to ensure collaborative care to achieve treatment goals. The algorithm states that there are four critical times at which assessment for DSMES referral should be made: (1) at diagnosis; (2) annually for assessment of needs; (3) when new complicating factors affect self-management; and (4) during transitions of care. Of course, for many patients, referral to DSMES may be indicated at other times. The 2024 ADA Standards of Care quotes this consensus report, but states that there are five critical times to evaluate the need for DSMES: ‘at diagnosis, when not meeting treatment goals, annually, when complicating factors develop, and when transitions in life and care occur’ [Citation92]. For T2D to be successfully managed, it is imperative that referrals are made when needed.
9. Conclusions
The efficacy, safety, convenience, and flexibility of longer-acting basal insulins for use in combination with most other classes of antihyperglycemic agents make them suitable for the majority of people with T2D who require restoration of glycemic control. Proactive, positive, and early discussion of the benefits of basal insulin with patients, acknowledgment of the barriers and fears that may be present, and use of a shared decision-making approach involving support from the wider healthcare team can ensure that primary care physicians and advance practice prescribers are able to provide timely initiation of basal insulin therapy and optimize glycemic control for people living with T2D.
Abbreviations
| A1C | = | glycated hemoglobin A1C |
| ADA | = | American Diabetes Association |
| CVD | = | cardiovascular disease |
| DSMES | = | diabetes self-management education and support |
| EASD | = | European Association for the Study of Diabetes |
| FPG | = | fasting plasma glucose |
| GLP-1 RA | = | glucagon-like peptide 1 receptor agonists |
| HCP | = | healthcare professional |
| ITAS | = | insulin treatment appraisal scale |
| MDT | = | multidisciplinary team |
| SGLT2 | = | sodium–glucose cotransporter 2 |
| TAR | = | time above range |
| TBR | = | time below range |
| TIR | = | time in range |
| T1D | = | type 1 diabetes |
| T2D | = | type 2 diabetes |
| UK CPRD | = | United Kingdom Clinical Practice Research Datalink |
Declaration of financial/other relationships
J Freeman has participated in speakers’ bureau for Bayer Corporation, MannKind, Novo Nordisk, and Sanofi. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed. Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author contributions
Susan Renda and Jeffrey Freeman made substantial contributions to conception and design of this manuscript, have revised it critically for important intellectual content, have given final approval of the version to be published, and have agreed to be accountable for all aspects of the work.
Data sharing statement
Data sharing is not applicable to this article as no new data were created or analyzed.
Acknowledgments
The authors thank Charlotte Singh (Sanofi) for her critical review of this manuscript. Medical writing support was provided by Helen Jones of Envision Pharma Group.
Correction Statement
This article has been corrected with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
References
- Mazur A. Why were “starvation diets” promoted for diabetes in the pre-insulin period? Nutr J. 2011;10(1):23. doi: 10.1186/1475-2891-10-23
- Vecchio I, Tornali C, Bragazzi NL, et al. The discovery of insulin: an important milestone in the history of medicine. Front Endocrinol. 2018;9:613. doi: 10.3389/fendo.2018.00613
- Hirsch IB, Juneja R, Beals JM, et al. The evolution of insulin and how it informs therapy and treatment choices. Endocr Rev. 2020;41(5):733–755. doi: 10.1210/endrev/bnaa015
- International Diabetes Federation. Type 2 diabetes [updated 2023; cited 2022 Nov 25]. Available from: https://www.idf.org/aboutdiabetes/type-2-diabetes.html
- Clements JM, Rosca M, Cavallin C, et al. Type 2 diabetes and chronic conditions disparities in Medicare Beneficiaries in the state of Michigan. Am J Med Sci. 2020;359(4):218–225. doi: 10.1016/j.amjms.2020.01.013
- Aktas G, Atak Tel BM, Tel R, et al. Treatment of type 2 diabetes patients with heart conditions. Expert Rev Endocrinol Metab. 2023;18(3):255–265. doi: 10.1080/17446651.2023.2204941
- American Diabetes Association Professional Practice Committee. 9. Pharmacologic approaches to glycemic treatment: standards of care in diabetes-2024. Diabetes Care. 2024;47(Suppl 1):S158–S178. doi: 10.2337/dc24-S009
- Andersen A, Knop FK, Vilsboll T. A pharmacological and clinical overview of oral semaglutide for the treatment of type 2 diabetes. Drugs. 2021;81(9):1003–1030. doi: 10.1007/s40265-021-01499-w
- Chavda VP, Ajabiya J, Teli D, et al. Tirzepatide, a new era of dual-targeted treatment for diabetes and obesity: a mini-review. Molecules. 2022;27(13):4315. doi: 10.3390/molecules27134315
- Defronzo RBL. From the triumvirate to the ominous octet: a new paradigm for the treatment of type 2 diabetes mellitus. Diabetes. 2009;58(4):773–795. doi: 10.2337/db09-9028
- Duckworth WC, Abraira C, Moritz TE, et al. The duration of diabetes affects the response to intensive glucose control in type 2 subjects: the VA diabetes trial. J Diabetes Complications. 2011;25(6):355–361. doi: 10.1016/j.jdiacomp.2011.10.003
- Hemmer A, Maiter D, Buysschaert M, et al. Long-term effects of GLP-1 receptor agonists in type 2 diabetic patients: a retrospective real-life study in 131 patients. Diabetes Metab Syndr. 2019;13(1):332–336. doi: 10.1016/j.dsx.2018.09.007
- Weiss T, Yang L, Carr RD, et al. Real-world weight change, adherence, and discontinuation among patients with type 2 diabetes initiating glucagon-like peptide-1 receptor agonists in the UK. BMJ Open Diabetes Res Care. 2022;10(1):e002517. doi: 10.1136/bmjdrc-2021-002517
- Weiss T, Carr RD, Pal S, et al. Real-world adherence and discontinuation of glucagon-like peptide-1 receptor agonists therapy in type 2 diabetes mellitus patients in the United States. Patient Prefer Adherence. 2020;14:2337–2345. doi: 10.2147/PPA.S277676
- Sikirica MV, Martin AA, Wood R, et al. Reasons for discontinuation of GLP1 receptor agonists: data from a real-world cross-sectional survey of physicians and their patients with type 2 diabetes. Diabetes Metab Syndr Obes. 2017;10:403–412. doi: 10.2147/DMSO.S141235
- Monami M, Liistro F, Scatena A, et al. Short and medium-term efficacy of sodium glucose co-transporter-2 (SGLT-2) inhibitors: a meta-analysis of randomized clinical trials. Diabetes Obes Metab. 2018;20(5):1213–1222. doi: 10.1111/dom.13221
- Frías JP, Maaske J, Suchower L, et al. Long-term effects of dapagliflozin plus saxagliptin versus glimepiride on a background of metformin in patients with type 2 diabetes: results of a 104-week extension to a 52-week randomized, phase 3 study and liver fat MRI substudy. Diabetes Obes Metab. 2022;24(1):61–71. doi: 10.1111/dom.14548
- Dagogo-Jack S, Pratley RE, Cherney DZI, et al. Glycemic efficacy and safety of the SGLT2 inhibitor ertugliflozin in patients with type 2 diabetes and stage 3 chronic kidney disease: an analysis from the VERTIS CV randomized trial. BMJ Open Diabetes Res Care. 2021;9(1):e002484. doi: 10.1136/bmjdrc-2021-002484
- Berard L, Antonishyn N, Arcudi K, et al. Insulin matters: a practical approach to basal insulin management in type 2 diabetes. Diabetes Ther. 2018;9(2):501–519. doi: 10.1007/s13300-018-0375-7
- Monnier L, Lapinski H, Colette C. Contributions of fasting and postprandial plasma glucose increments to the overall diurnal hyperglycemia of type 2 diabetic patients: variations with increasing levels of HbA(1c). Diabetes Care. 2003;26(3):881–885. doi: 10.2337/diacare.26.3.881
- Saisho Y. Obesity, type 2 diabetes and beta cell failure: an Asian perspective. J Mol Genet Med. 2014;S1:008. https://www.hilarispublisher.com/open-access/obesity-type-diabetes-and-beta-cell-failure-an-asian-perspective-1747-0862.S1-008.pdf
- Cersosimo E, Solis-Herrera C, Trautmann ME, et al. Assessment of pancreatic beta-cell function: review of methods and clinical applications. Curr Diabetes Rev. 2014;10(1):2–42. doi: 10.2174/1573399810666140214093600
- Lebovitz HE. Chapter 41: management of hyperglycemia with oral antihyperglycemic agents in type 2 diabetes. In: Kahn R, Weir GC King GL, et al. eds. Joslin’s diabetes mellitus, 14th ed., p. 687–710. Philadelphia (PA): Lippincott Williams & Wilkins; 2005.
- Garg SK, Rewers AH, Akturk HK Ever-increasing insulin-requiring patients globally. Diabetes Technol Ther. 2018;20(S2):S21–S24. doi: 10.1089/dia.2018.0101
- Lipska KJ, Yao X, Herrin J, et al. Trends in drug utilization, glycemic control, and rates of severe hypoglycemia, 2006-2013. Diabetes Care. 2017;40(4):468–475. doi: 10.2337/dc16-0985
- American Diabetes Association Professional Practice Committee 13. Older adults: standards of care in diabetes-2024. Diabetes Care. 2024;47(Suppl 1):S244–S257. doi: 10.2337/dc24-S013
- Wallia A, Molitch ME. Insulin therapy for type 2 diabetes mellitus. JAMA. 2014;311(22):2315–2325. doi: 10.1001/jama.2014.5951
- Sorli C, Heile MK. Identifying and meeting the challenges of insulin therapy in type 2 diabetes. J Multidiscip Healthc. 2014;7:267–282. doi: 10.2147/JMDH.S64084
- Peyrot M, Rubin RR, Kruger DF, et al. Correlates of insulin injection omission. Diabetes Care. 2010;33(2):240–245. doi: 10.2337/dc09-1348
- Karter AJ, Subramanian U, Saha C, et al. Barriers to insulin initiation: the translating research into action for diabetes insulin starts project. Diabetes Care. 2010;33(4):733–735. doi: 10.2337/dc09-1184
- Cheng AYY, Patel DK, Reid TS, et al. Differentiating basal insulin preparations: understanding how they work explains why they are different. Adv Ther. 2019;36(5):1018–1030. doi: 10.1007/s12325-019-00925-6
- Khunti K, Wolden ML, Thorsted BL, et al. Clinical inertia in people with type 2 diabetes: a retrospective cohort study of more than 80,000 people. Diabetes Care. 2013;36(11):3411–3417. doi: 10.2337/dc13-0331
- Okemah J, Peng J, Quiñones M. Addressing clinical inertia in type 2 diabetes mellitus: a review. Adv Ther. 2018;35(11):1735–1745. doi: 10.1007/s12325-018-0819-5
- Patil R, Nasrin AN, Datta SS, et al. Popular misconceptions regarding the diabetes management: where should we focus our attention? J Clin Diagn Res. 2013;7(2):287–291. doi: 10.7860/JCDR/2013/4416.2749
- Brunton SA, White JR Jr., Renda SM. The role of basal insulin in type 2 diabetes management. J Fam Pract. 2005;Suppl:S1–8.
- Davis SN, Renda SM Psychological insulin resistance: overcoming barriers to starting insulin therapy. Diabetes Educ. 2006;32(Suppl 4):146S–152S. doi: 10.1177/0145721706289S226
- Polonsky WH, Fisher L, Guzman S, et al. Psychological insulin resistance in patients with type 2 diabetes: the scope of the problem. Diabetes Care. 2005;28(10):2543–2545. doi: 10.2337/diacare.28.10.2543
- Bin Rsheed A, Chenoweth I Barriers that practitioners face when initiating insulin therapy in general practice settings and how they can be overcome. World J Diabetes. 2017;8(1):28–39. doi: 10.4239/wjd.v8.i1.28
- Hayes RP, Fitzgerald JT, Jacober SJ. Primary care physician beliefs about insulin initiation in patients with type 2 diabetes. Int J Clin Pract. 2008;62(6):860–868. doi: 10.1111/j.1742-1241.2008.01742.x
- Endocrine Society. Addressing insulin access and affordability: an Endocrine Society position statement. J Clin Endocrinol Metab. 2021;106(4):935–941. doi: 10.1210/clinem/dgaa817
- Office of the Assistant Secretary for Planning and Evaluation, U.S. Department of Health and Human Services. Report to Congress on the affordability of insulin 2022 Dec 16. [cited 2022 Dec 22]. Available from: https://aspe.hhs.gov/reports/insulin-affordability-rtc
- Wright EE Jr., Malone DC, Trujillo JM, et al. Real-world persistence, adherence, health care resource utilization, and costs in people with type 2 diabetes switching from a first-generation basal insulin to a second-generation (insulin glargine 300 U/mL) vs an alternative first-generation basal insulin. J Manag Care Spec Pharm. 2022;28(6):592–603. doi: 10.18553/jmcp.2022.21436
- Hanefeld M, Fleischmann H, Siegmund T, et al. Rationale for timely insulin therapy in type 2 diabetes within the framework of individualised treatment: 2020 update. Diabetes Ther. 2020;11(8):1645–1666. doi: 10.1007/s13300-020-00855-5
- The ORIGIN Trial Investigators. Basal insulin and cardiovascular and other outcomes in dysglycemia. N Engl J Med. 2012;367(4):319–328. doi: 10.1056/NEJMoa1203858
- Hanefeld M, Fleischmann H, Landgraf W, et al. EARLY study: early basal insulin therapy under real-life conditions in type 2 diabetics. Diabetes, Stoffwechsel und Herz. 2012;21(2):91–97.
- Hanefeld M, Fleischmann H, Schiffhorst G, et al. Predictors of response to early basal insulin treatment in patients with type 2 diabetes—the EARLY experience. Diabetes Technol Ther. 2014;16(4):241–246. doi: 10.1089/dia.2013.0246
- Yki-Järvinen H, Kauppinen-Mäkelin R, Tiikkainen M, et al. Insulin glargine or NPH combined with metformin in type 2 diabetes: the LANMET study. Diabetologia. 2006;49(3):442–451. doi: 10.1007/s00125-005-0132-0
- Balkau B, Home PD, Vincent M, et al. Factors associated with weight gain in people with type 2 diabetes starting on insulin. Diabetes Care. 2014;37(8):2108–2113. doi: 10.2337/dc13-3010
- Davies MJ, Aroda VR, Collins BS, et al. Management of hyperglycemia in type 2 diabetes, 2022. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2022;45(11):2753–2786. doi: 10.2337/dci22-0034
- American Diabetes Association Professional Practice Committee 6. Glycemic goals and hypoglycemia: standards of care in diabetes-2024. Diabetes Care. 2024;47(Suppl 1):S111–S125. doi: 10.2337/dc24-S006
- Blonde L, Umpierrez GE, Reddy SS, et al. American Association of Clinical Endocrinology clinical practice guideline: developing a diabetes mellitus comprehensive care plan—2022 update. Endocr Pract. 2022;28(10):923–1049. doi: 10.1016/j.eprac.2022.08.002
- Qaseem A, Wilt TJ, Kansagara D, et al. Hemoglobin A1c targets for glycemic control with pharmacologic therapy for nonpregnant adults with type 2 diabetes mellitus: a guidance statement update from the American College of Physicians. Ann Intern Med. 2018;168(8):569–576. doi: 10.7326/M17-0939
- Ford ES, Cowie CC, Li C, et al. Iron-deficiency anemia, non-iron-deficiency anemia and HbA1c among adults in the US. J Diabetes. 2011;3(1):67–73. doi: 10.1111/j.1753-0407.2010.00100.x
- Bry L, Chen PC, Sacks DB. Effects of hemoglobin variants and chemically modified derivatives on assays for glycohemoglobin. Clin Chem. 2001;47(2):153–163. doi: 10.1093/clinchem/47.2.153
- Nielsen LR, Ekbom P, Damm P, et al. HbA1c levels are significantly lower in early and late pregnancy. Diabetes Care. 2004;27(5):1200–1201. doi: 10.2337/diacare.27.5.1200
- Danne T, Nimri R, Battelino T, et al. International consensus on use of continuous glucose monitoring. Diabetes Care. 2017;40(12):1631–1640. doi: 10.2337/dc17-1600
- Battelino T, Danne T, Bergenstal RM, et al. Clinical targets for continuous glucose monitoring data interpretation: recommendations from the international consensus on time in range. Diabetes Care. 2019;42(8):1593–1603. doi: 10.2337/dci19-0028
- Beck RW, Bergenstal RM, Riddlesworth TD, et al. Validation of time in range as an outcome measure for diabetes clinical trials. Diabetes Care. 2019;42(3):400–405. doi: 10.2337/dc18-1444
- Mayeda L, Katz R, Ahmad I, et al. Glucose time in range and peripheral neuropathy in type 2 diabetes mellitus and chronic kidney disease. BMJ Open Diabetes Res Care. 2020;8(1):e000991. doi: 10.1136/bmjdrc-2019-000991
- Lu J, Ma X, Shen Y, et al. Time in range is associated with carotid intima-media thickness in type 2 diabetes. Diabetes Technol Ther. 2020;22(2):72–78. doi: 10.1089/dia.2019.0251
- Lu J, Wang C, Shen Y, et al. Time in range in relation to all-cause and cardiovascular mortality in patients with type 2 diabetes: a prospective cohort study. Diabetes Care. 2021;44(2):549–555. doi: 10.2337/dc20-1862
- Goldenberg RM, Aroda VR, Billings LK, et al. Effect of insulin degludec versus insulin glargine U100 on time in range: SWITCH PRO, a crossover study of basal insulin-treated adults with type 2 diabetes and risk factors for hypoglycaemia. Diabetes Obes Metab. 2021;23(11):2572–2581. doi: 10.1111/dom.14504
- Battelino T, Danne T, Edelman SV, et al. Continuous glucose monitoring-based time-in-range using insulin glargine 300 units/ml versus insulin degludec 100 units/ml in type 1 diabetes: the head-to-head randomized controlled InRange trial. Diabetes Obes Metab. 2023;25(2):545–555. doi: 10.1111/dom.14898
- Mehta R, Goldenberg R, Katselnik D, et al. Practical guidance on the initiation, titration, and switching of basal insulins: a narrative review for primary care. Ann Med. 2021;53(1):998–1010. 10.1080/07853890.2021.1925148
- Polonsky WH, Hajos TR, Dain MP, et al. Are patients with type 2 diabetes reluctant to start insulin therapy? An examination of the scope and underpinnings of psychological insulin resistance in a large, international population. Curr Med Res Opin. 2011;27(6):1169–1174. doi: 10.1185/03007995.2011.573623
- Khan H, Coyle F, Chowdhury T. Patients’ preference for subsequent therapy following secondary failure of metformin and sulphonylurea. Pract Diab Int. 2009;26(7):282–284. doi: 10.1002/pdi.1400
- DiBonaventura MD, Wagner JS, Girman CJ, et al. Multinational Internet-based survey of patient preference for newer oral or injectable type 2 diabetes medication. Patient Prefer Adherence. 2010;4:397–406. doi: 10.2147/PPA.S14477
- Polonsky W, Traylor L, Wei W, et al. More satisfied, but why? A pooled patient-level analysis of treatment satisfaction following the initiation of insulin glargine vs. comparators in insulin-naïve patients with type 2 diabetes mellitus. Diabetes Obes Metab. 2014;16(3):255–261. doi: 10.1111/dom.12214
- Mühlbacher AC, Juhnke C. Patient preferences versus physicians’ judgement: does it make a difference in healthcare decision making? Appl Health Econ Health Policy. 2013;11(3):163–180. doi: 10.1007/s40258-013-0023-3
- Snoek FJ, Skovlund SE, Pouwer F. Development and validation of the insulin treatment appraisal scale (ITAS) in patients with type 2 diabetes. Health Qual Life Outcomes. 2007;5(1):69. doi: 10.1186/1477-7525-5-69
- Lee AK, Warren B, Lee CJ, et al. The association of severe hypoglycemia with incident cardiovascular events and mortality in adults with type 2 diabetes. Diabetes Care. 2018;41(1):104–111. doi: 10.2337/dc17-1669
- Brown A, Guess N, Dornhorst A, et al. Insulin-associated weight gain in obese type 2 diabetes mellitus patients: what can be done? Diabetes Obes Metab. 2017;19(12):1655–1668. doi: 10.1111/dom.13009
- Trief PM, Cibula D, Rodriguez E, et al. Incorrect insulin administration: a problem that warrants attention. Clin Diabetes. 2016;34(1):25–33. doi: 10.2337/diaclin.34.1.25
- Kenny C, Hall G GLP-1 receptor agonist and basal insulin co-therapy in type 2 diabetes: clinical evidence and practicalities of use. Diabetes Prim Care 2015;17:80–85.
- Yang Y, Chen S, Pan H, et al. Safety and efficiency of SGLT2 inhibitor combining with insulin in subjects with diabetes: systematic review and meta-analysis of randomized controlled trials. Medicine (Baltimore). 2017;96(21):e6944. doi: 10.1097/MD.0000000000006944
- Dalal MR, Kazemi M, Ye F, et al. Hypoglycemia after initiation of basal insulin in patients with type 2 diabetes in the United States: implications for treatment discontinuation and healthcare costs and utilization. Adv Ther. 2017;34(9):2083–2092. doi: 10.1007/s12325-017-0592-x
- Davies M, Storms F, Shutler S, et al. Improvement of glycemic control in subjects with poorly controlled type 2 diabetes: comparison of two treatment algorithms using insulin glargine. Diabetes Care. 2005;28(6):1282–1288. doi: 10.2337/diacare.28.6.1282
- Becker RH, Dahmen R, Bergmann K, et al. New insulin glargine 300 units·mL−1 provides a more even activity profile and prolonged glycemic control at steady state compared with insulin glargine 100 units·mL−1. Diabetes Care. 2015;38(4):637–643. doi: 10.2337/dc14-0006
- Yang Y, Long C, Li T, et al. Insulin degludec versus insulin glargine on glycemic variability in diabetic patients: a systematic review and meta-analysis of randomized controlled trials. Front Endocrinol. 2022;13:890090. doi: 10.3389/fendo.2022.890090
- Goldman J, Kapitza C, Pettus J, et al. Understanding how pharmacokinetic and pharmacodynamic differences of basal analog insulins influence clinical practice. Curr Med Res Opin. 2017;33(10):1821–1831. doi: 10.1080/03007995.2017.1335192
- Gough SC, Bhargava A, Jain R, et al. Low-volume insulin degludec 200 units/mL once daily improves glycemic control similarly to insulin glargine with a low risk of hypoglycemia in insulin-naïve patients with type 2 diabetes: a 26-week, randomized, controlled, multinational, treat-to-target trial: the BEGIN LOW VOLUME trial. Diabetes Care. 2013;36(9):2536–2542. doi: 10.2337/dc12-2329
- Goldman J, Angueira-Serrano E, Gonzalez J, et al. Survey reveals patient and health care provider experiences and challenges with the use of high doses of basal insulin. Clin Diabetes. 2022;41(2):244–257. doi: 10.2337/cd22-0062
- Rosenstock J, Bajaj HS, Janež A, et al. Once-weekly insulin for type 2 diabetes without previous insulin treatment. N Engl J Med. 2020;383(22):2107–2116. doi: 10.1056/NEJMoa2022474
- Mathieu C, Asbjornsdottir B, Bajaj HS, et al. Switching to once-weekly insulin icodec versus once-daily insulin glargine U100 in individuals with basal-bolus insulin-treated type 2 diabetes (ONWARDS 4): a phase 3a, randomised, open-label, multicentre, treat-to-target, non-inferiority trial. Lancet. 2023;401(10392):1929–1940. doi: 10.1016/S0140-6736(23)00520-2
- Philis-Tsimikas A, Asong M, Franek E, et al. Switching to once-weekly insulin icodec versus once-daily insulin degludec in individuals with basal insulin-treated type 2 diabetes (ONWARDS 2): a phase 3a, randomised, open label, multicentre, treat-to-target trial. Lancet Diabetes Endocrinol. 2023;11(6):414–425. doi: 10.1016/S2213-8587(23)00093-1
- Polonsky WH, Arsenault J, Fisher L, et al. Initiating insulin: how to help people with type 2 diabetes start and continue insulin successfully. Int J Clin Pract. 2017;71(8):e12973. doi: 10.1111/ijcp.12973
- Krist AH, Tong ST, Aycock RA, et al. Engaging patients in decision-making and behavior change to promote prevention. Stud Health Technol Inform. 2017;240:284–302.
- Saheb Kashaf M, McGill ET, Berger ZD Shared decision-making and outcomes in type 2 diabetes: a systematic review and meta-analysis. Patient Educ Couns. 2017;100(12):2159–2171. doi: 10.1016/j.pec.2017.06.030
- Coronado-Vázquez V, Canet-Fajas C, Delgado-Marroquín MT, et al. Interventions to facilitate shared decision-making using decision aids with patients in primary health care: a systematic review. Medicine (Baltimore). 2020;99(32):e21389. doi: 10.1097/MD.0000000000021389
- Tamhane S, Rodriguez-Gutierrez R, Hargraves I, et al. Shared decision-making in diabetes care. Curr Diab Rep. 2015;15(12):112. doi: 10.1007/s11892-015-0688-0
- Rariden C. Diabetes distress: assessment and management of the emotional aspect of diabetes mellitus. J Nurse Pract. 2019;15(9):653–656. doi: 10.1016/j.nurpra.2019.06.020
- American Diabetes Association Professional Practice Committee 5. Facilitating positive health behaviors and well-being to improve health outcomes: standards of care in diabetes-2024. Diabetes Care. 2024;47(Suppl 1):S77–S110. doi: 10.2337/dc24-S005
- Boels AM, Vos RC, Hermans TGT, et al. What determines treatment satisfaction of patients with type 2 diabetes on insulin therapy? An observational study in eight European countries. BMJ Open. 2017;7(7):e016180. doi: 10.1136/bmjopen-2017-016180
- Gucciardi E, Xu C, Vitale M, et al. Evaluating the impact of onsite diabetes education teams in primary care on clinical outcomes. BMC Fam Pract. 2020;21(1):48. doi: 10.1186/s12875-020-01111-2
- Duncan I, Ahmed T, Li QE, et al. Assessing the value of the diabetes educator. Diabetes Educ. 2011;37(5):638–657. doi: 10.1177/0145721711416256
- James TL. Improving referrals to diabetes self-management education in medically underserved adults. Diabetes Spectr. 2021;34(1):20–26. doi: 10.2337/ds20-0001
- Powers MA, Bardsley JK, Cypress M, et al. Diabetes self-management education and support in adults with type 2 diabetes: a consensus report of the American Diabetes Association, the Association of Diabetes Care & Education Specialists, the Academy of Nutrition and Dietetics, the American Academy of Family Physicians, the American Academy of PAs, the American Association of Nurse Practitioners, and the American Pharmacists Association. Diabetes Care. 2020;43(7):1636–1649. doi: 10.2337/dci20-0023