ABSTRACT
With more than 30 available stimulant medications, choosing among therapeutic options for attention-deficit/hyperactivity disorder (ADHD) has become increasingly complex and patient specific. All ADHD stimulants owe their action to variants of either amphetamine or methylphenidate, yet formulation and delivery system differences create unique pharmacokinetic and clinical profiles for each medication. A benefit of the diversity within ADHD pharmacotherapy is that it facilitates tailoring treatment to meet patient needs. Historically, there has been a constant among long-acting stimulant options, regardless of formulation, which was morning dosing. The introduction of delayed-release and extended-release methylphenidate (DR/ER-MPH) is the first long-acting stimulant that patients take in the evening, with the clinical effect delayed until awakening in the morning. This paradigm shift has generated questions among clinicians and continued interest in real-world experience and data. This review used available clinical data, real-world evidence, emerging analyses, and clinical experience to evaluate the characteristics of DR/ER-MPH and its clinical utility within the greater context of ADHD medications and to provide clinicians with practical guidance on the use of DR/ER-MPH in children, adolescents, and adults with ADHD.
1. Introduction
The current era of attention-deficit/hyperactivity disorder (ADHD) management is marked by a rich and diverse treatment armamentarium that continues to expand on both the stimulant and nonstimulant fronts. Clinicians have at their disposal an array of medications, each with unique characteristics, which facilitates tailoring treatment to meet an individual patient’s needs. Finding an effective pharmacotherapy regimen is a journey that frequently involves trying different medications to find the treatment that is well tolerated and achieves a patient’s treatment goals [Citation1–3].
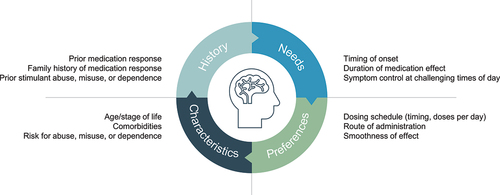
Medication selection involves a variety of factors from the easily ascertainable (e.g. age, comorbidities, medication history) to the more nuanced and personal (). The latter category includes addressing a patient’s need for symptom control during specific periods of the day; for example, to facilitate an early morning routine, to provide symptom control throughout the school or work day, or to allow for post-school or work activities (e.g. homework, family and home responsibilities, extracurricular and social interests). A relevant concern for patients, clinicians, and regulators when considering stimulant medication is the potential for abuse, misuse, and diversion. Patient management guidelines advocate the use of long-acting rather than short-acting stimulants to reduce the risk of abuse, misuse, and diversion [Citation4–6]. This sets up a dichotomy between clinical best practice and medication preference for a subset of patients who erroneously correlate the immediate energizing effects (‘the bump’) of short-acting stimulants with clinical benefit [Citation4].
The development of various long-acting stimulant formulations illustrates the increased appreciation for all-day control of ADHD-related symptoms to improve functional outcomes. Traditional long-acting stimulants are taken in the morning, but their ability to maintain effectiveness into the afternoon or evening is variable [Citation7]. The demand for extended duration is heightened by changing patient demographics, with greater recognition and diagnosis of ADHD in adults, particularly women [Citation8,Citation9]. For adults as well as adolescents, the need for focus and cognitive demand does not end when the school or work day is finished [Citation10,Citation11]. In recognition that the scope of a day varies across patients with ADHD, patient management guidelines recommend tailoring treatment to meet patient needs throughout the day [Citation4,Citation6].
In 2018, the first long-acting stimulant designed to be taken in the evening, delayed-release and extended-release methylphenidate (DR/ER-MPH; previously HLD200; Jornay PM®; Ironshore Pharmaceuticals, Inc.), was approved in the United States for the treatment of ADHD in patients aged 6 years and older. A delayed-release outer coating and an extended-release inner coating on DR/ER-MPH microbeads prevent medication release until 8 to 10 hours after it is taken, thus allowing for evening dosing with clinical effect deferred until awakening [Citation12]. The coatings also serve to delay medication release until the microbeads reach the lower gastrointestinal (GI) tract, resulting in slow absorption over the course of the day and providing an extended treatment effect [Citation13–15]. As a medication that uses a novel delivery platform and that is administered in the evening, DR/ER-MPH has raised questions in clinicians’ minds related to dosing, sleep, timing of efficacy, and suitability of the medication for patients without established or self-reported morning difficulties. Using the accumulated body of evidence (including recent data and analyses) and clinical experience, we evaluated the characteristics of DR/ER-MPH in the greater context of ADHD medications through the lens of a practicing clinician, with an eye toward the patient experience. Understanding the rationale for and mechanics of evening dosing and its utility for patients will help clinicians realize another tool for personalizing treatment of patients with ADHD.
2. Stimulant medications for ADHD
Psychostimulants used in the treatment of ADHD, amphetamine and methylphenidate, both increase extracellular concentrations of the monoamines dopamine and norepinephrine but do so through different mechanisms. Amphetamine is a substrate for monoamine transporters, through which it acts as a competitive inhibitor for monoamine uptake while also increasing cytosolic monoamine concentrations, thereby triggering efflux into the extracellular space [Citation16,Citation17]. Methylphenidate blocks reuptake of dopamine and norepinephrine by inhibiting monoamine transporters. All stimulant medications for ADHD contain either amphetamine or methylphenidate and have commensurate differences in mechanisms of action; however, the difference between individual medications of the same active ingredient is principally the formulation () [Citation7,Citation18,Citation19]. These formulation differences drive the unique pharmacokinetics of each agent [Citation18], including the delay between medication administration and first measurable concentration in the blood, peak concentration, presence or absence of multiple concentration peaks and associated low points (i.e. troughs) of circulating drug levels, and time it takes for medication to leave circulation. The pharmacokinetics of stimulant medications are, in turn, reflected in the formulation’s pharmacodynamics (i.e. efficacy profile) and in the dose timing and frequency. Notably, not all formulation variants are available in both amphetamine and methylphenidate versions. For example, formulations using an ion-exchange microparticle platform are available for amphetamine and methylphenidate [Citation18], but there is currently no amphetamine equivalent to DR/ER-MPH.
Figure 2. Evolution of drug delivery technologies. Characteristics of example short-, intermediate-, and long-acting stimulants used in the treatment of ADHD [Citation7,Citation18,Citation19]. Approximate timing of new technology introduction follows from left to right across the diagram. aTiming of dosing depends on whether the medication is being used as a single dose or in multiple doses and alone or in conjunction with a long-acting medication (either to provide rapid onset in the morning or to extend duration in the later part of the day). ADHD, attention-deficit/hyperactivity disorder; CR, controlled release; DR/ER-MPH, delayed-release and extended-release methylphenidate; ER, extended release; IR, immediate release; MEROS, methylphenidate extended-release oral suspension; OROS, osmotic-release oral system; SODAS, spheroidal oral drug absorption system.
![Figure 2. Evolution of drug delivery technologies. Characteristics of example short-, intermediate-, and long-acting stimulants used in the treatment of ADHD [Citation7,Citation18,Citation19]. Approximate timing of new technology introduction follows from left to right across the diagram. aTiming of dosing depends on whether the medication is being used as a single dose or in multiple doses and alone or in conjunction with a long-acting medication (either to provide rapid onset in the morning or to extend duration in the later part of the day). ADHD, attention-deficit/hyperactivity disorder; CR, controlled release; DR/ER-MPH, delayed-release and extended-release methylphenidate; ER, extended release; IR, immediate release; MEROS, methylphenidate extended-release oral suspension; OROS, osmotic-release oral system; SODAS, spheroidal oral drug absorption system.](/cms/asset/0fb3b687-17de-49ee-a0ed-09e9867b5f2c/ipgm_a_2370230_f0002_oc.jpg)
2.1. Pharmacokinetics of evening versus morning dosing
Long-acting ADHD medications are generally taken during the daytime, typically in the morning, whereas DR/ER-MPH is taken in the evening () [Citation7,Citation18]. Evening administration is made possible by two combined features: (1) the lack of an immediate-release component, and (2) the delayed-release and extended-release properties of the DR/ER-MPH microbeads. Most long-acting stimulants still rely on an immediate-release component within the formulation. If present, it is the immediate-release component that is responsible for the initial onset of clinical efficacy () [Citation18,Citation20]. The proportion of immediate-release to extended-release stimulant varies among combination long-acting formulations, ranging from 20% to 50% immediate-release, and has a direct influence on the medication’s pharmacokinetics [Citation18]. DR/ER-MPH and the prodrug lisdexamfetamine dimesylate are the only orally administered long-acting stimulant medications that do not contain an immediate-release component () [Citation18]. Unlike lisdexamfetamine dimesylate, the methylphenidate-based prodrug formulation, serdexmethylphenidate/dexmethylphenidate, includes an immediate-release component (30% by molar ratio) [Citation21].
Figure 3. Comparison of pharmacokinetic curves for long-acting stimulants given in the morning versus evening-dosed DR/ER-MPH. Simulated plasma concentration versus time curves for single doses of (a) OROS MPH 54 mg, (b) d-MPH ER 20 mg, (c) MEROS 60 mg, and (d) DR/ER-MPH 100 mg. Dashed vertical lines indicate when the medication dose was taken. Adapted from Gomeni et al.: Model-based approach for establishing the predicted clinical response of a delayed-release and extended-release methylphenidate for the treatment of attention-deficit/hyperactivity disorder. J Clin Psychopharmacol. 2020;40:350–358. Published by Wolters Kluwer Health, Inc. © Robert Gomeni, et al., 2020 licensed under Creative Commons Attribution 4.0 (CC BY). Proportions of immediate-release and extended-release methylphenidate data are from Childress et al. 2019 [Citation18]. conc, concentration; d-MPH ER, extended-release dexmethylphenidate; DR, delayed release; DR/ER-MPH, delayed-release and extended-release methylphenidate; ER, extended release; IR, immediate release; MEROS, methylphenidate extended-release oral suspension; MPH, methylphenidate; OROS, osmotic-release oral system.
![Figure 3. Comparison of pharmacokinetic curves for long-acting stimulants given in the morning versus evening-dosed DR/ER-MPH. Simulated plasma concentration versus time curves for single doses of (a) OROS MPH 54 mg, (b) d-MPH ER 20 mg, (c) MEROS 60 mg, and (d) DR/ER-MPH 100 mg. Dashed vertical lines indicate when the medication dose was taken. Adapted from Gomeni et al.: Model-based approach for establishing the predicted clinical response of a delayed-release and extended-release methylphenidate for the treatment of attention-deficit/hyperactivity disorder. J Clin Psychopharmacol. 2020;40:350–358. Published by Wolters Kluwer Health, Inc. © Robert Gomeni, et al., 2020 licensed under Creative Commons Attribution 4.0 (CC BY). Proportions of immediate-release and extended-release methylphenidate data are from Childress et al. 2019 [Citation18]. conc, concentration; d-MPH ER, extended-release dexmethylphenidate; DR, delayed release; DR/ER-MPH, delayed-release and extended-release methylphenidate; ER, extended release; IR, immediate release; MEROS, methylphenidate extended-release oral suspension; MPH, methylphenidate; OROS, osmotic-release oral system.](/cms/asset/3b190ab3-7fdc-4cdb-8e0b-b75b3fb6c029/ipgm_a_2370230_f0003_oc.jpg)
A key feature of the delayed-release and extended-release coatings on DR/ER-MPH microbeads is extending the time between when the medication is taken and when methylphenidate first appears in circulation (). Methylphenidate is not measurable in the blood until approximately 8 to 10 hours after DR/ER-MPH is taken [Citation12]. The pharmacokinetic curve is generally flat until approximately 8 hours postdose, with a subsequent initial slow rise in methylphenidate concentration and exposure to 5% or less of the total administered dose by 10 hours postdose. Pharmacokinetic analyses in children, adolescents, and adults have shown that the delay in methylphenidate release is consistent and predictable regardless of patient age or physiology [Citation12]. After the initial delay period, methylphenidate concentrations in the blood increase rapidly although at a seemingly more gradual slope than long-acting formulations that contain an immediate-release component.
2.2. Influence of absorption on pharmacokinetics
Another feature of the delayed-release and extended-release coatings on DR/ER-MPH microbeads is the delayed absorption of methylphenidate until reaching the lower GI tract [Citation13]. Other oral ADHD medications, and most oral medications in general, are absorbed in the upper GI tract, specifically the small intestine [Citation22,Citation23]. Structural and physiologic differences between GI regions, such as surface area, luminal fluid volume, presence of villi, mucus layer, drug transporter distribution, enzyme concentrations, and residence time, affect absorptive potential [Citation23,Citation24]. The influence of these regional differences on methylphenidate absorption was demonstrated by Incledon et al. [Citation13] who tested three variations of DR/ER-MPH designed to target absorption in the small intestine, proximal colon, or distal colon. The pharmacokinetic profile of the three varied formulations was markedly different, with absorption in the proximal colon versus the small intestine resulting in a more gradual increase in circulating methylphenidate over time, a lower peak concentration, and reduced bioavailability.
An attribute that was common to all three formulations tested by Incledon et al. [Citation13] was a smooth pharmacokinetic profile with a single peak. The lone pharmacokinetic peak is the direct result of having a single-entity formulation with no immediate-release component. As previously discussed, long-acting stimulants that combine immediate-release and extended-release components have multiple pharmacokinetic curve peaks resulting from the separate pulses of release: the first peak from the immediate-release component and the second (and, in some cases, third) peak from the delayed/controlled/extended-release component(s) () [Citation18]. After each peak, there is often a decrease in circulating stimulant concentration, creating a trough. Although there have not been studies evaluating the consequences of peaks and troughs on symptom control and rebound effects, anecdotal evidence suggests that, for at least some patients, the effects are noticeable [Citation25,Citation26]. Moreover, the occurrence of emotional lability, or sudden strong changes in mood, may be tied to waning stimulant levels [Citation27].
2.3. Measuring the smoothness of pharmacokinetic curves
A concept that has arisen to describe and compare pharmacokinetic curve fluctuations is smoothness. An idealized ‘smooth’ pharmacokinetic profile would have a consistent rate of increase during the initial absorption phase that is followed by a single peak and a consistent rate of decline in circulating drug concentration. In the context of ADHD, the initial slope of the pharmacokinetic curve should not be overly steep to avoid the sensation of the medication ‘kicking in,’ which is common to short-acting or immediate-release containing formulations and reflects the energizing effects of stimulants rather than clinical efficacy [Citation4]. The rapid ascending curve for short-acting formulations is also associated with increased likability, indicating greater abuse potential compared with formulations that have a more gradual initial absorption phase [Citation28]. A gradual rate of decrease may prevent the occurrence of rebound that has been speculatively linked to a rapid decline in circulating stimulant concentration [Citation28]. Through an innovative analysis that used mathematical equations to model pharmacokinetic curves, Po et al. [Citation29] quantified the smoothness of pharmacokinetic profiles for various long-acting methylphenidate formulations. Among the formulations tested, DR/ER-MPH exhibited the smoothest pharmacokinetic profile, which is consistent with visual comparisons of the pharmacokinetic curve characteristics among formulations ().
2.4. Pharmacokinetics enabling titratable duration of effect
An additional trait attributable to the mechanism of absorption and resulting pharmacokinetic profile of DR/ER-MPH is titratable duration of effect, wherein increasing the dose increases the duration of clinical efficacy. In comparison with other long-acting methylphenidate formulations, DR/ER-MPH has a protracted elimination phase that is likely the result of extended residence time (i.e. more time spent) and absorption in the lower GI tract [Citation12,Citation20]. As the dose is increased, the pharmacokinetic profile shifts upward along the Y-axis, potentially allowing methylphenidate levels to remain above the efficacy threshold for an extended period of time. Dose-dependent duration with DR/ER-MPH was established using a model-based approach that incorporated pharmacokinetic data from a phase 1 clinical trial, pharmacodynamic data from a phase 3 clinical trial, and simulated plasma concentration-time data [Citation20]. The resulting pharmacokinetic-pharmacodynamic model showed that clinical improvement with DR/ER-MPH was observed from early morning through late afternoon or evening and that the duration of effect increased with increasing dose. From a clinical standpoint, these data support an approach of increasing DR/ER-MPH dose to increase the duration of effect.
3. Clinical studies of DR/ER-MPH
3.1. Study design
The efficacy and safety of DR/ER-MPH were evaluated in two pivotal, phase 3, multicenter, randomized, placebo-controlled clinical trials that included data from 280 children (aged 6 to 12 years) with ADHD [Citation14,Citation15]. Following a ≤ 4-week screening period, patients in Study 1 began a 6 week, open-label, DR/ER-MPH dose-optimization phase [Citation14]. All patients initiated treatment with DR/ER-MPH 20 mg or 40 mg once daily (depending on treatment history) in the evening (8:00 pm ±30 minutes). After week 1, dose titration was permitted once per week in increments of 20 mg or 40 mg to achieve optimal daily dose, and dose timing could be adjusted within the range of 6:30 pm to 9:30 pm. The optimal dose was defined as that at which meaningful symptom control and relief of early morning functional impairment were achieved while remaining safe and well tolerated. After completing week 6, 119 patients were randomized (1:1) to DR/ER‑MPH at their optimized dose or placebo for 1 week that concluded with a laboratory classroom test day.
Study 2 was a 3-week, double-blind, placebo-controlled, forced-dose titration clinical trial conducted entirely outside the laboratory classroom setting (i.e. in a naturalistic setting) [Citation15]. After a ≤ 2-week screening period, patients were randomized (1:1) to DR/ER-MPH or placebo once daily in the evening for 3 weeks. The starting dose for all patients randomized to DR/ER-MPH was 40 mg/day, and the dose was increased to 60 mg/day on week 2 and 80 mg/day on week 3. Down titration by 20 mg/day was permitted once per patient for safety or tolerability reasons, and dose timing could be adjusted as per Study 1. A total of 161 patients were included in the efficacy and safety analyses.
3.2. Primary efficacy measure results
In Study 1, patients in the DR/ER-MPH treatment group performed significantly better than placebo-treated patients (p < 0.001) on the classroom-based primary endpoint measure, the model-adjusted postdose average Swanson, Kotkin, Agler, M-Flynn, and Pelham (SKAMP) combined score, which was assessed over a continuous 12-hour period (8:00 am to 8:00 pm) on the final day of the double-blind treatment phase [Citation14]. In Study 2, patients who were randomized to DR/ER-MPH experienced significant reductions in ADHD symptoms, as measured by the ADHD Rating Scale IV (ADHD-RS-IV) total score, at week 3 compared with patients in the placebo group (p = 0.002) [Citation15].
3.3. Achievement of clinical response and remission
In both of the phase 3 studies, DR/ER-MPH ameliorated ADHD-related symptoms and functional impairment from early morning through the late afternoon/evening as measured by an array of validated scales [Citation14,Citation15,Citation30–32]. Among these measures were the Before School Functioning Questionnaire (BSFQ) and the Parent Rating of Evening and Morning Behavior-Revised (PREMB-R). The BSFQ is focused on the early morning, before school time period (6:00 am to 9:00 am) [Citation32], and the PREMB-R captures time points (e.g. early morning, late afternoon, evening) and behaviors outside of the classroom setting (e.g. getting out of bed, completing homework, sitting through dinner, falling asleep) that are not accounted for by traditional scales [Citation15,Citation30]. The overall PREMB-R can be divided into questions that apply to morning symptoms (PREMB-R AM) and those that apply to later afternoon/evening symptoms (PREMB-R PM).
Faraone et al. [Citation33] categorized the severity of ADHD-related impairment by applying previously established thresholds for BSFQ and PREMB-R scores [Citation34] to patients treated with DR/ER-MPH or placebo in the 6-week, open-label, dose-optimization phase of Study 1. At baseline, the majority of patients (87%) demonstrated morning impairment of at least mild severity on the BSFQ () [Citation33]. After 6 weeks of treatment with DR/ER-MPH, 94% of patients scored in the nonimpaired range. Similarly, for the PREMB-R PM, 81% of patients demonstrated evening impairment of at least mild severity at baseline, whereas at week 6, 84% of patients scored in the nonimpaired range. In an identical analysis of data from DR/ER-MPH Study 2 [Citation35], a greater proportion of patients achieved ‘normalized’ scores at week 3 with DR/ER-MPH versus placebo.
Figure 4. Achievement of norm-referenced thresholds for impairment before and after DR/ER‑MPH dose optimization. Proportion of patients who met thresholds for impairment on the BSFQ and PREMB-R PM at baseline and after 6 weeks of DR/ER-MPH treatment in the phase 3, dose-optimization clinical trial (Study 1) [Citation33]. BSFQ, Before School Functioning Questionnaire; DR/ER-MPH, delayed-release and extended-release methylphenidate; PREMB-R PM, Parent Rating of Evening and Morning Behavior-Revised, Evening subscale.
![Figure 4. Achievement of norm-referenced thresholds for impairment before and after DR/ER‑MPH dose optimization. Proportion of patients who met thresholds for impairment on the BSFQ and PREMB-R PM at baseline and after 6 weeks of DR/ER-MPH treatment in the phase 3, dose-optimization clinical trial (Study 1) [Citation33]. BSFQ, Before School Functioning Questionnaire; DR/ER-MPH, delayed-release and extended-release methylphenidate; PREMB-R PM, Parent Rating of Evening and Morning Behavior-Revised, Evening subscale.](/cms/asset/4bb5847d-cd2f-49ee-8e95-bb963248f6a3/ipgm_a_2370230_f0004_oc.jpg)
A further post hoc analysis of Study 1 was conducted to assess achievement of thresholds for clinically meaningful response and remission of ADHD-related symptoms and impairment (i.e. normalization) [Citation36]. Response and remission criteria were based on previously established thresholds for the ADHD-RS-IV, BSFQ, PREMB-R AM, and PREMB-R PM [Citation34,Citation37,Citation38]. After 6 weeks of DR/ER-MPH dose optimization, response and remission targets were reached by 97% and 89% of patients for ADHD-RS-IV, 98% and 94% of patients for BSFQ, 94% and 98% of patients for PREMB-R AM, and 91% and 84% of patients for PREMB-R PM, respectively [Citation36]. Together, these analyses illustrate that the improvements with DR/ER-MPH represent remission of ADHD-related symptoms and functional impairment for a large proportion of patients.
3.4. Influence on family and caregivers
Symptoms and functional impairment associated with ADHD are not only troubling to the affected individual, but can generate stress for family members and caregivers that persists despite the patient receiving ADHD medication [Citation39–41]. The effect of DR/ER-MPH treatment of children with ADHD on relieving caregiver strain was measured as an exploratory endpoint in Study 2 using the Caregiver Strain Questionnaire (CGSQ) [Citation42]. Caregivers of children treated with DR/ER-MPH reported significant reductions from baseline in strain compared with caregivers of children in the placebo group (p < 0.001). A post hoc analysis further demonstrated that improvements in CGSQ were clinically meaningful for a greater proportion of caregivers of patients treated with DR/ER-MPH (53.2%) versus placebo (29.9%; p = 0.003).
3.5. Safety and tolerability
In Study 1, the most common treatment-emergent adverse events (TEAEs) reported during the 6-week, open-label, dose-optimization phase were any insomnia (41%), decreased appetite (27%), affect lability (22%), and headache (18%) [Citation14]. The majority of TEAEs were mild or moderate, and no serious TEAEs were reported. The occurrence of TEAEs was lower during the subsequent 1-week, double-blind, placebo-controlled phase of the study, with a similar percentage of patients reporting at least 1 TEAE in the DR/ER-MPH and placebo treatment groups (37% and 41%, respectively). TEAEs with an incidence of 5% or more occurred at a similar rate with DR/ER-MPH or placebo, including increased diastolic blood pressure (14% vs 13%) and any insomnia (8% vs 9%). During the 3-week, forced-dose titration phase of Study 2, the most common TEAEs in the DR/ER-MPH and placebo treatment groups were any insomnia (33% vs 9%), decreased appetite (19% vs 4%), and headache (10% vs 5%) [Citation43]. In both clinical trials, changes in vital signs were consistent with expectations for a methylphenidate formulation [Citation14,Citation15].
3.6. Sleep-related events
Difficulties with sleep onset, fragmentation, and maintenance are frequently seen in children, adolescents, and adults with ADHD, and may be exacerbated by stimulant medications [Citation44–47]. Given the potential concern about the effect of an evening-dosed stimulant on sleep, sleep disturbances were a TEAE of special interest in DR/ER-MPH clinical trials, with direct queries to participants and caregivers at each study visit [Citation14,Citation15]. As commonly seen in other stimulant trials, insomnia was among the most common TEAEs in both phase 3 clinical trials. In Study 2, which utilized a placebo control starting at the beginning of study treatment, any insomnia was reported by 33% of patients in the DR/ER-MPH treatment group and 9% of patients in the placebo group [Citation43]. All sleep-related TEAEs were mild or moderate in severity and resolved in 97% of patients by the end of the study (week 3) in the DR/ER-MPH treatment group [Citation15]. For 90% of those with sleep-related TEAEs, study medication dose was not reduced. Reassuringly, no patients treated with DR/ER-MPH in either phase 3 study withdrew from the study due to a sleep-related TEAE [Citation14,Citation15].
Context for these findings relative to other methylphenidate products is provided by a meta-analysis of 35 blinded, placebo-controlled, methylphenidate clinical trials that enrolled 3079 drug-exposed and 2606 placebo-treated children and adolescents with ADHD [Citation48]. Overall, methylphenidate treatment was associated with the occurrence of insomnia and sleep disorder. After adjustment for confounders, the relative risk for sleep-related TEAEs with DR/ER-MPH in this meta-analysis was similar to that observed in clinical trials of other methylphenidate products; moreover, the 95% confidence interval for the relative risk of insomnia overlapped 1, meaning that the risk with DR/ER-MPH was not statistically significantly different than that of placebo. Significant differences were observed among methylphenidate formulations, with higher relative risks observed for osmotic-release oral system methylphenidate, methylphenidate transdermal system, and methylphenidate controlled-release. Notably, a confounder identified in this analysis was if sleep-related events relied on spontaneous patient report or if providers asked patients about sleep. Asking about sleep rather than having patients report if they have sleep concerns has been attributed to higher sleep-related event rates [Citation48,Citation49]. The lack of effect on sleep beyond what is generally associated with other methylphenidate formulations aligns with the pharmacokinetics of DR/ER-MPH in which methylphenidate release is delayed for 8 to 10 hours after administration [Citation12], thereby allowing for evening administration without a clinical/pharmacodynamic response until awakening in the morning.
4. Real-world experience with DR/ER-MPH
4.1. Adherence
Adherence is a known issue with ADHD medications, with adherence rates comparable to those of other chronic psychiatric diseases associated with poor adherence such as schizophrenia and bipolar disorder [Citation50]. In the two phase 3 clinical trials of DR/ER-MPH, adherence was measured using the Medication Event Monitoring System (MEMS), which captures the time and date of study medication bottle opening [Citation51]. Patients who had a MEMS cap opening recorded for at least 80% of days during the week were considered adherent to treatment. During double-blind treatment in Study 1, 98% of patients were adherent to DR/ER-MPH, and for each of the 3 weeks evaluated in Study 2, 86% or more of patients were adherent to DR/ER-MPH. Of course, adherence is known to be higher in a clinical trial versus a nonclinical trial setting due to factors such as more frequent clinic visits, patient motivation, and selection bias [Citation52]. Using data from a large US claims database, Lloyd et al. [Citation53] evaluated adherence for an array of generic and branded long-acting stimulant medications over a 9-month period in a real-world setting. Average adherence, as measured by medication possession ratio, was significantly higher for DR/ER-MPH (92.1%) than for amphetamine prodrug (89.0%), generic long-acting amphetamine formulations (88.8%), branded long-acting stimulant formulations (88.1%), and generic long-acting methylphenidate formulations (87.8%; p < 0.0001 for all comparisons). The potential for a positive influence on adherence is intriguing but would benefit from additional study, including insights into the elements driving the observed differences.
4.2. Adults with ADHD
A retrospective, observational cohort study was conducted to evaluate real-world dosing, clinical efficacy, and safety/tolerability of DR/ER-MPH in the adult population [Citation54]. The study included 50 patients aged 16 years and older, with a mean age of 36 years (range, 16–56 years), enrolled from two clinics familiar with dose optimization of DR/ER-MPH. The population was notable for a large proportion of patients (86%) with at least one psychiatric comorbidity, including anxiety (82%) and depression (20%), and a high frequency (42%) of treatment with an amphetamine-based medication formulation as the most recent ADHD pharmacotherapy before starting DR/ER-MPH. Among patients who had baseline and on-treatment data (n = 41), treatment with DR/ER-MPH for at least 3 months significantly improved mean Adult ADHD Self-Report Scale scores from baseline (p = 0.001). Interestingly, 9 of 16 patients who were receiving combination therapy with more than one ADHD medication immediately before starting DR/ER‑MPH required only monotherapy with DR/ER-MPH after dose optimization. These data support the real-world efficacy of dose-optimized DR/ER-MPH in adults with ADHD and other comorbid conditions but would benefit from confirmation in additional real-world data sets.
4.3. Safety and tolerability
Overall, the accumulated evidence indicates that DR/ER-MPH is well tolerated and has a safety profile consistent with other methylphenidate formulations [Citation14,Citation15,Citation43,Citation55]. Postmarketing surveillance expands safety observations with DR/ER-MPH to approximately 74,000 children, adolescents, and adults treated outside of a clinical trial setting [Citation55]. No new safety signals arose from this postmarketing surveillance data.
5. Administration and titration of DR/ER-MPH
5.1. Starting dose
The recommended starting dose of DR/ER-MPH in patients aged 6 years and older is 20 mg once daily taken in the evening (starting at 8:00 pm, but with the ability to adjust timing to optimize tolerability and efficacy within the suggested range of 6:30 pm to 9:30 pm) [Citation43]. In Study 1, starting dose selection (20 mg/day or 40 mg/day) was based on treatment history, and in Study 2, all patients started treatment with 40 mg/day [Citation14,Citation15]. A post hoc analysis of data from Study 1 showed that patients who started treatment at 40 mg/day reached symptomatic and functional response and remission targets more quickly than those who started with 20 mg/day, whereas TEAEs during the first week of treatment were similar whether patients started treatment with the 20- or 40-mg/day dose [Citation36]. These data provide reassurance that clinicians should not be concerned about swiftly increasing the DR/ER-MPH dose from 20 mg/day to 40 mg/day, as the higher dose improved symptoms in less time without an increase in TEAEs.
5.2. Dose optimization
DR/ER-MPH dose can be increased weekly at 20-mg increments to achieve efficacy and duration goals. The available dosage strengths are 20 mg, 40 mg, 60 mg, 80 mg, and 100 mg. In Study 1, the maximum DR/ER-MPH dose was 100 mg/day or 3.7 mg/kg/day [Citation14]. The maximum achievable dose in Study 2 was 80 mg/day or 3.7 mg/kg/day, as the patients started at 40 mg/day and had the opportunity for only two dose increases over the 3-week study period [Citation15]. At the end of the 6-week, dose-optimization phase in Study 1, the mean dose of DR/ER-MPH was 66.2 mg/day [Citation14]. This achieved dose reflects dose optimization in a clinical trial setting for children aged 6 to 12 years. Real-world data indicate that higher optimized DR/ER-MPH doses are needed in the adult population. In a retrospective cohort of patients aged 16 years and older, the mean starting dose of DR/ER-MPH was 53.6 mg/day, and the mean optimized dose after more than 3 months of treatment was 81.9 mg/day [Citation54]. More than half of these patients were optimized at the 100 mg/day dose.
In clinical trials, DR/ER-MPH titration occurred once weekly, and dose optimization was measured by improvement from baseline on multiple validated ADHD scales [Citation14,Citation15]. In clinical practice, the pace of titration is likely slower due to less frequent clinic visits. A well-established rule of thumb is to follow-up frequently when starting or switching ADHD medications (e.g. 2- to 3-week increments) to allow for rapid dose optimization while monitoring efficacy and tolerability. This is preferred versus a ‘wait-and-see’ approach due to the speed at which these agents demonstrate their clinical effect. We also recommend objective testing to monitor the patient’s response to the medication during the titration period.
5.3. Switching from other long-acting stimulants
When transitioning from another medication to DR/ER-MPH, the dose cannot be determined based on milligram-per-milligram substitution because of fundamental differences in pharmacokinetic profiles and methylphenidate base composition [Citation43]. Due to its absorption in the lower GI tract, DR/ER-MPH has reduced bioavailability compared with other methylphenidate formulations. In adults, the relative bioavailability of DR/ER-MPH is 73.9% compared with the same dose of orally administered immediate-release methylphenidate [Citation56]. An exploratory post hoc analysis of data from phase 3 clinical trials determined that optimized doses of DR/ER-MPH were greater than stabilized doses of prior ADHD stimulant medications [Citation57]. Optimized doses of DR/ER-MPH ranged from 1.8 to 4.3 times greater than prior long-acting stimulants and 4.7 to 6.0 times greater than prior short-acting stimulants [Citation57]. Therefore, it should not be surprising if a patient’s optimized DR/ER-MPH dose is higher than that of their previous stimulant medication.
6. Clinical pearls
With the benefit of real-world evidence, emerging analyses, and clinical experience, we now have a better appreciation for how DR/ER-MPH fits in the ADHD treatment armamentarium and for whom it is an appropriate treatment option. The emphasis on improvements in morning symptoms and functioning when DR/ER-MPH first came to market may have pigeonholed it in clinicians’ minds as a treatment option reserved for patients with early morning dysfunction. Upon further analysis, DR/ER-MPH was more broadly beneficial to children, adolescents, and adults, with symptomatic and functional improvements observed throughout the day and into the late afternoon/evening for the vast majority of patients. Scenarios that would prompt clinicians to consider DR/ER-MPH as a potentially preferred treatment option are listed in [Citation4–6,Citation41,Citation43].
Table 1. Criteria for considering DR/ER-MPH.
A long-acting stimulant with efficacy that lasts into the late afternoon or evening and whose duration can be adjusted by titrating the dose is a particularly attractive treatment option for adolescent and adult patients with ADHD who have long days with obligations that extend beyond the school or work day [Citation10,Citation11]. Adults with ADHD, particularly women, have been historically overlooked [Citation58,Citation59]. Although there is a dearth of recent data, estimates from 10 to 15 years ago pointed to a rate of less than 20% of adults with ADHD currently diagnosed and/or receiving specialist treatment [Citation58]. There is also a longstanding myth that methylphenidate is for younger patients and amphetamine is for adults; however, adults can and do benefit from appropriate methylphenidate formulations [Citation60,Citation61].
With its smooth pharmacokinetic profile and single daily peak [Citation29], DR/ER-MPH may present a viable option for patients who are sensitive to fluctuating medication levels (e.g. feeling the effects of medication peaks and troughs, experiencing rebound symptoms or emotional lability), particularly those who have experienced these issues with prior stimulant medications.
Clinicians may have been hesitant to consider an evening-dosed stimulant over concerns about its effects on sleep. However, the available data indicate that the overall safety/tolerability profile and the effects on sleep with DR/ER-MPH are similar to other methylphenidate formulations [Citation14,Citation15,Citation43,Citation48,Citation55]. Lastly, although we would not limit DR/ER-MPH to patients with early morning dysfunction, having a medication onboard when awakening may make mornings easier, and easier mornings benefit the entire family [Citation41]. Moreover, clinical data have established that DR/ER-MPH has a long and titratable duration of effect, so that even with a treatment effect upon awakening, the medication can last into the late afternoon or evening, as needed.
6.1. Case studies
A 45-year-old male with a history of ADHD was referred from primary care to a specialty clinic. He had been prescribed short-acting mixed amphetamine salts (Adderall®; Teva Pharmaceuticals U.S.A., Inc.) 20 mg three times daily. He would occasionally increase this to four times per day if he ‘had a lot of work.’ While on medication, he reported that he felt really focused; however, his coworkers, spouse, and children described him as frequently irritable, moody, and difficult to get along with. He was switched to DR/ER-MPH at a starting dose of 40 mg/day and titrated through the 60-mg/day and 80-mg/day doses at subsequent clinic visits and is currently receiving DR/ER-MPH 100 mg/day. He reports that he is able to maintain focus throughout the day and evening and that his mood has improved, which has had a positive effect on his relationships as well.
A 17-year-old female presented with concerns regarding executive dysfunction (disorganization, forgetfulness, difficulty with follow through) and emotional dysregulation (irritability and hypersensitivity), both of which affected her in school, at home, and with sports. She had been diagnosed with ADHD in middle school and was started on a long-acting methylphenidate (osmotic-release oral system methylphenidate [Concerta®; Janssen Pharmaceuticals, Inc.]), which she described as giving her a ‘zombie-like’ feeling. She switched to lisdexamfetamine dimesylate (Vyvanse®; Takeda Pharmaceuticals America, Inc.), which made her feel agitated and markedly decreased her appetite. She was subsequently diagnosed with anxiety and depression, for which she began therapy and was prescribed fluoxetine. She reported that the fluoxetine helped some with her emotional dysregulation, but she still struggled with executive dysfunction. She had started on DR/ER-MPH at 20 mg/day and was titrated over time to 100 mg/day. She reports vast improvement in her executive dysfunction. She feels overall calmer and less emotional and is less stressed over schoolwork, but still has her ‘bubbly’ personality. She is doing better at home, in school, socially, at her part-time job, and in sports.
Parents of a 12-year-old boy receiving treatment for ADHD reported ongoing school performance issues and occasional disciplinary action for classroom disruption, typically trying to get laughs from friends. His grades have been declining. He is a talented football player, but when not playing football, he had a hard time getting work done and controlling his need to talk. His behavior at school was causing missed football practices due to detentions. His days are long because of football practice, and he had a hard time getting homework done after practice. He started psychotherapy, but when his grades placed him on academic probation from football, his parents were open to trying medication. Treatment with dexmethylphenidate hydrochloride (Focalin XR®; Novartis Pharmaceuticals Corporation) at 40 mg/day improved his symptoms without affecting appetite or sleep; however, symptom control did not last beyond lunchtime. Switching to Vyvanse at 40 mg/day gave a similar result. Because he had responded to methylphenidate and it was well tolerated, the decision was made to try a different once-daily, long-acting methylphenidate formulation to extend symptom control into later in the day. DR/ER-MPH at 20 mg/day was started and titrated over time to 60 mg/day. His grades and focus improved, and he no longer had issues completing his homework. His parents were happy to report that he was no longer getting in trouble for classroom disruptions and that he had better control of his need to talk.
7. Conclusions
Evening dosing for ADHD treatment has several unique advantages for patients that cannot be achieved with morning administration of long-acting stimulants. As demonstrated in the two phase 3 clinical trials, a single evening dose of DR/ER-MPH effectively improves ADHD-related symptoms and functional impairment upon awakening, throughout the day, through late afternoon, and into the evening. Moreover, accumulated evidence has shown that evening administration does not adversely affect the safety or tolerability profile of methylphenidate. Nonetheless, the evidence base could be made more robust through additional clinical trials and real-world studies. Evening dosing of DR/ER‑MPH is made possible by the microbead coatings, which delay methylphenidate release for 8 to 10 hours, thereby shifting absorption to the lower GI tract where it is slowly released over the course of the day. These attributes avoid the need for inclusion of an immediate-release component to generate rapid onset of effect and allow for optimizing symptom control duration with a purely delayed-release/extended-release medication. Understanding how evening dosing works and its potential benefits and tolerability for patients with ADHD will help clinicians realize another tool for personalizing their approach to care for patients with ADHD.
Declaration of financial/other relationships
GWM has served as a speaker for AbbVie, Alkermes, Axsome, Corium, Intra-Cellular, Ironshore, Janssen, Lundbeck, Neurocrine Biosciences, Noven, Otsuka, Sunovion, Supernus, Takeda, and Tris Pharma; has served a consultant for AbbVie, Alkermes, Axsome, Biogen, Corium, Eisai, Ironshore, Intra-Cellular, Janssen, Lundbeck, Neurocrine Biosciences, Noven, Otsuka, Redax, Roche, Sage Therapeutics, Sirona, Sunovion, Supernus, Takeda, and Teva; and has served as a researcher with AbbVie, Acadia, Alkermes, Akili, Axsome, Boehringer Ingelheim, Emalex Biosciences, Idorsia, Janssen, Karuna Therapeutics, Lumos Labs, Medgenics, Neurocrine Biosciences, NLS-1 Pharma AG, Otsuka, Redax, Relmada, Roche, Sage Therapeutics, Sirtsei, Sunovion, Supernus, Takeda, and Teva. JAC serves as a consultant to Karuna Therapeutics and has served on advisory boards for Karuna Therapeutics Supernus, and Janssen. PR speaks on behalf of Ironshore, Aytu Biopharma, Tris Pharma, Corium, and Supernus; has served on advisory boards for Aytu Biopharma, Corium, and Ironshore; has received research support from Ironshore; and serves on the ADHD Expert Consortium. FAL has served as a consultant to and received speaker fees and/or research support from Eli Lilly, GSK, Ironshore, Neos, Novartis, Noven, Pfizer, ShireUS, Shire Canada and Shire/UBC Europe, Sunovion, Supernus, and Tris; has served on the Experts on Call for Shire; and is presently a member of Team ADHD for Supernus. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author contributions
Conceptualization: Gregory W. Mattingly, Julie A. Carbray, Perry Roy, Frank A. López; Writing – review and editing: Gregory W. Mattingly, Julie A. Carbray, Perry Roy, Frank A. López.
Abbreviations
| ADHD | = | Attention-deficit/hyperactivity disorder |
| ADHD-RS-IV | = | ADHD Rating Scale IV |
| BSFQ | = | Before School Functioning Questionnaire |
| CGSQ | = | Caregiver Strain Questionnaire |
| DR/ER-MPH | = | Delayed-release and extended-release methylphenidate |
| GI | = | Gastrointestinal |
| MEMS | = | Medication Event Monitoring System |
| PREMB-R | = | Parent Rating of Evening and Morning Behavior-Revised |
| PREMB-R AM | = | Parent Rating of Evening and Morning Behavior-Revised, Morning subscale |
| PREMB-R PM | = | Parent Rating of Evening and Morning Behavior-Revised, Evening subscale |
| SKAMP | = | Swanson, Kotkin, Agler, M-Flynn, and Pelham |
| TEAE | = | Treatment-emergent adverse event |
Acknowledgments
Medical writing support for development of this article was provided by Crystal Murcia of JB Ashtin and funded by Ironshore.
Additional information
Funding
References
- Biederman J, DiSalvo M, Green A, et al. Rates of switching stimulants in consecutively referred medication naive adults with ADHD. Acta Psychiatr Scand. 2021 Dec;144(6):626–634. doi: 10.1111/acps.13370
- Schein J, Childress A, Cloutier M, et al. Reasons for treatment changes in adults with attention-deficit/hyperactivity disorder: a chart review study. BMC Psychiatry. [2022 Jun 3];22(1):377. doi: 10.1186/s12888-022-04016-9
- Schein J, Cloutier M, Gauthier-Loiselle M, et al. Reasons for treatment changes in children and adolescents with attention-deficit/hyperactivity disorder: a chart review study. Adv Ther. 2022 Dec;39(12):5487–5503. doi: 10.1007/s12325-022-02329-5
- CADDRA. Canadian ADHD resource alliance: Canadian ADHD Practice Guidelines. 4.1 ed. Toronto (ON): CADDRA; 2020.
- Kooij JJS, Bijlenga D, Salerno L, et al. Updated European consensus statement on diagnosis and treatment of adult ADHD. Eur Psychiatry. 2019 Feb;56(1):14–34. doi: 10.1016/j.eurpsy.2018.11.001
- National Institute for Health and Care Excellence (NICE) [Internet]. Attention deficit hyperactivity disorder: diagnosis and management (NG87) [updated 2019 Sep 13;cited 2023 Feb 14]. Available from: https://www.nice.org.uk/guidance/ng87
- Mattingly GW, Wilson J, Ugarte L, et al. Individualization of attention-deficit/hyperactivity disorder treatment: pharmacotherapy considerations by age and co-occurring conditions. CNS Spectr. 2021 Jun;26(3):202–221. doi: 10.1017/S1092852919001822
- Bellak L, Black RB. Attention-deficit hyperactivity disorder in adults. Clin Ther. 1992 Mar;14(2):138–147.
- Song P, Zha M, Yang Q, et al. The prevalence of adult attention-deficit hyperactivity disorder: a global systematic review and meta-analysis. J Glob Health. [2021 Feb 11];11:04009. doi: 10.7189/jogh.11.04009
- Brown TE, Romero B, Sarocco P, et al. The patient perspective: unmet treatment needs in adults with attention-deficit/hyperactivity disorder. Prim Care Companion CNS Disord. [2019 Jun 6];21(3):18m02397. doi: 10.4088/PCC.18m02397
- Pedersen SL, Kennedy TM, Joseph HM, et al. Real-world changes in adolescents’ ADHD symptoms within the day and across school and non-school days. J Abnorm Child Psychol. 2020 Dec;48(12):1543–1553. doi: 10.1007/s10802-020-00695-8
- Childress A, Mehrotra S, Gobburu J, et al. Single-dose pharmacokinetics of HLD200, a delayed-release and extended-release methylphenidate formulation, in healthy adults and in adolescents and children with attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol. 2018 Feb;28(1):10–18. doi: 10.1089/cap.2017.0044
- Incledon B, Incledon C, Gomeni R, et al. Effect of colonic absorption on the pharmacokinetic properties of delayed-release and extended-release methylphenidate: in vitro, and modeling evaluations. Clin Pharmacol Drug Dev. 2022 Aug;11(8):966–975. doi: 10.1002/cpdd.1089
- Childress AC, Cutler AJ, Marraffino A, et al. A randomized, double-blind, placebo-controlled study of HLD200, a delayed-release and extended-release methylphenidate, in children with attention-deficit/hyperactivity disorder: an evaluation of safety and efficacy throughout the day and across settings. J Child Adolesc Psychopharmacol. 2020 Feb;30(1):2–14. doi: 10.1089/cap.2019.0070
- Pliszka SR, Wilens TE, Bostrom S, et al. Efficacy and safety of HLD200, delayed-release and extended-release methylphenidate, in children with attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol. 2017 Aug;27(6):474–482. doi: 10.1089/cap.2017.0084
- Sitte HH, Freissmuth M. Amphetamines, new psychoactive drugs and the monoamine transporter cycle. Trends Pharmacol Sci. 2015 Jan;36(1):41–50. doi: 10.1016/j.tips.2014.11.006
- Mayer FP, Schmid D, Owens WA, et al. An unsuspected role for organic cation transporter 3 in the actions of amphetamine. Neuropsychopharmacology. 2018 Nov;43(12):2408–2417. doi: 10.1038/s41386-018-0053-5
- Childress AC, Komolova M, Sallee FR. An update on the pharmacokinetic considerations in the treatment of ADHD with long-acting methylphenidate and amphetamine formulations. Expert Opin Drug Metab Toxicol. 2019 Nov;15(11):937–974. doi: 10.1080/17425255.2019.1675636
- Kollins SH, Braeckman R, Guenther S, et al. A randomized, controlled laboratory classroom study of serdexmethylphenidate and d-methylphenidate capsules in children with attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol. 2021 Nov;31(9):597–609. doi: 10.1089/cap.2021.0077
- Gomeni R, Komolova M, Incledon B, et al. Model-based approach for establishing the predicted clinical response of a delayed-release and extended-release methylphenidate for the treatment of attention-deficit/hyperactivity disorder. J Clin Psychopharmacol. 2020 Jul;40(4):350–358. doi: 10.1097/JCP.0000000000001222
- Braeckman R, Guenther S, Mickle TC, et al. Dose proportionality and steady-state pharmacokinetics of serdexmethylphenidate/dexmethylphenidate, a novel prodrug combination to treat attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol. 2022 Jun;32(5):288–295. doi: 10.1089/cap.2022.0015
- Murakami T. Absorption sites of orally administered drugs in the small intestine. Expert Opin Drug Discov. 2017 Dec;12(12):1219–1232. doi: 10.1080/17460441.2017.1378176
- Vertzoni M, Augustijns P, Grimm M, et al. Impact of regional differences along the gastrointestinal tract of healthy adults on oral drug absorption: an UNGAP review. Eur J Pharm Sci. [2019 Jun 15];134:153–175. doi: 10.1016/j.ejps.2019.04.013
- Azman M, Sabri AH, Anjani QK, et al. Intestinal absorption study: challenges and absorption enhancement strategies in improving oral drug delivery. Pharmaceuticals (Basel). [2022 Aug 8];15(8):975. doi: 10.3390/ph15080975
- Buitelaar J, Medori R. Treating attention-deficit/hyperactivity disorder beyond symptom control alone in children and adolescents: a review of the potential benefits of long-acting stimulants. Eur Child Adolesc Psychiatry. 2010 Apr;19(4):325–340. doi: 10.1007/s00787-009-0056-1
- Shapiro D. Peaks and troughs: uneven medication coverage & ADHD. Attention Mag. 2017;Fall:16–21.
- Posner J, Kass E, Hulvershorn L. Using stimulants to treat ADHD-related emotional lability. Curr Psychiatry Rep. 2014 Oct;16(10):478. doi: 10.1007/s11920-014-0478-4
- Spencer TJ, Biederman J, Ciccone PE, et al. PET study examining pharmacokinetics, detection and likeability, and dopamine transporter receptor occupancy of short- and long-acting oral methylphenidate. Am J Psychiatry. 2006 Mar;163(3):387–395. doi: 10.1176/appi.ajp.163.3.387
- Po MD, Gomeni R, Incledon B. Quantitative characterization of the smoothness of extended-release methylphenidate pharmacokinetic profiles. Innov Clin Neurosci. 2022 Jul;19(7–9):32–37.
- Faraone SV, Childress A, Wigal SB, et al. Reliability and validity of the daily parent rating of evening and morning behavior scale, revised. J Atten Disord. 2018 Sep;22(11):1066–1073. doi: 10.1177/1087054715619009
- Sutton VK, Sumner C, Allen A, et al. Validity, reliability, and responsiveness of the DPREMB-R scale for ADHD. In: Scientific Proceedings of the 50th Anniversary Meeting of the American Academy of Child and Adolescent Psychiatry (AACAP); Miami Beach, FL. 169–170. [2003 Oct 14–19].
- Faraone SV, Hammerness PG, Wilens TE. Reliability and validity of the before-school functioning scale in children with ADHD. J Atten Disord. 2018 Sep;22(11):1040–1048. doi: 10.1177/1087054714564623
- Faraone SV, Wilens T, Childress AC, et al. Achievement and maintenance of normalized levels of early morning and late afternoon/evening functional impairment with delayed-release and extended-release methylphenidate (DR/ER-MPH) in children with ADHD: post hoc analyses of before-school functioning. J Am Acad Child Adolesc Psychiatry. 2019;58(10):S287–S288. doi: 10.1016/j.jaac.2019.08.443
- Faraone SV, DeSousa NJ, Komolova M, et al. Functional impairment in youth with ADHD: normative data and norm-referenced cutoff points for the before school functioning questionnaire and the parent rating of evening and morning behavior scale, Revised. J Clin Psychiatry. [2020 Dec 10];81(1):19m12956. doi: 10.4088/JCP.19m12956
- Faraone SV, Wilens TE, Pliszka SR, et al. Improvement in at-home functional impairment with DR/ER-MPH in children with ADHD: post hoc analysis of BSFQ and PREMB-R by norm-referenced cut-offs. In: American Psychiatric Association (APA) Annual Meeting; New York, NY; [2018 May 5–9].
- Childress AC, Cutler AJ, Po MD, et al. Symptomatic and functional response and remission from the open-label treatment-optimization phase of a study with DR/ER-MPH in children with ADHD. J Clin Psychiatry. [2021 Jun 22];82(4):21m13914. doi: 10.4088/JCP.21m13914
- Weiss M, Childress A, Nordbrock E, et al. Characteristics of ADHD symptom response/remission in a clinical trial of methylphenidate extended release. J Clin Med. [2019 Apr 5];8(4):461. doi: 10.3390/jcm8040461
- Wilens TE, Faraone SV, Hammerness PG, et al. Clinically meaningful improvements in early morning and late afternoon/evening functional impairment in children with ADHD treated with delayed-release and extended-release methylphenidate. J Atten Disord. 2022 Mar;26(5):696–705. doi: 10.1177/10870547211020073
- Faraone SV, Schachar RJ, Barkley RA, et al. Early morning functional impairments in stimulant-treated children with attention-deficit/hyperactivity disorder versus controls: impact on the family. J Child Adolesc Psychopharmacol. 2017 Oct;27(8):715–722. doi: 10.1089/cap.2016.0164
- Sallee FR. Early morning functioning in stimulant-treated children and adolescents with attention-deficit/hyperactivity disorder, and its impact on caregivers. J Child Adolesc Psychopharmacol. 2015 Sep;25(7):558–565. doi: 10.1089/cap.2014.0160
- Childress AC, Yu KR, Cuthbertson L. Early morning ADHD symptoms and functional impairment: impact on patients and caregivers, and pharmacological approaches to management. CNS Drugs. 2023 Jan;37(1):31–44. doi: 10.1007/s40263-022-00978-2
- Lopez FA, Faraone SV, Newcorn JH, et al. Effect of delayed-release and extended-release methylphenidate on caregiver strain and validation of psychometric properties of the caregiver strain questionnaire: results from a phase 3 trial in children with attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol. 2021 Apr;31(3):179–186. doi: 10.1089/cap.2020.0159
- JORNAY PM (methylphenidate hydrochloride) extended release capsules for oral use, CII. Prescribing information. Ironshore Pharmaceuticals Inc. 2023.
- Cortese S, Faraone SV, Konofal E, et al. Sleep in children with attention-deficit/hyperactivity disorder: meta-analysis of subjective and objective studies. J Am Acad Child Adolesc Psychiatry. 2009 Sep;48(9):894–908. doi: 10.1097/CHI.0b013e3181ac09c9
- Storebo OJ, Pedersen N, Ramstad E, et al. Methylphenidate for attention deficit hyperactivity disorder (ADHD) in children and adolescents - assessment of adverse events in non-randomised studies. Cochrane Database Syst Rev. [2018 May 9];5(5):CD012069. doi: 10.1002/14651858.CD012069.pub2
- Diaz-Roman A, Mitchell R, Cortese S. Sleep in adults with ADHD: systematic review and meta-analysis of subjective and objective studies. Neurosci Biobehav Rev. 2018 Jun;89:61–71. doi: 10.1016/j.neubiorev.2018.02.014
- Liang X, Qiu H, Li SX. Objectively measured sleep continuity in children and adolescents with ADHD: a systematic review and meta-analysis. Psychiatry Res. 2023 Oct;328:115447. doi: 10.1016/j.psychres.2023.115447
- Faraone SV, Po MD, Komolova M, et al. Sleep-associated adverse events during methylphenidate treatment of attention-deficit/hyperactivity disorder: a meta-analysis. J Clin Psychiatry. [2019 Apr 30];80(3):18r12210. doi: 10.4088/JCP.18r12210
- Wernicke JF, Faries D, Milton D, et al. Detecting treatment emergent adverse events in clinical trials: a comparison of spontaneously reported and solicited collection methods. Drug Saf. 2005;28(11):1057–1063. doi: 10.2165/00002018-200528110-00006
- Gajria K, Lu M, Sikirica V, et al. Adherence, persistence, and medication discontinuation in patients with attention-deficit/hyperactivity disorder - a systematic literature review. Neuropsychiatr Dis Treat. 2014;10:1543–1569. doi: 10.2147/NDT.S65721
- Childress AC, Pliszka SR, Cutler AJ, et al. 5.12 Electronically monitored adherence rates with evening-dosed delayed-release and extended-release methylphenidate in children with ADHD. J Am Acad Child Adolesc Psychiatry. 2020;59(10):S152–S153. doi: 10.1016/j.jaac.2020.08.073
- van Onzenoort HA, Menger FE, Neef C, et al. Participation in a clinical trial enhances adherence and persistence to treatment: a retrospective cohort study. Hypertension. 2011 Oct;58(4):573–578. doi: 10.1161/HYPERTENSIONAHA.111.171074
- Lloyd D, Gregg R, Morgan D. Higher persistence and adherence with DR/ER-MPH over other extended-release stimulants for the management of ADHD: real-world evidence from a large U.S. claims database analysis. J Am Acad Child Adolesc Psychiatry. 2021;60(10):S256–S257. doi: 10.1016/j.jaac.2021.09.411
- Stevenson AR, Roy P, Brugman RD. Early experience with delayed-release/extended-release methylphenidate for the treatment of adults with ADHD: a retrospective electronic medical record analysis. In: Poster presented at: American Professional Society of ADHD and Related Disorders (APSARD) Annual Meeting; Orlando, FL; [2023 Jan 12–15].
- Katzman MA, Otcheretko V, Po MD, et al. Adverse events during dosing of delayed-release/extended-release methylphenidate: learnings from the open-label phase of a registration trial and a real-world postmarketing surveillance program. Clin Ther. [2023 Sep 26];45(12):1212–1221. doi: 10.1016/j.clinthera.2023.09.009
- Liu T, Gobburu JVS, Po MD, et al. Pharmacokinetics of HLD200, a delayed-release and extended-release methylphenidate: evaluation of dose proportionality, food effect, multiple-dose modeling, and comparative bioavailability with immediate-release methylphenidate in healthy adults. J Child Adolesc Psychopharmacol. 2019 Apr;29(3):181–191. doi: 10.1089/cap.2018.0122
- Childress AC, Uchida CL, Po MD, et al. A post hoc comparison of prior ADHD medication dose and optimized delayed-release and extended-release methylphenidate dose in a pivotal phase III trial. Clin Ther. 2020 Dec;42(12):2332–2340. doi: 10.1016/j.clinthera.2020.10.004
- Ginsberg Y, Quintero J, Anand E, et al. Underdiagnosis of attention-deficit/hyperactivity disorder in adult patients: a review of the literature. Prim Care Companion CNS Disord. 2014;16(3). doi: 10.4088/PCC.13r01600
- Young S, Adamo N, Asgeirsdottir BB, et al. Females with ADHD: an expert consensus statement taking a lifespan approach providing guidance for the identification and treatment of attention-deficit/hyperactivity disorder in girls and women. BMC Psychiatry. [2020 Aug 12];20(1):404. doi: 10.1186/s12888-020-02707-9
- Lam AP, Matthies S, Graf E, et al. Long-term effects of multimodal treatment on adult attention-deficit/hyperactivity disorder symptoms: follow-up analysis of the COMPAS trial. JAMA Netw Open. [2019 May 3];2(5):e194980. doi: 10.1001/jamanetworkopen.2019.4980
- Jaeschke RR, Sujkowska E, Sowa-Kucma M. Methylphenidate for attention-deficit/hyperactivity disorder in adults: a narrative review. Psychopharmacol (Berl). 2021 Oct;238(10):2667–2691. doi: 10.1007/s00213-021-05946-0