?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
This overview paper describes the interaction of powder metallurgical iron-base alloys with the atmosphere during sintering. The methods of thermal analysis serve to clarify the processes that take place especially during the heating stage of the sintering cycle. After a discussion of the physical and chemical fundamentals of the sintering process, the methods of thermal analysis are explained. The differences between plain iron and alloyed systems are discussed in detail. Classical PM low alloy steels with alloying elements, such as Cu, Ni and Mo, react in a similar way as unalloyed carbon steels. The situation changes dramatically, when oxygen sensitive elements as chromium, manganese and even more silicon come into play. The removal of the surface oxygen is much more crucial, and there are several competing reactions, which have to be considered when these systems should be sintered in industrial scale to reach the desired mechanical and dimensional properties.
Introduction
The production of structural ferrous parts is a success story of powder metallurgy. Although the principles of sintering iron and steel parts are known to a wide public within the PM community, the interaction between the atmosphere and the parts was not of great importance over the years as productions ran smoothly as long as protective atmospheres were used that were reducing for iron oxides and thus also for the oxides of the common alloy elements copper, nickel and molybdenum. Removal of the oxides introduced through the starting powders thus was not a problem and usually ran virtually unnoticed. Decarburisation was definitely recognised as a problem but was usually coped with by adding carburising agents, as e.g. in endogas. The tendency towards using alloy steels containing chromium, manganese or even silicon, which are widely used in ingot metallurgy due to their interesting relationship cost/benefit in terms of mechanical properties, hardenability and microstructure evolution, however, confronted the structural parts industry with problems not known from classically alloyed powder metallurgical steels. This article is intended to give an overview about the investigations done to understand the reactions taking place between the pressed compacts and the atmosphere, like the considerations given by Boccini [Citation1] and Beiss [Citation2], to ensure proper sintering also in the presence of alloy element with high oxygen affinity. The analysis of the surface of metal powders was published by Nyborg et al. in numerous papers, where the composition and the thickness of powder surfaces of different steel powders were reported. This started with the much easier analysis of gas atomised steel powder [Citation3–7] and was furthermore applied on irregular shaped water atomised powders [Citation8–10]. This became of special interest when chromium low-alloyed powders appeared on the market.
Physical fundamentals
The main goal for the sintering process in precision parts production is significantly different from other powder metallurgical production processes in which the main goal is to reach full dense products, such as sintering of hardmetals or injection moulded parts. The sintering of precision parts is usually performed with hardly any shrinkage, which implies that the starting material (the pressed green compact) should have the maximum density already after compaction in order to reach acceptable density values – and thus mechanical properties. Especially impact energy and elongation to fracture show a strong increase after reaching a density level of about 7.6 g cm−3, which can be related to closed porosity [Citation11–14]
This means that if the material should be sintered without shrinkage, the compactibility of the powder will determine the final properties, but the compaction pressures that can be applied by technical presses are limited to 600–800 MPa maximum according to technical and economical reasons. Therefore, most of the precision parts are produced within a density range of 6.8–7.1 g cm−3. To attain the desired microstructure during sintering, the decisive process is the formation of stable metallic contacts between the compressed particles. The grain size and the phase distribution can be controlled by the alloying elements and the alloying technique chosen and by sintering temperature and time.
The principal driving force for sintering without external forces (free sintering) is defined by thermodynamics [Citation15]: The trend for a system to reach a minimum of energy. For a single component system, such as plain iron, the surface is the relevant feature for sintering. The surface of a solid (or a liquid) is connected with additional energy: specific surface free energy γ (J m−2) or surface tension γ (N m−1)
The energy of powder compacts is increased due to the large surface area of the material, the more the finer the powder is.
To reach a minimum of surface area, the driving force is defined by:(1)
(1)
where E is the energy of the System and A the surface area. This means that the driving force for sintering is the minimisation of the surface area of the dispersed system. Therefore, fine powders will sinter more intensely than course ones. The material transport in the powder compact during sintering is diffusion at the surface, along the grain boundaries and through the volume of the powder grains, i.e. surface, grain boundary and bulk diffusion, respectively. But precondition for this material transport is the presence of really metallic surfaces. Here the thermodynamics of oxide reduction on metal powders comes into play, which means the interaction of powder compact and the atmosphere.
Thermodynamic considerations
Most metallic elements are thermodynamically not stable in air (or oxidising environment in general) at room temperature, which means that the Gibbs free energy ΔG of the reaction 2x/yM + O2 = 2/yMxOy is negative. According to the base equation(2)
(2)
with ΔH as reaction enthalpy, T as absolute temperature and ΔS as reaction entropy, the oxides can be reduced either by heating until ΔG becomes positive – thermal decomposition of the oxide – which is realistic only for a few metals (e.g. silver) or by combination with a reducing agent for which ΔG of its oxide becomes more negative. The most common reducing agents in this sense are hydrogen and carbon.
The consequence for sintering of powder compacts is that it is decisive to remove the surface oxides covering every powder that has ever been exposed to air, since the material transport processes described above can only take place if a real metal–metal contact is given. As long as the contact region consists of metal oxides, the diffusion processes are hindered or at least slowed down such that there will be no contact formation in realistic times. Furthermore, the driving force for sintering is also lower because of the significantly lower surface energy of oxides compared to metals, i.e. an oxide-covered metal powder will sinter much less readily than a fully metallic one.
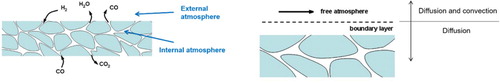
If a powder compact is introduced into a furnace, we have to keep in mind that in principle the system can be divided into four main components, which is schematically shown in .
The pressed powder itself: The compact consists of powder particles, pressed together with high forces to ensure high green density and green strength. Although high pressures (400–800 MPa) are used to consolidate the green body, the part still contains 10–15 vol.-% of pores when the sintered density is taken as a measure. Furthermore, the alloying techniques in powder metallurgy must be considered. In contrast to ingot metallurgy, powder metallurgy has several ways to introduce the alloying elements, as shown schematically in . The chemical activity α (the effective concentration of a species), which is responsible for the chemical interaction with the atmosphere, differs significantly between prealloyed and admixed variants, for the latter, the chemical activity of the alloying element being 1. While prealloying reduces the activity most pronouncedly, the master alloy variant shows medium chemical activities of the alloying elements.
The internal atmosphere: The atmosphere within the pores is the atmosphere the part carries when subjected to a sintering cycle. For sure, this atmosphere is strongly influenced by the interaction of the material with the atmosphere during this temperature cycle and can significantly differ from the atmosphere outside the body. It is possible that the internal atmosphere is more oxidising than the free atmosphere in the furnace, but it is also possible that it is the other way round. Owing to ‘internal gettering’-effects, the oxidising species can be trapped within the compact and ‘clean’ the atmosphere so that from thermodynamic point of view, the conditions turn to more reducing [Citation17].
External atmosphere: The atmosphere of the furnace, i.e. the gases introduced into the furnace together with the evaporated (or trapped) gases of the material-gas reactions, forms the external atmosphere. This is the only atmosphere we can measure and influence directly by analytical methods and gas introduction. In principal, it has to fulfil four tasks:
Task 1: The atmosphere protects the powder compacts against undesired reactions like oxidation, decarburisation, carburisation
Task 2: The atmosphere removes the reaction products of desired reactions as e.g. the water vapour produced by the reduction of oxides with hydrogen or the vapour of the lubricant in the burn-off zone of the furnace.
Task 3: The atmosphere is able to remove undesirable elements/contaminants like oxides by reduction
Task 4: The atmosphere is able to introduce interstitial elements (carbon, nitrogen or boron) by special reactions
Figure 2. Alloy variants for sintered steels (a) prealloyed; (b) elemental mixture; (c) master alloy, (d) diffusion bonded; (e) coated; (modified after [Citation16]).
![Figure 2. Alloy variants for sintered steels (a) prealloyed; (b) elemental mixture; (c) master alloy, (d) diffusion bonded; (e) coated; (modified after [Citation16]).](/cms/asset/bddde228-0327-4e58-b41a-4fe7adb82145/ypom_a_1810427_f0002_oc.jpg)
The possible atmospheres therefore can be grouped into:
Inert atmospheres: These atmospheres fulfil tasks T1 and T2. The most common ones are vacuum and noble gases (argon, helium). Nitrogen is frequently regarded as a member of this group, because there is hardly any reaction with steels, but there might be a reaction with chromium in stainless steels, vanadium [Citation18] and even stronger reaction with titanium or the formation of aluminium nitride during the sintering of aluminium [Citation19,Citation20]. Also, the effect of boron activated sintering is strongly dependent on the presence of nitrogen. Whereas in hydrogen or vacuum, even stable h-BN decomposes to form a eutectic melt between boron and iron, on the other hand, the reaction of boron, e.g. from borides, is completely inhibited by the nitrogen atmosphere through the formation of inert h-BN [Citation21–23] (see ).
Reactive atmospheres:
o Oxidising atmospheres: Air and inert atmospheres that contain amounts of oxidising species like water vapour, carbon dioxide and oxygen. The oxidising effect of these species is depending on their chemical activity, which in the gaseous phase usually is a function of the partial pressure. For carbon monoxide containing atmospheres this means that endogas atmospheres with 20 vol.-% CO (+40 vol.-% H2 and 40 vol.-%N2) may be oxidising for some systems and reducing for less sensitive ones. Usually these oxidising constituents of the sintering atmospheres mentioned above are undesirable for sintering of PM parts except for delubrication or binder burnout (‘rapid burnoff’ techniques [Citation24])
o Reducing atmospheres: hydrogen and to some extent carbon monoxide, depending on the partial pressure. These and their mixtures with inert gases are the most widely used protective gases in PM [Citation25,Citation26]
o Carburising atmospheres: Carbon monoxide, propane, acetylene or endogas; these gases are typically used during secondary treatment operations like gas carburising or low pressure carburising, but to some extent also in the sintering atmosphere, e.g. to compensate for the presence of decarburising agents.
o Decarburising atmospheres: All sintering atmospheres that contain too high amounts of water vapour, which is the most decarburising agent, but also carbon dioxide, oxygen and exogas. Typically, these constituents of the sintering atmosphere are extremely undesirable to appear within a sintering furnace, but may occur due to leakages or other malfunctions. They lead to unwanted decarburisation of the parts.
o Nitriding atmospheres: Ammonia, which forms nascent nitrogen, or atomic nitrogen in plasma are able to introduce nitrogen to a number of materials. For strong nitride formers as e.g. Ti or Zr, but also for stainless steels, also N2 is nitriding.
(4) Boundary layer: The interaction between external and internal atmosphere is usually performed by diffusion until a certain boundary layer is overcome and then by diffusion and convection in the free atmosphere. Convection in the body can only happen by ‘blowing’ of the pores during the heating process when the gases in the pores expand, by massive formation of gases within the pores by chemical reactions there or by ‘sucking’ during the cooling process.
Figure 3. Dilatometry of Fe–1.5Mo–0.6C–0.3B (h-BN) in N2 and H2, 60 min 1250°C isothermal, 10 K min−1 heating and cooling rate [Citation21].
![Figure 3. Dilatometry of Fe–1.5Mo–0.6C–0.3B (h-BN) in N2 and H2, 60 min 1250°C isothermal, 10 K min−1 heating and cooling rate [Citation21].](/cms/asset/a70c77e2-0fa8-4bac-aebc-49088de79c11/ypom_a_1810427_f0003_oc.jpg)
The problem for atmosphere control is that all decisive processes like deoxidation of the surface oxides occur within the pore network – in the internal atmosphere – and therefore cannot be directly measured by any known technology. The only atmosphere that can be measured and controlled is the external atmosphere.
As already mentioned, it is decisive to remove the oxides from the particles in the early stages of sintering – during the heating stage – either by a reducing atmosphere or by addition of a reducing agent to the powder mix, which usually means addition of carbon. The possible reactions to reduce the surface oxides are:(3)
(3)
(4)
(4)
(5)
(5)
(6)
(6)
The direct and indirect carbothermic reaction are connected by the Boudouard equilibrium(7)
(7)
At high temperatures (and low pressures), the equilibrium is shifted towards carbon monoxide, which means that the partial pressure of the specific gas components determines the equilibrium for industrial sintering. The driving force for the reactions therefore is the partial pressure of the oxides.
Methods to measure the interaction between atmosphere and PM compact
There are several techniques to measure the interaction, most of them belong to thermoanalytical methods, like dilatometry (DIL), differential thermal analysis (DTA), thermogravimetry (TG) or evolved gas analysis (EGA).
Dilatometry
Dilatometry (DIL) is usually employed to measure thermal expansion coefficients [Citation27], but for powder metallurgy, dilatometry is an instrument for process control during sintering, and the dilatometer is more or less a very precise sintering furnace [Citation28]. Since the dimensional stability, shrinkage and expansion effects during sintering are extremely important to describe the sintering process, in particular, for PM precision parts for which dimensional stability is crucial, dilatometry is very helpful.
Simultaneous thermal analysis
The method of simultaneous thermal analysis (STA) combines DTA and thermogravimetry (TG) in one operating unit, by placing the DTA sensor on a balance. The positive effect of one operating unit compared to separate DTA and TG systems is that the temperature signal is without any doubt the same for both methods. In STA systems, it is easier to correlate TG and DTA. One can be sure that the measurement happened simultaneously and is not influenced by differences in the operating units such as furnace shifts, etc.
Evolved gas analysis
In most cases, EGA is not operated as stand-alone system. EGA is usually combined with DTA, TG or both of them (STA) analysing the exhaust gases that had passed the systems. During thermal analyses, coupling techniques are typically used to identify and in part quantify the exhaust gases. The common techniques are:
Infrared spectrometry: This method is often used for the identification of decomposition products from organic compounds (feedstocks for metal injection moulding, polymer decomposition, etc.) [Citation29]
Mass spectrometry (MS): MS is the method of choice when inorganic gases should be detected. The quantification of mass spectrometry is known to be tricky since not all evolved gases are transferred into the measurement system. In principle, two methods of coupling a mass spectrometer to a thermoanalytical device are reported, the main task being the lowering of gas pressure from typically 1 bar (ambient) to the vacuum of 10−5 mbar required in the spectrometer (see ): The skimmer coupling, in which the vacuum is reached in two steps. The first step from 103 mbar to 10–1 mbar is performed by a rotary pump and an orifice system, and the second step is performed by a high vacuum pump. The so-called skimmer is placed within the furnace to prevent cooling of the evolved gases and condensation of material in the tiny orifice. The system is an expensive high-end system and only available for STA, but with high resolution, short transport distances and therefore almost perfect temperature-time correlation. The capillary coupling, in contrast, is the more flexible device since the capillary entrance can be placed e.g. also in the exhaust gas stream of a dilatometer. The necessary pressure drop is performed by the fused silica capillary only, which is usually heated to 250–300°C to prevent condensation problems. The most important disadvantage is that during the flight, which is performed at supersonic speed, the gas species may react with each other and the final analysis result therefore might differ from the original gases evolved from the specimen. For observation of sintering, both systems are used, but the most severe limitation for MS is the most widely used industrial sintering atmosphere which consists of nitrogen–hydrogen mixtures. The most abundant mass m28 can be related both to molecular nitrogen (N2) and carbon monoxide (CO), i.e. the signals interfere and cannot be separated except by high-resolution MS. In principle, the distinction between both gases often is realised by analysis of the breakdown species: masses m12 for carbon and m14 for atomic nitrogen, which are quite characteristic, but in excess of nitrogen the signals disappear due to too high base lines of mass m28. Therefore, for sintering studies often neutral atmospheres are chosen like argon or helium (or Ar–H2 in place of N2–H2), which do not interfere with masses of interest.
The first studies about the application of DTA/TG coupled with mass spectrometry in powder metallurgy were published by Gille and Leitner who focused on the system WC-Co (hardmetals/cemented carbides). They showed that sintering of the system only started after deoxidation of the WC powder particles. Sintering and deoxidation were further strongly influenced by alloying elements that form more stable oxides compared to WC, such as TaC, NbC, VC [Citation30–32]. This is particularly important for ultrafine hardmetal grades (in which esp. VC and Cr3C2 are necessary as grain growth inhibitors) since the presence of these alloy elements shifts the deoxidation to higher temperatures while the ultrafine grades tend to form closed porosity at lower and lower temperatures. Intersection of these two temperature ‘windows’ has to be avoided at any rate, otherwise pore formation or even blistering would occur.
Photoacoustic Spectroscopy (PAS): The principle is to detect an acoustic signal generated by light absorption. The method can be quantified from 0.1 to 2000 ppm. An example of a sintering simulation is shown by Chasoglou et al. [Citation33] in . It is obvious that sintering atmospheres containing nitrogen are not a limiting factor for detecting carbon monoxide in PAS. The main limitation of this technique is that for PAS, gas has to be sampled, and this limits the time–temperature correlation. For the usual number of gases (CO, CO2, CH4, H2O), the sampling time is approximately 60 s [Citation34], which means, a temperature resolution of 10 K for the usual heating rates (10 K min−1).
Figure 4. EGA coupling systems (schematic), left: skimmer coupling, right: capillary coupling (courtesy of Netzsch Gerätebau GmbH).
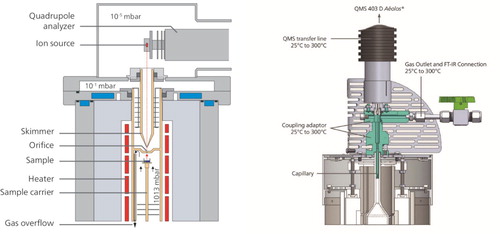
Figure 5. PAS curve from sintering simulation of Fe–3Cr–0.5Mo–0.5C, 10 K min−1, Tmax 1120°C, 30 min in N2/3%H2 [Citation32].
![Figure 5. PAS curve from sintering simulation of Fe–3Cr–0.5Mo–0.5C, 10 K min−1, Tmax 1120°C, 30 min in N2/3%H2 [Citation32].](/cms/asset/a5425425-8d96-40e6-aec1-6eb00192d796/ypom_a_1810427_f0005_oc.jpg)
Other gas analysing systems
In industrial practice, but also in laboratory scale, there are numerous systems that allow the analysis of the sintering atmosphere. Some of them are operating on-line and can directly be used as a control system for the atmosphere.
The most common sensors are infrared sensors for quantification of CO or CO2, which usually are combined with lambda sensors for the measurement of oxygen content to control the atmosphere in industrial sintering furnaces. Also dewpoint meters are useful instruments to prevent decarburisation.
For sintering furnaces, there has been a system introduced by Linde and Höganäs AB which allows the determination and control of the sintering atmosphere (Sinterflex). The system needs some CO in the atmosphere (about 1%) and is operated not completely on-line, as it extracts some gas from the sintering atmosphere to analyse it in this extra unit (see [Citation35–37]).
Reduction process during sintering
The following chapter describes in examples the reactions of the powder compacts with the atmosphere. The chapter is divided into three main parts.
Reactions with systems of low oxygen affinity
Reactions with systems of high oxygen affinity
Special reactions in oxygen-sensitive systems
Systems with low oxygen affinity
The systems with low oxygen affinity do not contain elements with higher oxygen affinity than iron in higher amounts. All alloying elements described in this chapter are less sensitive to oxygen, namely nickel, copper and molybdenum. In principle, also tungsten also belongs to this group but usually is not used in PM due to its high price.
Plain iron
To remove surface oxides from plain iron compacts, hydrogen-containing atmospheres are necessary. If neutral atmospheres like vacuum of argon are used, the traces of carbon still present in the powder are able to reduce some surface oxides to enable sintering, but the reaction is far away from being complete, which is, however, the case in hydrogen atmosphere ().
Figure 6. Dilatometry +MS of Fe, heating ramp of plain iron compacts (600 MPa) in (a) argon and (b) hydrogen, 10 K min−1, Tmax 1300°C (please note the different ion current scales for the MS graphs), sample size 55 × 10 × 8 mm³ [Citation27].
![Figure 6. Dilatometry +MS of Fe, heating ramp of plain iron compacts (600 MPa) in (a) argon and (b) hydrogen, 10 K min−1, Tmax 1300°C (please note the different ion current scales for the MS graphs), sample size 55 × 10 × 8 mm³ [Citation27].](/cms/asset/81cf1bc5-3515-47ba-b6e8-4dcda6dc6176/ypom_a_1810427_f0006_oc.jpg)
For sintering of plain iron, an extremely unsymmetrical phase transformation behavior has been reported [Citation38,Citation39] as shown in . This coincides with unexpected longitudinal growth of the sample due to slight temperature gradients during the austenite–ferrite phase transformation that causes excessive and directional grain growth [Citation40]. During the formation of the α-iron crystals, the oxygen is trapped and enriched at the grain boundaries, and this causes severe embrittlement when sintering in argon and vacuum. The samples sintered in hydrogen also show enlarged grains [Citation41, p. 119], but the impact energy is much higher (71.1 J cm−2 compared to 2.3 J cm−2). It is important to mention that interstitials like carbon or nitrogen (see [Citation42]) completely inhibit this behavior by extending the phase transformation over a wider temperature range. In addition, alloyed systems do not show this effect either because of the same reason ().
Figure 7. Dilatometry of plain iron compacts (600 MPa) in (a) argon and (b) hydrogen, Tmax 1300°C, 60 min, 10 K min−1, sample size 55 × 10 × 8 mm³ [Citation27].
![Figure 7. Dilatometry of plain iron compacts (600 MPa) in (a) argon and (b) hydrogen, Tmax 1300°C, 60 min, 10 K min−1, sample size 55 × 10 × 8 mm³ [Citation27].](/cms/asset/138776b6-fb13-4634-a7d4-4e5a23ed11f5/ypom_a_1810427_f0007_oc.jpg)
The system iron-carbon
In contrast to plain iron compacts, the system Fe–C contains a strongly reducing agent within the compact in the form of carbon, which is intentionally admixed to the iron-base powder as alloying element, typically as fine graphite powder. Here it has to be considered that carbon consumed for reduction purposes leaves the compact as gaseous CO/CO2 and is therefore no more available for alloying, which means that carbothermal reduction comes with the penalty of carbon loss, the more, the more effective the deoxidation is. However, this carbon loss is homogeneous within the entire specimen and usually well controllable, in contrast to e.g. surface decarburisation through impure atmospheres. It can be compensated for by adding slightly more graphite.
The system Fe–C was systematically studied in [Citation43] where the respective deoxidation behavior of compacts prepared from different base powders such as atomised and sponge grades (Höganäs ASC 100.29; Höganäs ABC 100.30 and Höganäs SC 100.26) admixed with carbon was studied in neutral (argon) and hydrogen (reducing) atmosphere (both plain hydrogen and N2/H2 mixture 90/10) by dilatometry (sample size: 10 × 10 × 8 mm³). The results showed that – if desorption of humidity at low temperatures is neglected – the deoxidation takes place in two stages for all atmospheres. In neutral atmospheres the first, very pronounced peak of CO appears at 640–730°C, depending on the purity of the base powder, but always before the α−γ-transition. This peak marks the reduction of the surface oxides and is followed at higher temperature by reduction of the internal oxides (including those trapped in the pressing contacts) [Citation44]. Since the oxygen from the internal oxides must diffuse to the surface before being able to react with the reducing agent to form gaseous products, the deoxidation peaks are much broader since the diffusion distance is not the same for each oxide. It is also obvious that the particularly pure grade ABC 100.30, with extra low manganese content of 0.074 mass% Mn compared to ASC 100.29 with 0.113 mass% Mn [Citation41], shows markedly less reduction of internal oxides by the admixed carbon (). Furthermore, it has also been shown that the ratio of the two peaks depends on the particle size of the base powder, with finer powders the first peak being more pronounced – because of the larger specific surface – while the second one is smaller and is shifted to lower temperatures [Citation43].
Figure 9. Dilatometric and MS graphs of Fe–0.8C in argon, heating stage, Tmax 1300°C, 10 K min−1 (a) ABC 100.3 (b) ASC 100.29, sample size 55 × 10 × 8 mm³ [Citation43].
![Figure 9. Dilatometric and MS graphs of Fe–0.8C in argon, heating stage, Tmax 1300°C, 10 K min−1 (a) ABC 100.3 (b) ASC 100.29, sample size 55 × 10 × 8 mm³ [Citation43].](/cms/asset/5a0b83fd-f9c5-42db-8070-d55df699ba96/ypom_a_1810427_f0009_oc.jpg)
In reducing atmospheres, the reduction of the surface oxides is performed at much lower temperatures, but now by the hydrogen in the atmosphere. The CO peak at about 700°C completely disappears while a water peak (mass m18) appears at around 400°C, depending on the hydrogen content of the atmosphere. Reduction of the internal oxides, in contrast, is once more performed by the added carbon, as indicated by the pronounced CO peak in (a). This shows that at high temperatures, above about 900°C, carbon is the preferred reducing agent even in presence of H2, because of the increased thermodynamic stability of CO with higher temperatures compared to H2O. In the nitrogen–hydrogen mixture, this carbothermal reduction can only be detected by the breakdown peak of mass 12, since, as already mentioned before; mass m28 for carbon monoxide will be masked by nitrogen from the atmosphere ((b)).
Figure 10. Dilatometric and MS graphs of Fe (ASC 100.29)–0.8C in (a) hydrogen and (b) nitrogen–hydrogen mixture 90/10, heating stage, Tmax 1300°C, 10 K min−1, sample size 10 × 10 × 8 mm³; please note the different ion current scales for the MS signals [Citation43].
![Figure 10. Dilatometric and MS graphs of Fe (ASC 100.29)–0.8C in (a) hydrogen and (b) nitrogen–hydrogen mixture 90/10, heating stage, Tmax 1300°C, 10 K min−1, sample size 10 × 10 × 8 mm³; please note the different ion current scales for the MS signals [Citation43].](/cms/asset/595699fc-8ef4-4a2b-a6bf-9b51829f59ac/ypom_a_1810427_f0010_oc.jpg)
The production process of the metal powders also implies differences in oxygen content and allocation of the oxides. Especially sponge iron powders with its internal porosity, which is to a large extent closed during pressing, show a significant shift of ratio between surface and internal oxides towards the latter ones (see ).
Figure 11. TG/DTG graphs for Fe–0.8% C prepared from water atomised (black) and sponge iron powders (green), respectively. 10 K min−1, flowing He [Citation44].
![Figure 11. TG/DTG graphs for Fe–0.8% C prepared from water atomised (black) and sponge iron powders (green), respectively. 10 K min−1, flowing He [Citation44].](/cms/asset/8b45a7fb-7986-40ce-93d1-092f576f83c5/ypom_a_1810427_f0011_oc.jpg)
When full-size Charpy bars (ISO 5754, 55 × 10 × 8 mm³) are used for the dilatometric measurement, the resolution of the mass peaks is not that sharp any more – the gaseous reaction products being released more slowly and gradually – but in principle, the results are the same [Citation28]. In argon, there are once more two m28 deoxidation peaks, and in hydrogen, the surface deoxidation is performed by hydrogen, a corresponding water (m18) peak being formed at lower temperatures, <500°C. Since the mass is much higher with these large specimens, the first peak of mass m28 does not disappear completely even in plain H2, indicating some carbothermal reduction of the surface oxides. It stands out rather clearly that the dissolution of graphite in the austenite matrix takes place slightly faster in hydrogen, although the phase transformation starts at higher temperatures. The former effect can be seen by the more pronounced expansion in the austenite range after the ferrite–austenite transition ().
Figure 12. Dilatometric and MS graphs of Fe (ASC 100.29)–0.5C in (a) argon and (b) hydrogen, heating stage, Tmax 1300°C, 10 K min−1, sample size 55 × 10 × 8 mm³ [Citation28].
![Figure 12. Dilatometric and MS graphs of Fe (ASC 100.29)–0.5C in (a) argon and (b) hydrogen, heating stage, Tmax 1300°C, 10 K min−1, sample size 55 × 10 × 8 mm³ [Citation28].](/cms/asset/dcb87d60-75bd-430d-9216-4aacf38126c8/ypom_a_1810427_f0012_oc.jpg)
Chromium and manganese alloyed systems
Chromium alloyed steels have been thoroughly investigated as described in [Citation28,Citation44,Citation45]. All these studies show that chromium as an alloying element is responsible for decisive changes in the deoxidation behaviour. In argon atmosphere, the first intense peak that indicates the deoxidation of the surface oxides is shifted to much higher temperatures and somehow overlaps with the peak for the internal oxides, only for small samples, for which better resolution is attained, the separation is possible (see ).
Figure 13. Dilatometric and MS graphs of Fe–3Cr–0.5Mo–0.6C in (a) argon and (b) hydrogen, heating stage, Tmax 1300°C, 10 K min−1, sample size 10 ×10 ×8 mm³ (please consider different y axis scalings) [Citation45].
![Figure 13. Dilatometric and MS graphs of Fe–3Cr–0.5Mo–0.6C in (a) argon and (b) hydrogen, heating stage, Tmax 1300°C, 10 K min−1, sample size 10 ×10 ×8 mm³ (please consider different y axis scalings) [Citation45].](/cms/asset/474ece99-654e-406d-b9dd-47a2ef7c47c8/ypom_a_1810427_f0013_oc.jpg)
This means that deoxidation does not start before 900–950°C and is not even finished at 1300°C, the critical temperatures depending on the chromium content, as also shown in [Citation46]. The consequence for the mechanical properties is a pronounced effect of the sintering temperature: below the first degassing peak, there is virtually no formation of metallic sintering contacts [Citation47], and even at temperatures above this threshold, higher temperatures have a strongly beneficial effect on both monotonic and cyclic mechanical properties [Citation47, Citation48]. Molybdenum as an alloying element (also contained in these steel grades) hardly affects deoxidation, Mo alloyed steels being comparable to Fe–C [Citation49]. In hydrogen atmosphere, there is somewhat less difference between the Fe–C system and Fe–Cr(–Mo)–C. There is a rather pronounced water peak in the low-temperature region, just as it is in Fe–C, which means that at least part of the deoxidation is performed around 400°C by hydrogen, and only the more stable oxides have to be reduced at higher temperatures by the added carbon, which is clearly confirmed by [Citation50]. Nevertheless, when comparing the peak areas for m18 and m28, it stands out clearly that the vast majority of the initial oxygen content is removed carbothermally at temperatures >900°C (see ). It has been shown that prealloyed manganese steels react in a very similar way when the manganese content is in a similar range (>1.5 mass %) [Citation51]. In [Citation52], a comprehensive study on the efficiency of reducing atmospheres was performed on Cr-alloyed steels. The results show clearly that it needs hydrogen-containing atmospheres for effective reduction. CO addition is only helpful, when lean H2/CO (concentration of active gas ≤ 5 vol.-%) is used, otherwise, oxidation and/or carburisation is observed.
Figure 14. Dilatometric and MS graphs of Fe–1.5Cr–0.2Mo–0.5C (solid lines) and Fe–3Cr–0.5Mo–0.6C (dashes lines) in (a) argon and (b) hydrogen, heating stage, Tmax 1300°C, 10 K min−1, sample size 55 × 10 × 8 mm³ [Citation28].
![Figure 14. Dilatometric and MS graphs of Fe–1.5Cr–0.2Mo–0.5C (solid lines) and Fe–3Cr–0.5Mo–0.6C (dashes lines) in (a) argon and (b) hydrogen, heating stage, Tmax 1300°C, 10 K min−1, sample size 55 × 10 × 8 mm³ [Citation28].](/cms/asset/b5c4e5e9-ff11-49d1-9cd0-7667ce528f5d/ypom_a_1810427_f0014_oc.jpg)
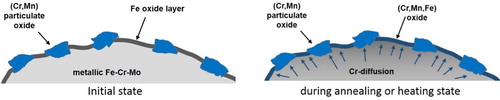
The main reason for this behavior was found by applying high-resolution electron microscopy and photoelectron spectroscopy (XPS) on the surfaces of the prealloyed powders. The analysis showed that alloyed steel powders are mainly covered by iron oxides (90% of the area) which form a homogeneous thin layer of about 6–7 nm thickness. The rest of the surface is covered by islands formed of mixed oxides [Fe, Mn, Cr]xOy and oxides of spinel type like FeCr2O4, MnFe2O4, MnCr2O4, etc. (). The size of these particulate features rises up to 300 nm with higher alloy element content [Citation53]. According to the calculations in [Citation54], these oxide islands contain about 50% of the surface oxygen in as delivered powder.
Figure 15. Qualitative SEM + EDX analysis of particulate features on the surface of manganese alloyed steel powder [Citation54].
![Figure 15. Qualitative SEM + EDX analysis of particulate features on the surface of manganese alloyed steel powder [Citation54].](/cms/asset/2bdf6bcb-ee9f-48be-b14c-32d669d42cc6/ypom_a_1810427_f0015_oc.jpg)
The influence of the green density on deoxidation is marginal for classical PM steels until the parts reach 7.4 g cm−³, as shown in [Citation55]. For the chromium alloyed variants, the deoxidation is shifted to higher temperatures as the density increases. As green densities reach 7.5 g cm−³, which can be performed, e.g. by high-velocity compaction, the deoxidation virtually stops at temperatures above 1150°C indicating a fast closing of the pore channels with resulting trapping of the remaining oxygen. However, in [Citation56], it has been shown that the effect of this trapped oxygen on the mechanical properties is apparently insignificant, even in the gigacycle fatigue regime.
Special reactions in oxygen-sensitive systems
In the above mentioned oxygen-sensitive systems, there is a certain tendency to special reactions that are described in the following sub-chapters.
Methane formation
As can be seen in (b), there is some methane formation, indicated by the mass16. This methane formation has always been a topic of discussions since it influences carbon control, which is essential for carbon containing steels. The common reason for inhomogeneous decarburisation observed in sintering of PM steels (in contrast to the ‘natural’, homogeneous carbon loss described above) is the presence of water vapour in the atmosphere, carbon removal taking place according to(8)
(8)
but in the literature [Citation29], also the reaction(9)
(9)
is described. However, the deoxidation experiments with the system Fe–C have never shown significant amounts of methane; neither in the old literature [Citation57, Citation58] nor in the more recent one [Citation26, Citation59], significant carbon loss via methane formation is reported. However, when the advanced chromium and manganese prealloyed steels were introduced, the appearance of methane in the temperature range of about 700–950°C was observed. This was first reported in [Citation60, Citation61] and also in [Citation62]. The calculation of the Gibbs free energies of possible reactions [equations (10–13)] showed that the probability of CH4 formation in the system Fe–C is rather low because the ΔG values for the direct reactions of carbon containing species with hydrogen become positive at rather low temperatures (), i.e. the reactions are thermodynamically inhibited, while at lower temperatures at which ΔG would be negative, the kinetics of the reactions are not favourable.(10)
(10)
(11)
(11)
(12)
(12)
(13)
(13)
Figure 16. Gibbs free energies of reactions possibly forming CH4 [Citation60].
![Figure 16. Gibbs free energies of reactions possibly forming CH4 [Citation60].](/cms/asset/c093c07a-a5a6-4307-b8f4-50dc4a678c53/ypom_a_1810427_f0016_oc.jpg)
The analysis of the chromium and manganese prealloyed steels sintered in hydrogen showed distinct methane peaks in the range of 600–1100°C, which disagrees with the performed calculations. The introduction of oxides of silicon, chromium and manganese opened new ways of probable reactions. The temperature thresholds when ΔG = 0 of some of these reactions are at much higher temperature levels compared to the reactions cited above. Especially the findings in [Citation63] showed that silicon is that particular element that promotes methane formation very strongly. By the formation of methane, steels containing 1 wt-% of silicon lost about 0.5 wt-% of carbon during a soaking period of 2 h in the mentioned temperature range. For Cr prealloyed materials, it is striking that it is not the material with the highest Cr content that shows the strongest methane formation (see (b)), but the 1.8% chromium material. The proposed reaction mechanisms all include the presence of CO, which for sure will be higher when there is a reduction of the surface oxides by carbon at the given temperature range. In [Citation64], it is shown that different steel compositions result in the formation of methane at different temperature ranges, and the intensity of the methane formation depends on the alloying elements used. There seems to be a correlation between the oxygen sensitivity of the elements, i.e. the Gibbs free energy of the oxides, and CH4 formation, following the sequence Cr < Mn < Si. Manganese-containing mixes show more intense methane formation than chromium and even more does silicon. However, binary mixes Si + 0.5C mixes do not show any methane formation at all, which indicates that the presence of iron is necessary (see ).
Figure 17. Thermogravimetric signals and MS graphs of different powder mixes sintered in H2, (a) Fe–0.5C–4.0X (X = Cr, Mn or Si) (b) Si–0.5C [Citation64].
![Figure 17. Thermogravimetric signals and MS graphs of different powder mixes sintered in H2, (a) Fe–0.5C–4.0X (X = Cr, Mn or Si) (b) Si–0.5C [Citation64].](/cms/asset/48b16df2-cefb-4bc6-ac48-e5d870b86150/ypom_a_1810427_f0017_oc.jpg)
The current working hypothesis is that the methane formed and measured is a final product of an ‘internal getter’ effect. Systematic experiments have shown that both iron as base material and oxygen-sensitive elements have to be present to produce significant amounts of methane. Carbon monoxide seems to be a necessary intermediate product which finally oxidises the sensitive alloying elements (Si, Mn, Cr) by the reaction Me + 2H2 + CO → MeO + CH4. The metallographic section shown in prove that there is much more homogeneous carbon loss in hydrogen compared to sintering in argon atmosphere, which was also confirmed by combustion analysis [Citation65]. This supports the hypothesis that the homogeneous decarburisation through CH4 formation is preceded by homogeneous carbothermal reduction. If a direct reaction of C with H2 would be responsible for CH4 formation, heterogeneous decarburisation from the surface would be expected, similar to the decarburisation caused by H2O from impure atmospheres.
Figure 18. Metallographic sections of masteralloy-containing steels Fe–4MA–0.5Cadmixed (MA: Fe–40Mn–17Si) sintered in the dilatometer at 1300 °C for 60 min in Ar or H2 (a) Argon: O: 0.025%, combined: 0.43% (b) Hydrogen: O: 0.013%, combined: 0.31% [Citation65].
![Figure 18. Metallographic sections of masteralloy-containing steels Fe–4MA–0.5Cadmixed (MA: Fe–40Mn–17Si) sintered in the dilatometer at 1300 °C for 60 min in Ar or H2 (a) Argon: O: 0.025%, combined: 0.43% (b) Hydrogen: O: 0.013%, combined: 0.31% [Citation65].](/cms/asset/620a546b-36ab-4b1c-9307-1a459a52b61f/ypom_a_1810427_f0018_oc.jpg)
‘Internal gettering’
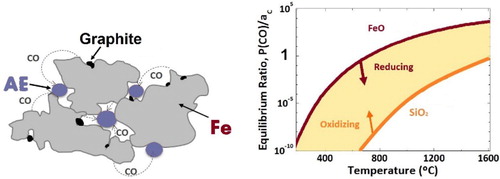
From a thermodynamic point of view, an elemental or masteralloy powder mix is in extreme inequilibrium, which has pronounced consequences if elements with widely differing oxygen affinity are present. The presence of agents that become reducing for the iron oxide surfaces above a given temperature during the heating stage generates reaction products (e.g. H2O and/or CO) which are, however, heavily oxidising for the more oxygen-sensitive elements at the same temperature. The largest source of oxygen is usually not the elemental or master alloy powder (unless treated improperly), but the base iron powder itself. Therefore, the gaseous reaction products, as oxidising agents, will oxidise silicon, manganese and chromium, present as elemental powders or combined in a masteralloy, by simply transferring the oxygen from the iron via the gas phase to the elemental or master alloy particles (see schematic in ).
This phenomenon usually does not occur at low temperatures (300–450°) at which only reduction by H2 is possible and when water is the reaction product, since the kinetics of the oxidising reactions is usually still too slow at these temperatures. However, in inert atmospheres when the reduction is performed by the added graphite, the temperatures of first CO/CO2 formation are significantly higher, and the effect can be observed indirectly, by the disappearance of the first CO peak, as given in (b) for admixed elemental Mn. As the CO does not leave the compound, the absence or at least the diminution of the expected CO peak at about 700°C is a clear hint for this internal oxygen transfer. In fact, the net reaction mechanism is a metallothermic reduction of the iron oxide surfaces by the alloy element(s) via gaseous intermediate products (see [Citation66]).
Figure 20. Thermogravimetric signals and MS graphs of Fe–0.5C and Fe–0.5C–4.0Mn (elemental) powder mixes heated in Ar, Tmax 1300°C, 20 K min−1, (a) Fe + 0.5C, (b) Fe + 0.5C + 4Mn [Citation66].
![Figure 20. Thermogravimetric signals and MS graphs of Fe–0.5C and Fe–0.5C–4.0Mn (elemental) powder mixes heated in Ar, Tmax 1300°C, 20 K min−1, (a) Fe + 0.5C, (b) Fe + 0.5C + 4Mn [Citation66].](/cms/asset/2f025993-c067-4f6e-88f6-c22bed925e2b/ypom_a_1810427_f0020_oc.jpg)
A similar phenomenon was reported by Hryha [Citation67], when using elemental manganese as alloying element during sintering at 1120°C. Intergranular decohesion facets were found around Fe–Mn residues with a huge amount of point inclusions consisting of oxides, forming networks. This observation also is an indicator for internal gettering since the manganese that diffuses along the grain boundaries reduces the surface iron oxides of the particles, forming a network of point-like Mn oxides that locally weaken the grain boundaries and finally lead to intergranular fracture in this area. The mechanical behavior is strongly improved at a higher sintering temperature, because then also these Mn oxides can be reduced.
The practical consequence is that effective deoxidation of such powder compacts requires temperatures typical for carbothermal reduction of the alloy element oxides, although initially the oxygen contained has been present mainly as – easily reducible – iron oxides. To some extent, the temperature thresholds for reduction are lowered by the dissolution of the alloy metal formed in the iron matrix, with a resulting decrease of the chemical activity. In any case, there is not much difference in the deoxidation behavior between mixed and prealloyed systems, respectively, in both cases, high sintering temperatures, >1200°C, are required for reasonably complete deoxidation.
‘Internal gettering by diffusion’
As stated above, the introduction of alloy elements into sintered steels can be done by admixing these elements, as elemental powders or through masteralloys, but also by prealloying the entire powder. The deoxidation of chromium or manganese prealloyed powders in inert atmospheres shows a macroscopically similar effect as the ‘internal gettering’ observed with mixes, although here the transport of the reaction partners does not occur via gaseous products but by diffusion. As already shown, the surface of commercially available prealloyed powders consists primarily of iron oxide, and the oxides of the more sensitive elements are located in particulates on the surface and within the powder particle [Citation46,Citation68]. This is confirmed by the fact that sintering in H2 results in a pronounced H2O peak at about 400°C, as observed for Fe–C. When this powder is, however, heat treated (or heated) in inert atmosphere, the chromium (or the manganese) dissolved in the iron matrix starts to diffuse towards the surface where oxygen is present bonded to iron, the driving force being the much more negative Gibbs free energy for the formation of, e.g. MnO compared to iron oxides (see ). There, the transfer from iron oxide to more stable oxides – which is in fact also a metallothermic reduction of the iron oxide – can happen if the kinetics of the reaction is fast enough. As can be seen in , heat treatment at 400°C in argon does not change the deoxidation behaviour in hydrogen at low temperatures compared to as delivered powder (see (b) and (a)), the typical m18 peak at 400°C still being present. This confirms that the surface oxides still have been iron oxides in this case. The situation changes dramatically when the material is heated to 650°C (even without holding time). For the Fe3Cr0.5Mo–C system, the water peak disappears almost completely, which is a clear indicator for the presence of more stable oxides on the surface that require much higher temperatures for (carbothermal) reduction. The kinetics aspects of this oxide transformation have also been discussed in [Citation53], where it is shown, that the reduction of the surface oxides by hydrogen is beneficial, especially when it is performed before a part the stable oxides are entrapped within the growing sintering necks. The critical temperature identified by this study for oxygen transfer is 800–1000°C, where an unfavourable balance between thermodynamics and kinetics of oxide reduction exists during the heating stage. This means increased mass transfer of oxygen-sensitive elements but still poor conditions for oxygen reduction.
Figure 22. Dilatometry and mass spectra of Fe–3wt-%Cr–0.5 wt-%Mo–0.5wt-%C in H2. Tmax 1300°C, heating rate 10 K min−1. In part, pre-annealed in Ar. (a) Reference run, no pre-anneal. (b) Pre-annealed in Ar at 400°C. (c) Pre-annealed in Ar at 650°C [Citation66].
![Figure 22. Dilatometry and mass spectra of Fe–3wt-%Cr–0.5 wt-%Mo–0.5wt-%C in H2. Tmax 1300°C, heating rate 10 K min−1. In part, pre-annealed in Ar. (a) Reference run, no pre-anneal. (b) Pre-annealed in Ar at 400°C. (c) Pre-annealed in Ar at 650°C [Citation66].](/cms/asset/cda91114-127a-4a8c-8df0-9537c1e79de3/ypom_a_1810427_f0022_oc.jpg)
The transformation of oxides towards more stable ones has been shown through XRF and SEM studies and microanalysis, e.g. by Karamchedu et al. [Citation37], but at much higher temperature levels (900°C), at 450°C delubrication temperature, no effects having been measured. Chasoglou et al. [Citation69] demonstrate the change in morphology of stable oxides and the incorporation of these in the sintering necks. This for sure influences the mechanical properties, not so much the static ones but it can be expected and was already demonstrated in [Citation47] that dynamic properties suffer from these inclusions. The present study, however, shows that such ‘internal getter’ phenomena occur at quite moderate temperature levels, below 700°C.
One possible solution might be a reduction/presintering treatment in H2 at temperatures at which the internal getter effect does not yet appear, but for PM precision parts, this collides with the necessity to do effective lubricant burnout, which in industrial practice requires temperatures well above 400°C. Furthermore, as indicated by the graphics in (a,b), even at optimal conditions presintering in H2 removes only part of the oxygen – that one bonded to iron – while the remaining oxygen must be removed by carbothermal reduction at much higher temperatures. Therefore, it is as necessary to apply high sintering temperatures for Cr/Mn/Si prealloyed powders as it is for the mixed systems described above, at least if the potential of these advanced alloy elements is to be fully exploited.
Conclusions
It has been shown that thermoanalytical techniques, such as dilatometry or DTA/TG combined with EGA by mass spectrometry, are powerful tools to understand the interaction of powder components with the sintering atmosphere, in particular, the deoxidation processes in the early stages of sintering that are essential for obtaining excellent mechanical properties. The main findings are:
The classical materials for ferrous structural parts are more or less easy to sinter, since the deoxidation processes are dominated by the base iron powder. The classical alloying elements, such as nickel, copper and molybdenum form oxides, that are less stable than the iron oxides and are therefore easier to reduce, and the system is fairly robust towards the quality of the sintering atmosphere, the limiting factor being rather surface decarburisation.
Advanced high-performance sintered steels contain alloying elements, such as chromium, manganese (and in the future possibly silicon), and the presence of these oxygen-sensitive elements changes the chemical behaviour dramatically.
The reducing of the oxides of these elements in the early sintering stage becomes decisive for a successful sintering.
One of the most critical points is the transfer of oxygen from the surface of the starting powder, which consists mainly of iron oxides, to the alloying elements.
In inert atmospheres, a transformation of the original iron oxides to more stable complex oxides occurs by diffusion of the alloying elements to the particle surface during the heating stage of the sintering process in case prealloyed powders are used.
When powder mixes – or more modern – master alloys are used, this transfer takes place via the gas phase, in a process designated ‘internal gettering’, the transporting agent being CO within the pore structure of the compact.
In reducing atmospheres, it is possible that this CO is reduced by hydrogen to form methane in different temperature ranges depending on the alloying system.
All these reactions influence the final carbon and oxygen content of the material and therefore the mechanical properties. Therefore, it is important to be aware of these reactions also in industrial practice.
The high-performance steels based on advanced alloying systems are much more sensitive to the quality of the furnace atmosphere than are traditional grades. Therefore, more attention has to be paid to keep the processing conditions as stable as possible for a successful sintering of these materials. On-line measurement systems to ensure proper atmospheres are available for industrial practice
Furthermore, reduction of the ‘natural’ oxides present on the powders requires significantly higher temperatures than applied for standard PM steels, and therefore high-temperature sintering is a must if the full benefit of the new alloy systems is to be exploited.
It can be expected that the alloy development will continue and alloying elements that are today in use for wrought steels like silicon or vanadium become more and more interesting for powder metallurgy. Therefore, investigations in this field will be still necessary to further improve the performance of PM steels. In any case, however, tools for these studies are available.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Notes on contributors
Christian Gierl-Mayer studied Technical Chemistry at TU Wien, Vienna Austria. He made his Master Degree in Inorganic Chemistry in 1996. He got his PhD in 2000 from TU Wien for ‘Production of PM structural parts by optimised sintering‘. After three years as scientist in private research institute (ofi-Austrian Institute for Chemistry and Technology) he became senior scientist at TU Wien, Institute for Chemical Technologies and Analytics (2003). 2017 he was promoted Associate professor and 2019 after his habilitation in powder metallurgy he became Associate Professor for metallic sintered materials. He is currently leading the research group ‘Powder metallurgy’ at the above mentioned Institute. He is currently leading the research group ‘Powder metallurgy’ at the above mentioned Institute.
References
- Boccini GF. Influence of controlled atmosphere on the proper sintering of carbon steels. Powder Metall Prog. 2004;4(1):1–33.
- Beiss P. Control of protective atmospheres during sintering. Proceedings Euro PM 2000 – Sintering and Equipment. Munich; 2000. p. 147–157.
- Bracconi P, Nyborg L. Quantitative phase analysis and thickness measurement of surface oxide layers in metals and alloy powders by the chemical granular method. Appl Surf Sci. 1998;133:129–147. doi: 10.1016/S0169-4332(98)00194-9
- Oleford I, Nyborg L. Surface analysis of gas atomized ferritic steel powder. Powder Metall. 1985;28:237–243. doi: 10.1179/pom.1985.28.4.237
- Nyborg L, Nylund A, Olefjord I. Thickness determination of oxide layers on spherical shaped metal powders by ESCA. Proc. of ESCASA 1987. Stuttgart; 1987.
- Nyborg L, Norell M, Olefjord I. Surface studies of powder metallurgical stainless steel. Surf Interface Anal. 1992;19:607–614. doi: 10.1002/sia.7401901113
- Norell M, Nyborg L, Tunberg T, et al. Thickness determination of surface oxides on metal powder by AES depth profiling. Surf Interface Anal. 1992;19:71–76. doi: 10.1002/sia.740190116
- Tunberg T, Nyborg L. Surface reactions during water atomisation and sintering of austenitic stainless steel powder. Powder Metall. 1995;38:120–139. doi:10.1179/pom.1995.38.2.120.
- Karlsson H, Nyborg L, Berg S. Surface chemical analysis of pre-alloyed water-atomized steel powder. Powder Metall. 2005;48(1):51–58. doi: 10.1179/0032589005X37675
- Karlsson H, Nyborg L, Berg S, et al. Surface product formation of chromium alloyed steel powder particles. Proc. EuroPM 2001 Vol. 1. Shrewsbury: Nice: EPMA; 2001. p. 22–27.
- Bocchini G. The influence of porosity on the characteristics of sintered materials. Int J Powder Metall. 1986;22(3):185–202.
- Dlapka M, Danninger H, Gierl C, et al. Defining the pores in PM components. Met Powder Rep. 2010;65(1):30–33. doi: 10.1016/S0026-0657(10)70093-X
- Murphy T, Lindsley B. Metallographic analysis of PM fracture surfaces. In: Murphy CB, editor. Advances in powder metallurgy and particulate materials. Princeton, NJ: Metal Powder Industries Federation; 2007. p. 11-1–11-15.
- Danninger H, Spoljaric D, Jangg G, et al. Characterization of pressed and sintered ferrous materials by quantitative fractography. Pract Metallogr. 1994;31(2):56–59.
- Schatt W. Sintervorgänge – Grundlagen. Düsseldorf: VDI; 1992.
- Zapf G. Handbuch der Fertigungstechnik, Bd.1. Urformen. (G. Spur, Hrsg.). München: Hanser Verlag; 1981.
- Gierl-Mayer C, de Oro Calderon R, Danninger H. The role of oxygen transfer in sintering of low alloy steel powder compacts: a review of the “internal getter” effect. JOM. 2016;68(3):920–927. doi: 10.1007/s11837-016-1819-z
- Danninger H, Gierl C. New alloying systems for ferrous powder metallurgy precision parts. Sci Sinter. 2008;40(1):33–46. doi: 10.2298/SOS0801033D
- Sercombe T, Schaffer G. The effect of trace elements in the sintering of Al-Cu alloys. Acta Mater. 1999;2:689–697. doi: 10.1016/S1359-6454(98)00353-X
- Schaffer G, Huo S, Drennan J, et al. The effect of trace elements on the sintering of an Al-Zn-Mg-Cu alloy. Acta Mater. 2001;49(14):2671–2678. doi: 10.1016/S1359-6454(01)00177-X
- Gierl C, Ul Mohsin I, Danninger H. Boron activated sintering of PM steels – alterntive boron sources. Powder Metall Prog. 2008;8(2):135–141.
- Momeni M, Gierl C, Danninger H, et al. Thermoanalytical sintering studies of Fe–C admixed with ferroboron performed in different atmospheres. Powder Metall. 2012;55(1):54–64. doi: 10.1179/1743290111Y.0000000020
- Vassileva V, Danninger H, Strobl S, et al. The role of the atmosphere on boron-activated sintering of ferrous powder compacts. Powder Metall Prog. 2018;18(1):6–20. doi: 10.1515/pmp-2018-0002
- Crease, Jr, A. Rapid burn-off system for removing lubricant from P/M parts. J Powder Metall. 1977;13(1):21–24.
- anonymous. Funace atmospheres No.6 sintering of steelsLinde AG; 2011. https://www.linde-gas.com/en/images/Furnace.
- Nayar H. Sintering atmospheres. In: Metal handbook Vol. 7 powder metal technologies and applications. Materials Park, OH: ASM International; 1998. p. 1066–1094.
- ASM International. Thermal expansion. In: Cverna F, Editor. Thermal properties of metals. Materials Park, OH: ASM International; 2002. p. 9–12.
- Gierl-Mayer C, Danninger H. Dilatometry coupled with MS as instrument for process control in sintering of powder metallurgy steels. Powder Metall Prog. 2015;15(1):3–12.
- Quadbeck P, Schreyer B, Strauß A, et al. In-Situ monitoring of gas atmospheres during debinding and sintering of PM steel components. (EPMA, Edt.) Proceedings of PM21010 World Congress, Vol. 2 Sintering; 2010.
- Gille G, Leitner G, Roebuck B. Sintering behaviour and properties of WC-Co hardmetals in relation to the WC powder properties. Proceedings of European Conference of Advanced Hard Metals Production, Stockholm (S. 195–201). Shrewsbury: EPMA (Edt.); 1996.
- Gestrich T. In situ Charakterisierung der Vorgänge beim Entbindern, Ausgasen und Sintern von WC-Co-Hartmetallmischungen mittlels komplexer thermischer Analyse [PhD Thesis]. Dresden, TU; 2005.
- Gestrich T, Kaiser A, Pötschke J, et al. Thermal behavior of cermets and hardmetals during debinding and sintering. Int J Refract Met Hard Mater. 2018;73:210–214. doi: 10.1016/j.ijrmhm.2018.01.008
- Chasoglou D, Hryha E, Bergman O. Efficient sintering of Cr-prealloyed powders by careful adjustment of process parameters. Adv Powder Metall Part Mater. 2014:142–153.
- Frisk K, Söderberg H, Caddeo S. Studies of surface oxides on steel powders using photo acoustic spectroscopy coupled with thermodynamic calculations. Proc. Euro PM2009, Copenhagen. Shrewsbury, EPMA (Edt.); 2009.
- Hryha E, Nyborg L, Malas A, et al. Carbon control in PM sintering: industrial applications and experience. Powder Metall. 2013;56(1):5–10. doi: 10.1179/0032589912Z.00000000085
- Hryha E, Dudrova E, Nyborg L. On-line control of processing atmospheres for proper sintering of oxidation-sensitive PM steels. J Mater Process Technol. 2012;212(4):977–987. doi: 10.1016/j.jmatprotec.2011.12.008
- Karamchedu S, Hryha E, Nyborg L. Changes in surface chemistry of chromium alloyed powder metallurgical steel during delubrication and their impact on sintering. J Mater Process Technol. 2015;223:171–185. doi: 10.1016/j.jmatprotec.2015.03.054
- Danninger, H. Asymmetrical ferrite-Austenite transformation during vacuum sintering of plain iron compacts. Powder Metall Prog 2003, 3(2), p. 75–85.
- Danninger H, Gierl C, Vassileva V. Asymmetry effects in ferrite-austenite transformation during sintering of carbon-free ferrous alloys. Proceedings of EuroPM2005, Vol. 1. Prague, Shrewsbury: EPMA (Edt); 2005. p. 15–20.
- Kuroki, H., Suzuki, H. Y. Coarse columnar structure of transformation-grown ferrite in pure iron – on wrought iron and sintered iron. Mater Trans 2006, 47(10), p. 2449–2456. doi: 10.2320/matertrans.47.2449
- Jaliliziyaeian M. Sintering of manganese prealloyed powder metallurgy steels [PhD thesis], TU Wien. 2008.
- Gierl C, Danninger H. Thermal analysis of plain iron – the effect of “inert” atmospheres. Proceedings EUROPM2005, Vol. 3. Prague: EPMA (Edt.), Shrewsbury; 2005. S. 3–8.
- Gierl C, Jaliliziyaeian M, Danninger H, et al. Dilatometry of PM carbon steels in different atmospheres – deoxidation effects. Proceedings Euro PM2009. Copenhagen. Shrewsbury: European Powder Metallurgy Association; 2009. Paper-Nr. 29-1, on CD.
- Danninger H, Gierl C, Kremel S, et al. Degassing and deoxidation processes during sintering of unalloyed and alloyed PM steels. Powder Metall Prog. 2002;2(3):125–140.
- Kent D, Drennan J, Schaffer G. A morphological study of nitride formed on Al at low temperature in the presence of Mg. Acta Mater. 2011;59(6):2469–2480. doi: 10.1016/j.actamat.2010.12.050
- Ortiz P, Castro F. Thermodynamic and experimental study of role of sintering atmospheres and graphite additions on oxide reduction in Astaloy CrM powder compacts. Powder Metall. 2004;47(5):291–298. doi: 10.1179/003258904225020747
- Kremel S, Danninger H, Yu Y. Effect of sintering conditions on particle contacts and mechanical properties of PM steels prepared from 3%Cr prealloyed powders. Powder Metall Prog. 2002;2(4):211–221.
- Dlapka M, Danninger H, Gierl C, et al. Fatigue behavior and wear resistance of sinter-hardening steels. Int J Powder Metall. 2012;48(5):49–60.
- Danninger H, Leitner, G. Gas formation in Fe-Mo-C below the α−γ transition. In: German R, Messing G, Cornwall R. editors. Sintering technology (Proc. Conf. “Sintering'95”). University Park, PA; 1996; New York-Basel-Hong Kong: Marcel Dekker. p. 165–172.
- Hryha E, Wendel J. Effect of heating rate and process atmosphere on the thermodynamics and kinetics of the sintering of pre-alloyed water-atomized powder metallurgy steels. J Am Cer Soc. 2019;102:748–756.
- Danninger H, Jaliliziyaeian M, Gierl C, et al. Chemical reactions during sintering of Mn and Mn-Cr prealloyed steels in inert versus reducing atmospheres. Mater Sci Forum. 2011;672:203–206. doi: 10.4028/www.scientific.net/MSF.672.203
- Hryha E, Nyborg L. Thermogravimetry study of the effectiveness of different reducing agents during sintering of Cr-prealloyed PM steels. J Therm Anal Calorim. 2014;118(2):825–834. doi: 10.1007/s10973-014-3915-z
- Hryha E, Nyborg L. Surface oxides on metal powders: an overview of the effect of alloy composition and manufacturing method. Proc. World PM 2016; Hamburg. Shrewsbury: EPMA (Edt.); 2016.
- Hryha E, Gierl C, Nyborg L, et al. Surface composition of the steel powders pre-alloyed with manganese. Appl Surf Sci. 2010;256(12):3946–3961. doi: 10.1016/j.apsusc.2010.01.055
- Danninger H, Xu C. Degassing and reduction during sintering of high density PM steels. Proc. EuroPM 2003, Vol. 1. Valencia; 2003. p. 269–274.
- Danninger H., Xu C., Khatibi G., Weiss B., Lindqvist B., Gigacycle fatigue of ultra high density sintered alloy steels. Powder Metall. 2012, 55(5), p. 378–387 doi: 10.1179/1743290112Y.0000000001
- Kieffer R, Hotop W. Sintereisen und Sinterstahl. Springer-Verlag: Wien; 1948.
- Jones W. Fundamental principles of powder metallurgy. London: Edward Arnold; 1960.
- Lenel F. Powder metallurgy – principles and applications. Princeton (NJ): MPIF; 1980.
- de Oro Calderon R, Campos M, Gierl C, et al. Atmosphere effects on liquid phase sintering of PM Steels modified with master alloy additions. Proc. PM2010 World Congress Florence, Vol. 2 Sintering. Shrewsbury: EPMA (Edt.); 2010.
- Gierl C, Danninger H. Charakterisierung von Sintervorgängen in Eisenbasis- Pulverpresslingen mittels Prozessanalytik durch thermische Analysenmethoden. In: Kolaska H. Hrsg. Pulvermetallurgie in Wissenschaft und Praxis Band 27: Sintern – der zentrale Prozess der Pulvermetallurgie. Hagen: Fachverband Pulvermetallurgie; 2011. p. 215–236.
- Gierl-Mayer C, Danninger H, de Oro Calderon R, et al. “Internal gettering” – metallothermic reduction processes in the early stage of sintering. Int J Powder Metall. 2015;51:47–53.
- Danninger H, Avakemian A, Gierl-Mayer C, et al. Methane formation through substrate-atmosphere interaction during sintering of Si containing steels. Proc. EuroPM2014 Salzburg. Shrewsbury, UK: EPMA Edt; 2014. p. 6.
- Gierl-Mayer C, de Oro Calderon R, Danninger H. Sintering of ferrous metallic compacts: chemical reactions that involve interstitial elements. J Am Cer Soc. 2018;102:695–705.
- Gierl-Mayer C, de Oro Calderon R, Danninger H. Methane formation during sintering of PM steels alloyed with chromium and/of manganese. Proc. World PM 2016 Hamburg. Shrewsbury, UK: EPMA (Edt.); 2016.
- Danninger H, de Oro Calderon R, Gierl-Mayer C. Chemical reactions during sintering of PM steel compacts as a function of the alloying route. Powder Metall. 2018;6(3):241–250. doi: 10.1080/00325899.2018.1458489
- Hryha E, Nyborg L. Oxide transformation in Cr-Mn-prealloyed sintered steels: thermodynamic and kinetic aspects. Metall Mater Trans A. 2014;45(4):1736–1747. doi: 10.1007/s11661-013-1969-3
- Hryha E, Nyborg L, Dudrova E, et al. Brittleness of structural PM steels admixed with manganese studied by advanced electron microscopy and spectroscopy. Powder Metall Prog. 2008;8(2):109–114.
- Chasoglou D, Hryha E, Nyborg L. Effect of atmosphere composition on the surface interactions during sintering of chromium-alloyed PM steels. Proceedings Euro PM2011 Congress and Exhibition on Powder Metallurgy, Barcelona, Vol. 3. Shrewsbury: EPMA; 2011, p. 111–117.