ABSTRACT
Aim
To compare the ablation techniques’ efficacy of endovenous microwave ablation (EMA) combined with high ligation (HL), foam sclerotherapy (FS) and compression therapy (CT) and endovenous laser ablation (EVLA) combined with HL-FS-CT in the treatment of VLUs.
Method
301 consecutive patients with VLUs from 2013 to 2022 in a 3200-bed hospital were intervened by EMA combined with HL-FS-CT and EVLA combined with HL-FS-CT were retrospectively compared.
Results
One hundred thirty-four patients underwent EMA+HL-FS-CT and 167 patients underwent EVLA+HL-FS-CT. The primary outcome of the ulcer healing time was 1.45(0.75–1.5) months and 1.86(0.5–2.5) months, respectively, in the two groups (HR for ulcer healing was 1.26, 95% CI [0.96–1.66], p = 0.097). Secondary outcomes included that no significant difference was found in ulcer recurrence and GSV recanalization and complications between the two groups, and the postoperative VCSS and AVVQ were significantly lower than the baseline values in the respective groups (p = 0.0001).
Conclusion
EMA+HL-FS-CT and EVLA+HL-FS-CT are both effective at treating VLUs. Both of the two comprehensive treatments were beneficial to the healing of ulcers, but no evidence showed which one was superior in the ulcer healing time.
Introduction
The clinical classification of venous leg ulcers (VLUs) is the highest clinical classification of C6 in Clinical-Etiology-Anatomy-Pathophysiology (CEAP) [Citation1], and the pooled prevalence of C6 in the CEAP classification was 0.4% [Citation2]. The current standard of treatment is to use a variety of treatments individually or in combination to promote ulcer healing [Citation3–5]. The annual cost associated with venous leg ulcers in the United States is approximately $14.9 billion [Citation6]. However, VLU also has a high 50–70% recurrence rate, likely due to noncompliance with compression therapy, failure of surgical procedures, or poorly understood pathophysiology [Citation7]. The aims of current treatment options are to reduce or completely stop the superficial and deep venous reflux of the lower limbs to reduce the skin damage caused by the pressure of superficial venous reflux and to promote the healing of the ulcer.
Clinically, in order to better reduce the pressure of the superficial veins of the lower limbs, some combined treatment methods have emerged, such as the ablation techniques of endovenous laser ablation (EVLA) and microwave ablation (EMA) combined with the high ligation (HL) of great saphenous vein (GSV) and foam sclerotherapy (FS) for the varicose veins (VVs), which also has good effects in promoting the healing of VLUs [Citation8–10]. In addition, compression therapy (CT) is undeniable to resist venous hypertension of the lower extremities and promote the healing of VLUs [Citation11–13].
However, there are few reports on the efficacy of EMA combined with HL-FS-CT and EVLA combined with HL-FS-CT in the treatment of VLUs. A lack of reliable evidence has resulted in weak support for endovenous ablation combined with HL-FS-CT in current management guidelines [Citation2,Citation14]. In this study, we retrospectively compared the 1-year clinical data of patients treated with EMA combined with HL-FS-CT and EVLA combined with HL-FS-CT for the treatment of VLUs. In addition, ulcer recurrence, GSV recanalization, complications, VCSS and AVVQ were also compared between the two groups.
Patients and methods
Patients
We performed a retrospective analysis of a real-world study of the EMA combined with HL-FS-CT versus EVLA combined with HL-FS-CT for VLUs with 1 year follow up. The study was conducted at a regional medical center, a 3200-bed general university-affiliated hospital. Consecutive patients with VLUs at Ganzhou People’s Hospital from 2013 to 2022 were included in this retrospective study. The baseline characteristics of the patients included sex, age, occupation, course of varicose vein, ulcer duration, ulcer diameter, reflux time of the GSV [Citation15], number of perforators, baseline VCSS, and baseline AVVQ.
Inclusion criteria
1. Patients who received EMA+HL-FS-CT or EVLA+HL-FS-CT; 2. Lower extremity active venous ulcers (VLUs) (CEAP, C6).
Exclusion criteria
1. A history of surgery for VVs; 2. a history of deep vein thrombosis (DVT) in the ipsilateral limb; 3. GSV thrombosis; 4. The diameter of the VVs >1 cm; 5. The ipsilateral limb artery was occluded.
Procedures
According to the information provided in the medical record, procedures included EMA+HL-FS-CT or EVLA+HL-FS-CT. The procedures conducted in both groups included HL to the GSV and FS to the VVs and compression therapy (CT) to the limbs after surgery. The choice of procedures was made by the patients themselves based on the surgeon’s experience and the available equipment at that time. The operation was performed by the same surgical team. Patients satisfying the inclusion criteria were fully informed about EVLA or EMA and gave their written consent to undergo these specific procedures.
HL-FS
All the VVs were marked before surgery. After epidural or general anesthesia, the lower limbs were sterilized. An oblique incision was made in the groin, all the collateral branches of the GSV were ligated, and the HL of GSV was performed at the sapheno-femoral junction (SFJ) [Citation16]. FS of the VVs were injected with sclerosing agent foam (foam was produced with the use of the Tessari technique at a ratio of 1 ml of 1% lauromacrogol to 4 ml of CO2) [Citation8]. Foam was also injected into the VVs around the ulcer and perforators. The injection of the perforators must be performed under ultrasound guidance [Citation4]. The total amount of lauromacrogol injected in each limb was generally not more than 10 ml.
EMA
The GSV was punctured at the medial malleolus, and a microwave ablation needle (Shanxi Danhui Biotechnology, Shanxi, China.) was inserted into the GSV. The microwave needle followed the GSV up to the groin. The distal end of the needle was linked to the microwave generator (HBS-B, Nanjing Huabai Electronic Medical, Nanjing, China). Tumescence infiltration was injected around the GSV from 10 cm below the knee to the groin ensuring that the distance of the vein-to skin more than 1 cm (patients were under spinal or general anesthesia, so we used normal saline alone as swelling fluid). At a power of 40W and a withdrawn speed of 1 cm/6 sec, the microwave generator was activated to close the GSV trunk from 10 cm below the knee to the groin. The microwave wire was withdrawn 1 cm each time. For those patients in whom the microwave wire could not be inserted upward from the ankle, such as patients with local infection or distortion or narrowing of the GSV, we chose to place the catheter downward at the inguinal incision and then retrograde cauterization of the GSV.
EVLA
The GSV trunk was punctured at the medial malleolus, and the laser fiber was inserted through a catheter into the GSV trunk to the groin. No tumescence infiltration was injected around the GSV. At a power of 15W and a withdrawn speed of 0.3 cm/sec with manual pressure being applied to achieve venous wall apposition around the laser fiber tip [Citation17], the 810 nm laser (AngioDynamics, Germany) was activated to close the GSV trunk from 10 cm below the knee to the groin. For those patients in whom the fiber could not be inserted upward from the ankle, we chose to place the fiber downward at the inguinal incision and then retrograde cauterization of the GSV [Citation8].
CT
After cleaning the ulcer, the wound was covered with vaseline gauze. Four layers of compression bandages were applied to the limbs, ensuring that the pressure at the ankle was 40 mmHg [Citation18,Citation19]. Three days later, they were replaced with grade II (30-40 mmHg) compression stockings, with the wound covered with thin dressing. We suggested the CT of stockings should be maintained for more than 1 year.
Prophylactic anticoagulation and antibiotics
All patients received prophylactic anticoagulant therapy with low molecular weight heparin after surgery, except those who were contraindicated to anticoagulation. Sensitive antibiotics were used to prevent surgical site infection in some patients with positive cultures of ulcer secretions and enlarged inguinal lymph nodes.
Discharge criteria
The patient could be mobile after 6 hours of bed rest following spinal anesthesia. If there were no complications such as wound bleeding, infection, DVT, etc. which require prolonged hospitalization, the patient was discharged 3 days after the procedure.
Follow-up and quality of life (QoL) assessment
Follow-up was conducted by the physicians involved in the clinical management of the patients. The reexamination methods included physical examination, duplex scanning and a questionnaire survey. All patients were examined for occlusion of the GSV by duplex ultrasound scanning by the same group of sonographers at 1 week after surgery and as outpatients at 1, 6 and 12 months after discharge. The follow-up staff recorded the ulcer healing time provided by the patients during the follow-up visit. The impact of disease relationships on quality of life (QoL) was assessed using the AVVQ [Citation20] and VCSS [Citation21]. The AVVQ ranges from 0 to 100, with lower scores indicating better QOL. For VCSS, 0 indicates no significant venous disease and a score of 30 is the highest score, which is a valid and sensitive indicator of VVs severity.
Outcomes
The primary outcome was ulcer healing time. Ulcer healing time was defined as the time from the start of the intervention to ulcer healing. Ulcer healing is complete epithelialization of leg ulcers [Citation8]. The secondary outcomes were the procedure-related outcomes, complications, the QoL assessments, the occlusion rate of GSV at 1 week after surgery, the recurrence of ulcer and recanalization of the GSV at 6 and 12 months. The postoperative complications included the numbness, skin burns, endothermal heat induced thrombosis (EHIT), DVT/pulmonary embolism (PE), induration ecchymosis, thrombophlebitis and groin infection. Ulcer recurrence is the return of ulceration at the healed ulcer site, presenting as incomplete epithelium. Recanalization of the GSV or treatment failure was defined as an open segment of the treated GSV segment greater than 10 cm in length [Citation22]. Recanalization refers to the restoration of blood flow, which can be attributed to the reflux through the accessory saphenous vein, recanalized collaterals, or perforating veins.
Statistical analysis
All data were checked and entered in excel form to establish a database. The homogeneity of variance of measurement data was tested by two independent samples t-test, and the heterogeneity of variance was tested by rank sum test. Count data were analyzed by χ2 test. Kaplan-Meier survival curve analysis was used to compare the difference in ulcer healing time between the two groups. GraphPad Prism 8 was used for plotting. All tests were two-sided, with a significance level of 0.05. Statistical analyses were performed with the SPSS software (IBM SPSS Statistical software, version 26).
Results
Study population and baseline characteristics
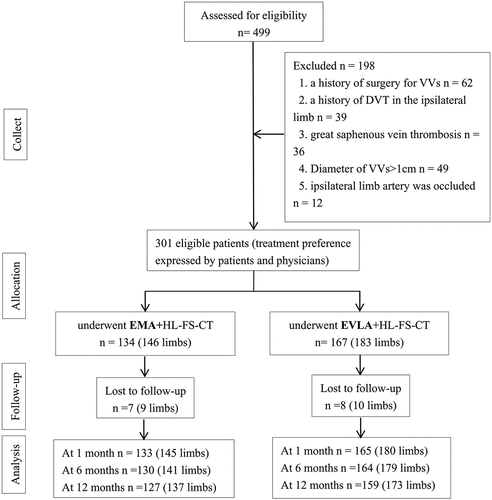
In total, 499 patients with VLUs were assessed for inclusion in our single-center hospital from 2013 to 2022 and 198 patients did not meet the inclusion criteria were excluded. Treatment was successful in both groups. In the EMA group, 1, 3 and 3 patients were lost to follow-up at 1, 6 and 12 months after operation. In the EVLA group, 2, 1 and 5 patients were lost to follow-up at 1, 6 and 12 months after operation (). There were no statistically significant differences in baseline characteristics between the two groups ().
Table 1. Baseline Characteristics in the EMA+HL-FS-CT and EVLA+HL-FS-CT groups.
EMA group
One hundred thirty-four patients/146 limbs underwent EMA+HL-FS-CT. Eighty-four of these patients were engaged in manual labor. A total of 73 male with a mean age of (61.7 ± 8.9) years and a course of varicose vein of 19.3(12.0–25.0) years were included. Ulcer duration was (22.9 ± 68.1) months, Baseline VCSS and baseline AVVQ were 12.6 (11.0–14.0) and 52.9(46.2–59.0).
EVLA group
A total of 167 patients/183 limbs underwent EVLA+HL-FS-CT. Ninety-eight of these patients were engaged in manual labor. A total of 97 male patients with a mean age of (60.5 ± 10.5) years and a course of varicose veins of 20.2(12.0–30.0) years. Ulcer duration was (26.0 ± 72.6) months. Baseline VCSS and baseline AVVQ were 12.6 (10.0–15.0) and 52.5 (43.6–59.0).
Primary outcome
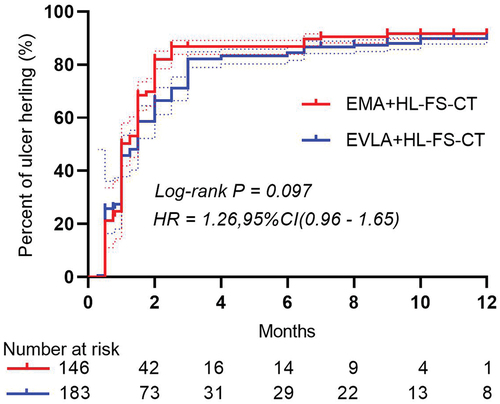
After 12 months of intervention, 129 limbs (88.4%) ulcer healed in EMA group, and the median time to ulcer healing was 1.45 months (range: 0.75–1.5 months). Ulcer healing occurred in 160 limbs (87.4%) in the EVLA group, with a median time to ulcer healing was 1.86 months (range: 0.5–2.5 months) (). No difference was found in ulcer healing time between the two groups. (HR for ulcer healing was 1.26, 95% CI [0.96–1.65], p = 0.097) ().
Table 2. The follow-up outcomes in the EMA and EVLA groups.
Secondary outcomes
The procedure-related outcomes
The mean operation time was longer in the EMA group than in the EVLA group ([80.4 ± 12.8] min vs. [74.3 ± 11.8] min, p = 0.001), There was no significant difference between the two groups in the number and amount of sclerosing agent ([9.6 ± 0.7] ml vs. [9.7 ± 0.7] ml, p = 0.592). One week after surgery, the GSV was detected to be occluded by duplex ultrasound scanning in both groups ().
Table 3. The procedure-related outcomes in the EMA and EVLA groups.
Recurrences
Recurrence of ulcer occurred in 1 case (0.7%) and 1 case (0.5%) in the two groups at 6 months after operation, and increased to 6 cases (4.1%) and 7 cases (3.8%) at 12 months after operation. The recanalization rate of GSV was 0 (0.0%) and 1 (0.6%) at 6 months and 6 (4.4%) and 8 (4.6%) at 12 months. However, there was no significant difference in ulcer recurrence and GSV recanalization between the two groups ().
Complications
In each cohort, a single case of DVT was reported, with resolution following anticoagulant therapy. No instances of EHIT were documented in either group. There were 7 and 11 occurrences of skin burns in the respective groups; however, these incidents were non-severe and exhibited complete recovery within two weeks. There were 5 cases of groin wound infection in the patients’ cohort, and all of them were cured by dressing change. No statistically significant difference was found in complications between the two groups ().
Table 4. The incidence of complications in the EMA and EVLA groups.
Disease-specific QoL assessment
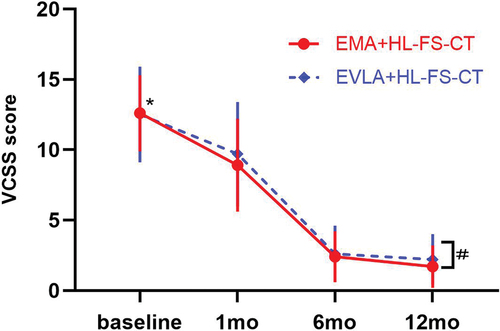
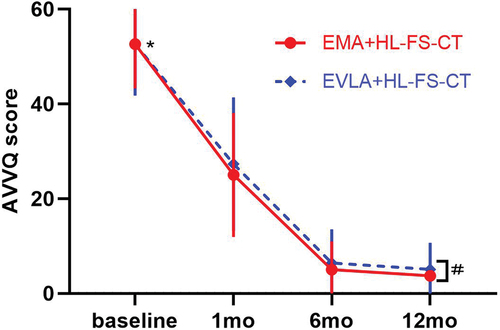
Postoperative VCSS () and AVVQ () were significantly lower than the baseline values in the respective groups (p = 0.0001). In addition, there were significant differences in VCSS and AVVQ scores between the two groups at 12 months (p = 0.008 and 0.001, respectively).
Discussion
This real-world study demonstrated that EMA+FS-HL-CT and EVLA+FS-HL-CT were both beneficial to the healing of VLUs and no difference was found in ulcer healing time between the two groups. Shortening the healing time of VLUs has been our long-pursuing goal.
Twenty years ago, we utilized the traditional method of high ligation and stripping the GSV for treating VLUs, which effectively eradicated reflux in the GSV, yielding favorable outcomes at a lower cost. However, complete GSV stripping carries a risk of subcutaneous injury, bleeding, ecchymosis formation, and potential saphenous nerve injury, leading to lower limb numbness [Citation23]. Laser (810 nm) ablation was introduced to our hospital in 2010. Following HL of the GSV, EVLA closure of the main trunk of the GSV replaced traditional stripping procedures. This resulted in a significant reduction in postoperative subcutaneous hemorrhage and saphenous nerve injury. Nowadays, there is even EVLA using a wavelength of 1940 nm for the treatment of VVs [Citation24]. EMA was introduced after 2018. For patients with VLUs, a more aggressive approach is taken, including HL of the GSV followed by EMA of the GSV trunk. The efficacy of these interventions in the management of VVs has been well established [Citation2,Citation9,Citation14,Citation25–27]. The treatment of the two cohorts in the study was not performed in the same period. These two groups were from different historical cohorts.
In the early stage, we compared the clinical effects of CT alone and EVLA combined with HL-CT in the treatment of VLUs, and found that the EVLA combined with HL-CT treatment had a shorter ulcer healing time [Citation8]. Later, we also compared the clinical effects of traditional high ligation – stripping (HL-S) and EVLA in the treatment of venous ulcer, also concluded that EVLA could accelerate ulcer healing [Citation28]. EMA has been compared with HL in the treatment of VLUs, it was found that the postoperative healing time of the ulcers was faster in patients in the EMA group than in the HL group [Citation9]. Early ablation is recommended for VLUs. Compared with CT alone, early ablation helped to accelerate ulcer healing and prolong ulcer-free survival time for the patients [Citation5].
Although a benefit of endovenous intervention was observed in the current studies, the best method of ablation among those currently available remains unclear [Citation5,Citation26,Citation29–31]. Interestingly, in our retrospective study, there was no difference in ulcer recurrence between the two groups at 6 and 12 months of follow-up.
Both EMA and EVLA are ablation techniques of the GSV, which damage the endothelium of the reflux GSV by thermal effect, and promote the contraction of the GSV followed by fibrosis until the vein is completely occluded [Citation32–34]. Sclerotherapy has a good occlusion effect on VVs, VVs around ulcers and perforators [Citation26,Citation35–37]. HL further sever the reflux of the GSV [Citation10,Citation38,Citation39]. All these measures can synergistically block the venous reflux of the lower extremity, reduce the pressure of the superficial veins of the lower extremity, and facilitate the healing of VLUs [Citation40].
The use of thermal ablation combined with HL for the treatment of varicose veins in the lower extremities increases trauma. However, it has been reported that EMA-HL can promote VLU healing [Citation9]. In addition, a meta‑analysis was performed on the effects of EVLA-HL on the GSV which indicated that combining EVLA with high ligation provides stable long-term clinical efficacy in treating VVs of the lower extremities, although it increases the invasiveness of the surgery. The use of EVLA alone may be less effective at preventing vein occlusion [Citation25]. Additionally, HL can prevent the detachment of thrombus formed in the proximal GSV after EVLA or EMA ablation, thus reducing the risk of EHIT [Citation41] and pulmonary embolism [Citation42].
We previously utilized tumescent anesthesia during EVLA with HL of the GSV under local anesthesia, followed by the injection of foam sclerosing agent through superficial VVs puncture without additional anesthesia. Despite the avoidance of spinal anesthesia, the patient experienced significant discomfort during the procedure, particularly when the foam sclerosing agent was injected into the superficial VVs, resulting in considerable pain with each injection. We so chose spinal or general anesthesia for all the patients. Some researchers have also utilized spinal or general anesthesia in these same procedures [Citation43,Citation44].
The comparison of recanalization of GSV at 6 and 12 months of follow-up did not differ between the two groups, which is similar to the results of earlier studies [Citation22]. However, some studies have found that the recanalization rate of GSV after EVLA is higher than that after EMA, which may be related to the lack of HL of the GSV in their treatment methods [Citation45].
Thermal ablation of GSVs maybe results in heat-related complications, such as skin burns, induration ecchymosis and numbness for nerve damage [Citation9,Citation45]. However, there was no difference between the two cohorts in our study. In addition, paresthesia of the skin also improved over time, which was the same result as previous studies [Citation22]. DVT is another complication of variceal surgery [Citation23]. However, DVT occurred in one and two patients in our two groups and was improved by later anticoagulation without occurring serious consequences. Induration ecchymosis occurred in the two groups may be related to the perforation of the GSV after thermal ablation [Citation46] or to the injection of sclerosing agent in the VVs. VLUs often manifests as local infection of the ulcer, so multiple dressing changes are needed before surgery to improve the infection of the ulcer. Even prophylactic antibiotics were used, wound infection may occur. There were no more other serious consequences. The occurrence of thrombophlebitis in the two groups may be related to the FS to the VVs [Citation47].
VVs significantly affects the QoL of patients and limits their daily activities and work. Various ablation procedures can significantly improve the QoL of patients [Citation27]. The QoL scale obtained after VVs intervention can not only assess the improvement in the quality of life of individual patients, but also be used to compare the differences between before and after different treatments [Citation26,Citation48]. Most studies have shown no difference in QoL improvement after different thermal ablation treatments [Citation22,Citation29,Citation49]. Our study also showed that the AVVQ and VCSS values improved after surgery in both groups, and both decreased significantly compared with the baseline values. However, our study showed that there were significant differences in VCSS and AVVQ scores between the two groups at 12 months. In the long term, patients with VLUs treated with EMA appear to have better QoL.
Limitations
Our study has several limitations. First, the follow-up period was only 12 months. Longer follow-up is needed to confirm the durability of the benefit. Second, this study was a retrospective study within a single center. In addition, multiple combined methods were used to reduce the venous pressure of the lower extremities, such as HL and EMA or EVLA of the GSV and sclerotherapy injection of the VVs. Whether there is a suspicion of over manipulation requires more research to clarify the effect of the combination of surgical approaches on long-term outcomes for VLUs. The retrospective observational study could not determine whether EMA or EVLA is superior in promoting VLUs healing. Further randomized controlled multi-center studies are needed to extend the follow-up time to verify the accuracy of the conclusion and effectively apply it to clinical practice.
Conclusion
Both EMA combined with HL-FS-CT and EVLA combined with HL-FS-CT are effective in the treatment of VLUs. The complications of both EMA and EVLA are acceptable, and they can improve the QoL of patients with VLUs after intervention. However, no evidence showed which one was superior in the ulcer healing time.
Declaration of financial/other relationships
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties. Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author contributions
GF Zheng, B Qin, XC Liu conceived the study, made the design, performed the statistical analyses, and drafted the manuscript. GF Zheng, XC Liu participated in the design and statistic work. HL Xie, MG Lai, B Qin collected data. HL Xie and XC Liu revised the paper. All authors gave final approval and agree to be accountable for all aspects of work ensuring integrity and accuracy.
Ethic statement
This study was approved by the ethics committee and institutional review board of the Ganzhou People’s Hospital (approval number: TY-ZKY2024-011-01). Written informed consent was obtained from all the subjects involved in the study.
Acknowledgments
The authors would like to thank the American Journal Experts (AJE) for English language polishing of the paper.
Data availability statement
The data that support the findings of this study are available on request from the corresponding author XCL. The data are not publicly available due to the containing information that could compromise the privacy of research participants.
Additional information
Funding
References
- Lurie F, Passman M, Meisner M, et al. The 2020 update of the CEAP classification system and reporting standards. J Vasc Surg Venous Lymphat Disord. 2020;8(3):342–352. doi: 10.1016/j.jvsv.2019.12.075
- De Maeseneer MG, Kakkos SK, Aherne T, et al. Editor’s Choice - European Society for Vascular Surgery (ESVS) 2022 clinical practice guidelines on the management of chronic venous disease of the lower limbs. Eur J Vasc Endovasc Surg. 2022;63(2):184–267. doi: 10.1016/j.ejvs.2021.12.024
- Liu X, Zheng G, Ye B, et al. Comparison of combined compression and surgery with high ligation-endovenous laser ablation-foam sclerotherapy with compression alone for active venous leg ulcers. Sci Rep. 2019;9(1):14021. doi: 10.1038/s41598-019-50617-y
- O’Hare JL, Earnshaw JJ. Randomised clinical trial of foam sclerotherapy for patients with a venous leg ulcer. Eur J Vasc Endovasc Surg. 2010;39(4):495–499. doi: 10.1016/j.ejvs.2009.11.025
- Gohel MS, Heatley F, Liu X, et al. A randomized trial of early endovenous ablation in venous ulceration. N Engl J Med. 2018;378(22):2105–2114. doi: 10.1056/NEJMoa1801214
- Rice JB, Desai U, Cummings AK, et al. Burden of venous leg ulcers in the United States. J Med Econ. 2014;17(5):347–356. doi: 10.3111/13696998.2014.903258
- Raffetto JD, Ligi D, Maniscalco R, et al. Why venous leg ulcers have difficulty healing: overview on pathophysiology, clinical consequences, and treatment. J Clin Med. 2020;10(1):29. doi: 10.3390/jcm10010029
- Liu X, Zheng G, Ye B, et al. Comparison of combined compression and surgery with high ligation-endovenous laser ablation-foam sclerotherapy with compression alone for active venous leg ulcers. Sci Rep. 2019;9(1):1–8. doi: 10.1038/s41598-019-50617-y
- Yang L, Wang XP, Su WJ, et al. Randomized clinical trial of endovenous microwave ablation combined with high ligation versus conventional surgery for varicose veins. Eur J Vasc Endovasc Surg. 2013;46(4):473–479. doi: 10.1016/j.ejvs.2013.07.004
- Kalteis M, Adelsgruber P, Messie-Werndl S, et al. Five-year results of a randomized controlled trial comparing high ligation combined with endovenous laser ablation and stripping of the great saphenous vein. Dermatol Surg. 2015;41(5):579–586. doi: 10.1097/DSS.0000000000000369
- Perry C, Atkinson RA, Griffiths J, et al. What promotes or prevents greater use of appropriate compression in people with venous leg ulcers? A qualitative interview study with nurses in the north of England using the theoretical domains framework. BMJ Open. 2022;12(8):e061834. doi: 10.1136/bmjopen-2022-061834
- Klonizaki M, Gumber A, McIntosh E, et al. Testing the feasibility of a co-designed intervention, comprising self-managed, home-based, exercise training with embedded behavioural support and compression therapy for people with venous leg ulcers receiving treatment at home (FISCU-II). Clin Exp Dermatol. 2023;49(2):135–142. doi: 10.1093/ced/llad342
- Shi C, Dumville JC, Cullum N, et al. Compression bandages or stockings versus no compression for treating venous leg ulcers. Cochrane Database Syst Rev. 2021;7(7):CD013397. doi: 10.1002/14651858.CD013397.pub2
- Gloviczki P, Lawrence PF, Wasan SM, et al. The 2023 Society for Vascular Surgery, American Venous Forum, and American Vein and Lymphatic Society clinical practice guidelines for the management of varicose veins of the lower extremities. Part II: endorsed by the Society of Interventional Radiology and the Society for Vascular Medicine. J Vasc Surg Venous Lymphat Disord. 2023;12(1):101670. doi: 10.1016/j.jvsv.2023.08.011
- Jeanneret C, Labs KH, Aschwanden M, et al. Physiological reflux and venous diameter change in the proximal lower limb veins during a standardised valsalva manoeuvre. Eur J Vasc Endovasc Surg. 1999;17(5):398–403. doi: 10.1053/ejvs.1998.0797
- Kuserli Y, Kavala AA, Turkyilmaz S. Comparison of high saphenous ligation and stripping, radiofrequency ablation, and subfascial endoscopic perforator surgery for the treatment of active venous ulcers: retrospective cohort with five-year follow-up. Vascular. 2022;30(2):375–383. doi: 10.1177/17085381211011356
- Lu X, Ye K, Li W, et al. Endovenous ablation with laser for great saphenous vein insufficiency and tributary varices: a retrospective evaluation. J Vasc Surg. 2008;48(3):675–679. doi: 10.1016/j.jvs.2008.04.017
- O’Meara S, Cullum N, Nelson EA, et al. Compression for venous leg ulcers. Cochrane Database of Systematic Reviews. 2012;11(11):CD000265. doi: 10.1002/14651858.CD000265.pub3
- Arundel CE, Welch C, Saramago P, et al. A randomised controlled trial of compression therapies for the treatment of venous leg ulcers (VenUS 6): study protocol for a pragmatic, multicentre, parallel-group, three-arm randomised controlled trial. Trials. 2023;24(1):357. doi: 10.1186/s13063-023-07349-2
- Smith JJ, Garratt AM, Guest M, et al. Evaluating and improving health-related quality of life in patients with varicose veins. J Vasc Surg. 1999;30(4):710–719. doi: 10.1016/S0741-5214(99)70110-2
- Passman MA, McLafferty RB, Lentz MF, et al. Validation of Venous Clinical Severity Score (VCSS) with other venous severity assessment tools from the American Venous Forum. J Vasc Surg. 2011;54(6 Suppl):2S–9S. doi: 10.1016/j.jvs.2011.05.117
- Yang L, Wang X, Wei Z, et al. The clinical outcomes of endovenous microwave and laser ablation for varicose veins: a prospective study. Surgery. 2020;168(5):909–914. doi: 10.1016/j.surg.2020.06.035
- Liao CJ, Song SH, Li T, et al. Randomized clinical trial of radiofrequency-induced thermotherapy combined with transilluminated powered phlebectomy versus high ligation and stripping for the treatment of lower limb varicose veins. J Vasc Surg Venous Lymphat Disord. 2021;9(1):95–100. doi: 10.1016/j.jvsv.2020.04.028
- Setia A, Schmedt CG, Beisswenger A, et al. Safety and efficacy of endovenous laser ablation (EVLA) using 1940 nm and radial emitting fiber: 3-year results of a prospective, non-randomized study and comparison with 1470 nm. Lasers Surg Med. 2022;54(4):511–522. doi: 10.1002/lsm.23500
- Huang Y, Zhang D, Zhou C, et al. The first meta-analysis research on the effects of endovenous laser ablation combined with sapheno-femoral junction high ligation of the great saphenous vein. Lasers Med Sci. 2023;38(1):175. doi: 10.1007/s10103-023-03833-y
- Brittenden J, Cotton SC, Elders A, et al. A randomized trial comparing treatments for varicose veins. N Engl J Med. 2014;371(13):1218–1227. doi: 10.1056/NEJMoa1400781
- Rasmussen L, Lawaetz M, Serup J, et al. Randomized clinical trial comparing endovenous laser ablation, radiofrequency ablation, foam sclerotherapy, and surgical stripping for great saphenous varicose veins with 3-year follow-up. J Vasc Surg Venous Lymphat Disord. 2013;1(4):349–356. doi: 10.1016/j.jvsv.2013.04.008
- Liu X, Zheng G, Ye B, et al. A retrospective cohort study comparing two treatments for active venous leg ulcers. Medicine. 2020;99(8):e19317. doi: 10.1097/MD.0000000000019317
- Beteli CB, Rossi FH, de Almeida BL, et al. Prospective, double-blind, randomized controlled trial comparing electrocoagulation and radiofrequency in the treatment of patients with great saphenous vein insufficiency and lower limb varicose veins. J Vasc Surg Venous Lymphat Disord. 2018;6(2):212–219. doi: 10.1016/j.jvsv.2017.09.010
- Vahaaho S, Halmesmaki K, Alback A, et al. Five-year follow-up of a randomized clinical trial comparing open surgery, foam sclerotherapy and endovenous laser ablation for great saphenous varicose veins. Br J Surg. 2018;105(6):686–691. doi: 10.1002/bjs.10757
- Whiteley MS, Shiangoli I, Dos Santos SJ, et al. Fifteen year results of radiofrequency ablation, using VNUS closure, for the abolition of truncal venous reflux in patients with Varicose Veins. Eur J Vasc Endovasc Surg. 2017;54(3):357–362. doi: 10.1016/j.ejvs.2017.06.001
- van Ruijven PW, Poluektova AA, van Gemert MJ, et al. Optical-thermal mathematical model for endovenous laser ablation of varicose veins. Lasers Med Sci. 2014;29(2):431–439. doi: 10.1007/s10103-013-1451-x
- Wang XH, Wang XP, Su WJ, et al. Microwave ablation versus laser ablation in occluding lateral veins in goats. J Huazhong Univ Sci Technolog Med Sci. 2016;36(1):106–110. doi: 10.1007/s11596-016-1550-6
- Subwongcharoen S, Praditphol N, Chitwiset S. Endovenous microwave ablation of varicose veins: in vitro, live swine model, and clinical study. Surg Laparosc Endosc Percutan Tech. 2009;19(2):170–174. doi: 10.1097/SLE.0b013e3181987549
- Cuffolo G, Hardy E, Perkins J, et al. The effects of foam sclerotherapy on ulcer healing: a single-centre prospective study. Ann R Coll Surg Engl. 2019;101(4):285–289. doi: 10.1308/rcsann.2018.0218
- Pihlaja T, Torro P, Ohtonen P, et al. Ten years of experience with first-visit foam sclerotherapy to initiate venous ulcer healing. J Vasc Surg Venous Lymphat Disord. 2021;9(4):954–960. doi: 10.1016/j.jvsv.2020.11.012
- Abreu GCG, Camargo Jr O, Abreu MFM, et al. Ultrasound-guided foam sclerotherapy for chronic venous disease with ulcer. A prospective multiple outcome cohort study. J Vasc Bras. 2020;19:e20180108. doi: 10.1590/1677-5449.180108
- Flessenkamper I, Hartmann M, Hartmann K, et al. Endovenous laser ablation with and without high ligation compared to high ligation and stripping for treatment of great saphenous varicose veins: Results of a multicentre randomised controlled trial with up to 6 years follow-up. Phlebology. 2016;31(1):23–33. doi: 10.1177/0268355514555547
- Rass K, Frings N, Glowacki P, et al. Same site recurrence is more frequent after endovenous laser ablation compared with high ligation and stripping of the great saphenous vein: 5 year results of a randomized clinical trial (RELACS study). Eur J Vasc Endovasc Surg. 2015;50(5):648–656. doi: 10.1016/j.ejvs.2015.07.020
- Lawrence PF, Hager ES, Harlander-Locke MP, et al. Treatment of superficial and perforator reflux and deep venous stenosis improves healing of chronic venous leg ulcers. J Vasc Surg Venous Lymphat Disord. 2020;8(4):601–609. doi: 10.1016/j.jvsv.2019.09.016
- Marsh P, Price BA, Holdstock J, et al. Deep vein thrombosis (DVT) after venous thermoablation techniques: rates of endovenous heat-induced thrombosis (EHIT) and classical DVT after radiofrequency and endovenous laser ablation in a single centre. Eur J Vasc Endovasc Surg. 2010;40(4):521–527. doi: 10.1016/j.ejvs.2010.05.011
- Healy DA, Kimura S, Power D, et al. A systematic review and Meta-analysis of thrombotic events following endovenous thermal ablation of the great saphenous vein. Eur J Vasc Endovasc Surg. 2018;56(3):410–424. doi: 10.1016/j.ejvs.2018.05.008
- Flessenkamper I, Hartmann M, Stenger D, et al. Endovenous laser ablation with and without high ligation compared with high ligation and stripping in the treatment of great saphenous varicose veins: initial results of a multicentre randomized controlled trial. Phlebology. 2013;28(1):16–23. doi: 10.1258/phleb.2011.011147
- Golbasi I, Turkay C, Erbasan O, et al. Endovenous laser with miniphlebectomy for treatment of varicose veins and effect of different levels of laser energy on recanalization. A single center experience. Lasers Med Sci. 2015;30(1):103–108. doi: 10.1007/s10103-014-1626-0
- Mao J, Zhang C, Wang Z, et al. A retrospective study comparing endovenous laser ablation and microwave ablation for great saphenous varicose veins. Eur Rev Med Pharmacol Sci. 2012;16(7):873–877.
- Kemaloglu C. Saphenous vein diameter is a single risk factor for early recanalization after endothermal ablation of incompetent great saphenous vein. Vascular. 2019;27(5):537–541. doi: 10.1177/1708538119837110
- Alder G, Lees T. Foam sclerotherapy. Phlebology. 2015;30(2 Suppl):18–23. doi: 10.1177/0268355515589536
- Rocha FA, Lins EM, de Almeida CC, et al. Quality of life assessment before and after surgery for lower limb varicose veins. J Vasc Bras. 2020;19:e20190108. doi: 10.1590/1677-5449.190108
- Shepherd AC, Gohel MS, Brown LC, et al. Randomized clinical trial of VNUS ClosureFAST radiofrequency ablation versus laser for varicose veins. Br J Surg. 2010;97(6):810–818. doi: 10.1002/bjs.7091