?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Owing to the different characteristics of each of the multiple polymers, obtaining a homogenous metal injection moulding (MIM) feedstock blend is challenging. Therefore, studying interactions between the polymeric components is vital for achieving suitable MIM feedstocks. We report on the effects of different compatibilisers – EGMA and E40 – on the interactions in polyoxymethylene (POM) and polypropylene (PP) blends. We measured contact angles and performed Fourier transform infrared spectroscopy and atomic force microscopy analyses to identify the suitable compatibiliser that yields a feedstock with excellent properties. It was found that the binder system based on EGMA-3 demonstrates the lowest contact angle and best miscibility for the POM/PP blends compared to E40. This enhanced interaction lies in the chemistry of EGMA, having an active site that reduces the interfacial tension between the components of the POM/PP blend. Subsequently, this creates a positive interaction between the polymers and metal powders, ensuring good adhesion within the feedstock.
1. Introduction
Metal injection moulding (MIM) is an advanced net-shape metal forming technique that combines the advantages of plastic injection moulding and conventional powder metallurgy [Citation1,Citation2]. Owing to its low production cost and reduced material wastage, it is suitable for expensive and difficult-to-machine metals such as titanium and its alloys [Citation3]. Typically, MIM comprises four sequential technological stages; mixing, injection moulding, debinding and sintering [Citation4]. This process is based on injecting a mixture of the desired metal powder with a binder of several polymers, known as feedstock, into the desired part. The part is then subjected to debinding to extract the binder and sintered to obtain the final dense part [Citation5].
The initial stages, such as mixing and moulding, are considered critical in the MIM process [Citation6, Citation7]. During mixing, it is ideal that every individual metal powder particle is covered with a thin layer of binder to provide sufficient fluidity to fill the mould during injection [Citation8]. Hence, the significance of binders cannot be overstressed to achieve such characteristics, as explained in detail by German [Citation9]. Since the initial interaction is to wet the metal powder surface, good compatibility between binder components is essential to prevent phase separation or segregation of feedstock during processing via injection moulding [Citation10].
In MIM feedstocks, binders are usually composed of multicomponent polymeric mixtures containing a small amount of additives, such as stearic acid. The additives are used to promote adhesion to the powder surface, coating the particles and preventing powder aggregation [Citation11]. However, due to the relatively short chain lengths of stearic acid, interlinking strength among binder components is often weak [Citation7,Citation12]. Hence, including polymers with a polar group as interfacial agents among binder components is beneficial in enhancing the interlinking strength of binder components and improving the adhesion force on the powder surface [Citation13,Citation14].
At present, the interaction of the binder and powder is mainly assessed by rheological and thermogravimetry measurements [Citation3,Citation15–17]. Very little is known about the specific interactions among binder components and how such interaction affects the MIM of reactive powders, such as Ti-MIM. In one attempt, Hausnerova et al. [Citation7] studied the adhesion of binder components to ceramic powders via contact angle measurement. The results show that among the binder components, the acrawax/polyethylene glycol (PEG) polymer blend having the highest values of polar component is more suited for powder injection moulding. In addition, Subuki et al. [Citation6] investigated the molecular interactions of three different binder systems comprising polystyrene/polyethylene (PS/PE), PS/polypropylene (PP) and thermoplastic natural rubber/paraffin wax (TPNR/PW) for stainless steel (316L) using Fourier-transform infrared (FTIR). It is reported that PS/PP blends displayed the highest interaction with the metal powder. On the other hand, Bleyan et al. [Citation18] propose that studying the interaction of low-molecular analogues of binder components via FTIR and calorimetric measurements could provide necessary information on the miscibility of the polymers. It is claimed that the interactions between the two polymers can noticeably enhance the debinding and sintering characteristics.
In this work, polyoxymethylene (POM) was used as a primary binder, polypropylene (PP) as the backbone binder and stearic acid (SA) as a surfactant. Thus far, no reports are available in the literature studying the interactions of POM-based binders. Considering the molecular structure of POM and PP, blending these two polymers is expected to be incompatible and demonstrates a two-phase system [Citation19–21]. Therefore, to improve the homogeneity of the POM-based binder system for Ti-MIM, two different types of compatibiliser, poly(ethylene methyl acrylate-co-glycidyl methacrylate) (EGMA) and ethylene-vinyl acetate (EVA) will be investigated. This study aims to better understand the interactions between the POM-based binder system and titanium metal powder, which can lay the foundation for developing a novel binder system for Ti-MIM.
2. Experimental procedures
2.1. Materials
Commercial purity Ti powder was purchased from TLS Technik Spezialpulver (Germany). The powder was produced by gas-atomisation, resulting in a spherical shape with a nominal size of 45 μm. The polymeric binder system consisted of laboratory-modified POM (density = 1.42 g cm−3, melt flow index = 9.73 g/10 min). Isotactic polypropylene (PP), poly(ethylene-co-methyl acrylate-co-glycidyl methacrylate) (EGMA), poly(ethylene-co-vinyl acetate) with 40% vinyl acetate (E40) and stearic acid (SA) were supplied by Sigma-Aldrich.
2.2. Methodology
Initially, the POM-based binder component without metal powder was prepared in a ThermoHaake Brabender mixer (Germany) at 180°C for 10 min with a rotor speed of 60 rev min−1. The binder system was based on the polymer blend containing POM as the primary binder, PP as the secondary binder, EGMA and E40 as a compatibiliser, and SA as a surfactant. The composition of the binder content is tabulated in . After mixing, the polymeric binder mixture is hot pressed into a thin film with a thickness of 1 mm for characterisation. Subsequently, the selected binder formulation based on the contact angle measurement and AFM was used to prepare the titanium feedstock. In feedstock preparation, mixing was performed at 180°C, 60 rev min−1 for 40 min. In this study, the formulation of 63 vol.-% powder loading of titanium powder was used for investigation.
Table 1. Binder weight content (wt-%) used for feedstock preparation.
2.3. Fourier-transform infrared
FTIR using a Perkin Elmer 100 spectrophotometer (Waltham, MA, USA) was performed to evaluate the chemical interactions between the POM-based binder system and the feedstock. The spectra were collected over a scan range of 450–4000 cm−1 at a resolution of 16 cm−1.
2.4. Atomic force microscopy and scanning electron microscopy
Tapping mode AFM was used to characterise the polymer blend samples. Atomic force microscopy was performed on an Asylum Research MFP-3D Origin with Budget Sensors silicon 150 probes. The nominal scans include a line rate from 0.5 to 1 Hz, with a frequency of 150 kHz and a spring constant of 1.5–15 Nm−1. The images obtained were analysed using commercial Gwyddion software. Fractured surfaces of the feedstock were analysed using a Hitachi SU-70 Scanning Electron Microscope (SEM).
2.5. Contact angle measurement
The contact angle measurement of the POM binder constituents was determined on four different test liquids (deionised water, ethylene glycol, di-iodomethane and hexadecane). A drop test was performed by depositing a single drop of liquid (volume, V = 3–5 µL) on the sample surface, and the sessile drop and the contact angle were measured. Contact angles measured on three different areas for each sample were averaged to obtain one representative value. Using the above data, the total surface energy γ(tot), disperse part γ(d) and polar part γ(p) were obtained, following the Owens–Wendt method built on the theory of Fowkes [Citation22–24]. Afterwards, the interfacial tension between the polymers was calculated using the geometric and harmonic mean equations (Equations (1) and (2)):
(1)
(1)
(2)
(2)
Further, to study the powder-binder interactions, contact angle measurement using deionised water was performed on the formulated POM binder system presented in and on selected titanium feedstock. Each measurement was repeated four times for each specimen for an accurate measurement.
2.6. Gas pycnometer
Accupyc II Gas Pycnometer is another tool to determine the interaction between the powder and binder. A homogeneous feedstock will demonstrate low standard deviation values. On the other hand, an inhomogeneous feedstock caused by poor powder-binder interaction will lead to varying density within the feedstock and can further cause distortion within the moulded part. The homogeneity of the feedstock was determined by randomly selecting five samples from the prepared batch. Subsequently, to ensure the highest possible density with no voids, the injection moulded part and the crushed injected part were compared to the feedstock's theoretical density [Citation25].
2.7. Rheological properties
Rheological experiments were performed on a CFT-500D capillary rheometer (Shimadzu) in the temperature range of 180–200°C. Different pressure values and die sets with different length–radius (L/D) ratios (D1 = 1 mm, L1 = 1 mm; D2 = 1 mm, L2 = 2 mm) were used for calculations based on the Bagley & Rabinowitsch [Citation26]. The mouldability index (α) can be calculated using Equation (3) [Citation27]:
(3)
(3) where E is the activation energy, R is the gas constant and ηo is the reference viscosity at 190°C and a shear rate of 6000 s−1. A high value of α denotes better rheological stability of the feedstock [Citation28].
3. Results and discussion
3.1. FTIR analysis
depicts the FTIR spectra of the titanium MIM feedstocks based on formulations C–O, EGMA-3, and E40-3 and compares them with their respective binder constituents. (a) shows that there is not much significant change in the wavenumber arising from the interaction between the metal powder and the binders. Moreover, the FTIR spectra of Feedstock C-0 display all the characteristic peaks for pure POM and pure PP, indicating the absence of interaction.
Figure 1. The FTIR spectra of feedstock (a) C-0, (b) EGMA-3 and (c) E40-3 with its respective binder constituent.
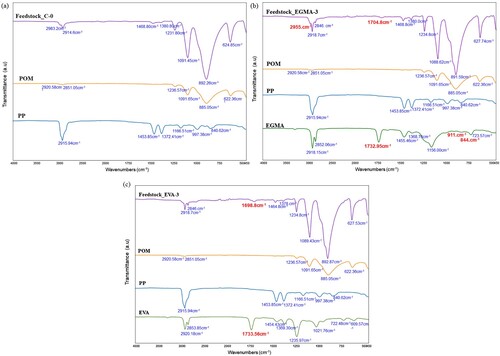
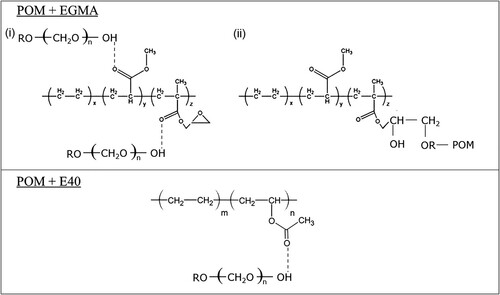
However, after the addition of compatibiliser, a significant shift in the wavenumber of the feedstock is observed. From (b and c), both EGMA and E40 have a carbonyl group (C=O) at absorption band 1733 and 1734 cm−1, respectively. It has been reported that a shift towards a lower wavenumber, usually less than 30 cm−1, can be expected after the formation of a Lewis acid–base reaction between binder and powder [Citation29]. However, it is noteworthy that the presence of stearic acid (SA) as a surfactant in the MIM feedstock is often one of the reasons for this interaction. Nonetheless, it can be seen that Feedstock EGMA-3 ((b)) demonstrates a shift from 1733 to 1705 cm−1 with a difference of 28 cm−1, while Feedstock E40-3 ((c)) displays a difference of 35 cm−1 after the band shifts from 1734 to 1699 cm−1. This stipulates that in the presence of EGMA-3, the POM-based binder system has a stronger bond or acid–base interaction towards titanium powder than E-40. It is suggested that compatibilisers – EGMA and E40 – seem to interact and overlap with the binder system, forming possible hydrogen bonding between the OH group from POM and C=O group from compatibiliser ().
Besides, a new peak can be distinguished at 2955 cm−1 in (b). This corresponds to the symmetrical and asymmetrical CH2 stretching vibrations. Interestingly, the peaks of the epoxy group located at wavenumber 844 and 911 cm−1 on the EMA−GMA group have disappeared. This indicates a chemical reaction between the epoxy groups of EGMA and the end hydroxyl groups of POM () [Citation30,Citation31]. In the case of Feedstock E40-3 ((c)), no new peaks are observed. This proves that the addition of EGMA has a more effective interaction in improving the compatibility between POM and PP blends than E40. Hence, developing the ternary blend system based on EGMA-3 would contribute to better binder adhesion on powder particles.
3.2. Microstructural observations
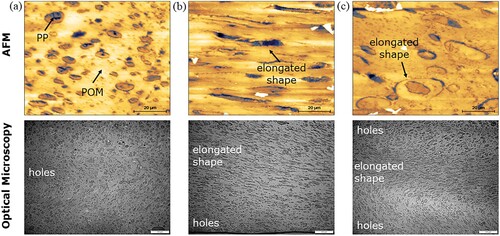
The compatibility of the POM-based binder system with and without compatibiliser is illustrated in . Typically, a blend with poor miscibility exhibits distinctly separate phases of components, while a homogeneous surface morphology is observed for the blend with good compatibility [Citation32]. In (a), the island-like morphology shows a clear phase separation between POM and PP blends, where the darker regions correspond to PP and the lighter regions correspond to POM. This indicates the high level of immiscibility between the two polymer phases. However, an elongated structure can be seen in the ternary blends after the addition of compatibiliser. A compatible blend has been reported to demonstrate surface morphology with smaller holes and larger extended-like morphology [Citation33]. As depicted in (b), the POM-based binder system with EGMA-3 revealed better miscibility, dominated by more elongated-like morphology dispersed in the continuous phase. On the other hand, the morphology of the POM-based binder system with E40-3 shows both island-like and elongated-like characteristics ((c)). Similarly, from the optical micrograph, the POM-based binder system with E40-3 presents many holes dispersed in the continuous phases compared to the binder system with EGMA-3 formulation.
Figure 3. AFM phase images (with optical micrograph) of the POM-based binder system with formulation (a) C-0, (b) EGMA-3 and (c) E40-3.
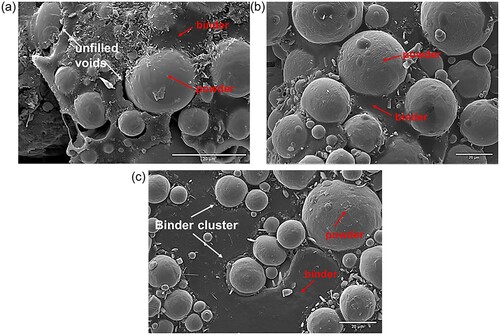
Further, the SEM micrograph of the MIM feedstock is presented in . It is apparent that some unfilled voids or gaps were observed at the interface between titanium particles and the binders for feedstock C-0 ((a)). The presence of voids or gaps in the feedstock blend is usually a result of poor compatibility and interfacial stability [Citation34]. For feedstock EGMA-3 and E40-3, the binder is melted and uniformly distributed in the feedstock matrix. No voids were found, indicating a good adhesion of the binder on metal powder. Nonetheless, from (b and c), feedstock EGMA-3 shows better wetting between powder and binder than feedstock E40-3. The clusters of binders are embedded surrounding the powder particles in feedstock E40-3 ((c)). The difference in wetting can be ascribed to the presence of oxygen-containing functional groups in the binder system. Feedstock EGMA-3 containing more carboxyl groups reveals better interaction between binder-binder components, subsequently enhancing the powder-binder adhesion, as reflected from the FTIR.
3.3. Contact angle measurements
Contact angle measurement for each binder is studied to determine the binder components’ wettability and interaction. The Owens–Wendt theory (geometric and harmonic method) was applied to calculate the surface and interfacial tensions of POM, PP and compatibiliser blends [Citation22]. shows that the contact angles for all given binder components are highest with deionised water (DI) and lowest with hexadecane (HD). It is well known that a decrease in contact angle allows the binder to spread over the powder particles easily, resulting in better wettability [Citation35]. As can be seen from , POM has a lower contact angle value in the polar liquids with the highest polar component, γ (p) = 3.9 m J m−2. This can be related to POM's polar functional group, (–CH2O–), which can easily form hydrogen bonds with powder via acid–base interactions. This proves that POM is suitable for acting as a primary binder. On the other hand, PP demonstrates a hydrophobic characteristic. Since molecular interactions among binder components are critical to achieve good homogeneity of feedstocks and necessary green strength, compatibiliser is introduced.
Table 2. Contact angle [o] and calculated free surface energy (10−3 J m−2) of the binder components on four different liquids (deionised water (DI), ethylene glycol (EG), di-iodomethane (DIM) and hexadecane (HD.)).
The Owens–Wendt theory was used to predict the compatibility of POM/PP blends. From , it is revealed that POM and PP blends demonstrated the highest interfacial tension. This signifies that the combination of these two polymers is immiscible. The high polarity POM blended with the apolar PP is not favourable from a thermodynamic point of view, which results in clearly separated phases, as seen previously by morphological investigations. shows that POM/EGMA/PP blends have the lowest interfacial tension value compared to E40. Besides, EGMA has a similar surface tension to POM with respect to the polar component (γp) and PP in terms of the dispersive component (γd). This demonstrates the preferential miscibility of EGMA over E40 in POM/PP ternary blends.
Table 3. The interfacial tension of the binder components using geometric and harmonic mean equations.
As can be seen from , the POM-based binder system with no compatibiliser (C-0) has the highest contact angle value, 94.3°. However, when compatibiliser is introduced into the POM-based binder system, the contact angle values in all the samples are less than 90°, displaying a hydrophilic characteristic. Nonetheless, the POM-based binder system with EGMA demonstrated greater wettability than E40. The wettability trend is as follow: EGMA-3 (66.9°) > E40-3 (73.3°) > C–O (94.3°). The low contact angle of the binder is critical to ensure sufficient wetting of the metallic powder, subsequently promoting appropriate mixing and moulding of metal powders [Citation36].
Figure 5. Contact angle of the POM-based binder system with different compatibiliser compositions. Note that F.C-0, F. EGMA-3 and F.E40-3 represent feedstock C-O, EGMA-3 and E40-3.
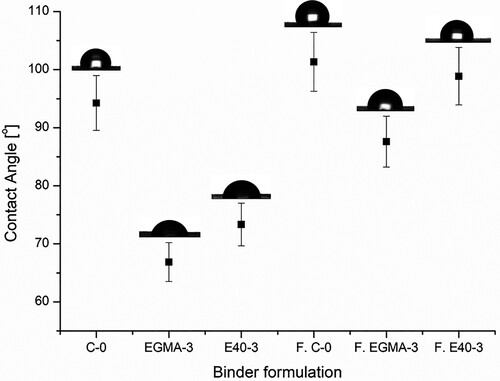
As anticipated, the resulting titanium MIM feedstock based on the EGMA-3 binder formulation shows the lowest contact angle value of 87.6° (presented in ), when compared to feedstock based on C-0 (101.3°) and E40-3 (98.9°) composition, respectively. This better dispersibility is the result of a stronger powder-binder adhesion. The powder particles are dispersed more homogeneously with the EGMA-3 binder formulation, which will induce less powder-binder separation during injection moulding at high shear stress.
3.4. Gas pycnometer
The homogeneity was then compared between the best and worst feedstocks using the gas pycnometer. demonstrates the pycnometer density of the best feedstock (EGMA-3) and compares it to the worst feedstock, C-0. It is observed that Feedstock EGMA-3 exhibits a much smaller standard deviation, 0.009, compared to Feedstock C-0 (0.012). This indicates that Feedstock EGMA-3 is more homogeneous and has higher powder-binder interaction. Interestingly, Feedstock C-0 exhibits a density of more than 100%. It is believed that some of the primary binders have been lost during the moulding process [Citation11].
Table 4. Pycnometer density of feedstock C-0 and EGMA-3 at three different conditions.
Furthermore, when comparing the density of the moulded part, we found that Feedstock EGMA-3 reveals a slightly higher value than C-0 (99.56% cf. 99.28%). It is noteworthy that a high-density green part is ideal for improving the final MIM product [Citation37]. It is important to ensure that the moulded part is filled before continuing the MIM process, as this could affect the final product.
3.5. Rheological behaviour
shows the viscosity versus shear rate for Feedstocks C-0 (worst feedstock) and EGMA-3 (best feedstock). The results demonstrate that the viscosity for both feedstocks decreases with an increasing shear rate, indicating pseudoplastic behaviour. Both feedstock C-0 and EGMA-3 have viscosities well below 1000 Pa.s, which is considered suitable for injection moulding [Citation38,Citation39]. Nonetheless, a lower viscosity can be seen associated with Feedstock EGMA-3. and show that Feedstock EGMA-3 also demonstrates more consistent and higher shear sensitivity value at most temperatures. In contrast, a significant variation of the shear rate dependency values can be observed for Feedstock C-0. This better shear sensitivity of Feedstock EGMA-3 can be attributed to the excellent compatibility between the binder system. Since the flowability of a feedstock depends on the bonding between metal powder and polymeric binder, the addition of compatibiliser, EGMA, into the immiscible POM/PP blends improves the interaction between powder and the polymeric binder. Consequently, this increased binder adhesion on the metal powder surface leads to better flowability ((b)). On the other hand, a poor powder-binder interaction can be seen in Feedstock C-0 ((a)).
Figure 6. The log-log plot of viscosity vs shear rate plot of feedstock (a) C-0 and (b) EGMA-3 in the temperature range of 180–200°C.
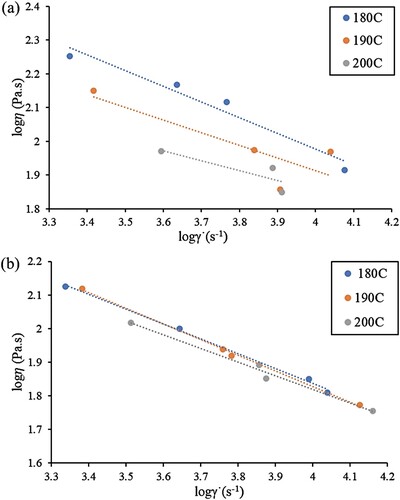
Figure 7. The comparison between the poor (a) and good (b) flow behaviour of feedstock. A stable feedstock will have a consistent flow and extrude nicely through the capillary die.

Table 5. The (n−1) value and moldability index for feedstock C-0 and EGMA-3 at three different temperatures.
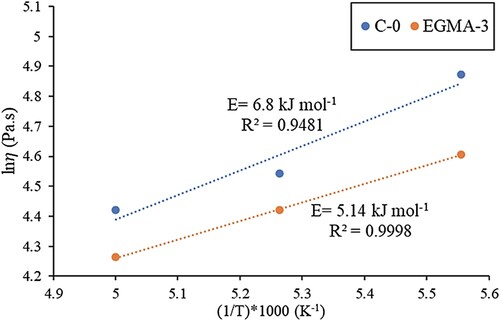
Another critical characteristic of MIM feedstock is temperature sensitivity. Low activation energy, E, indicates that viscosity is less affected by temperature [Citation40]. Thus, any small temperature fluctuation during injection moulding will not result in sudden viscosity change that can cause moulding defects. From , the lowest E value was obtained for Feedstock EGMA-3 at 5.41 kJ mol−1. Further, the moldability index, α, from reveals the highest value for feedstock EGMA-3 at all temperatures. It is worth concluding that a binder system must have miscible or partially miscible blends to ensure better compatibility between metal powders and binders. The addition of compatibiliser such as EGMA helps modify the interfaces between the immiscible POM/PP blends. As a result, it reduces the binder system's interfacial energy and improves the binder interlinking strength, which leads to the creation of MIM feedstock with enhanced performance. Nonetheless, it is worth mentioning that titanium has high affinity towards oxygen and carbon. Hence, we could expect that the addition of compatibiliser, which introduce more oxygen group within the binder system, might influence the impurity uptake during the subsequent debinding and sintering processes.
4. Conclusions
This study investigated the effects of compatibiliser on the properties of the powder feedstock. It has been demonstrated that the addition of compatibiliser, EGMA and E40, aids in lowering the interfacial tension between the immiscible POM/PP blends. The POM-based binder system with EGMA-3 demonstrates the lowest contact angle and best miscibility for the POM/PP blends compared to E40-3. Owing to the active site of EGMA molecule (i.e. epoxy and carbonyl functional group), chemical reaction and hydrogen bonding are possible within the ternary polymeric blend. As reflected, the ternary binder blends containing POM, EGMA-3 and PP have provided feedstock with a more homogeneous and higher-density moulded part than feedstock C-0. Besides, from the rheological analysis, feedstock-containing compatibiliser EGMA-3 demonstrates low viscosity, high shear sensitivity, low-temperature sensitivity and high mouldability index, which is suitable for MIM production.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Ismail MH, Muhamad N, Jumahat A, et al. Study on stability of a novel binder system based on palm stearin in metal injection moulding application. Sci Res J. 2007;4(2):1–12.
- Min LY, Quan LX, Hua LF, et al. Effects of surfactant on properties of MIM feedstock. T Nonferr Metal Soc China. 2007;17(1):1–8.
- Hayat MD, Wen G, Li T, et al. Compatibility improvement of Ti-MIM feedstock using liquid surfactant. J Mater Process Technol. 2015;224:33–39.
- German RM. Powder metallurgy and particulate materials processing: the processes, materials, products, properties and applications. Sci Sinter. 2005;38(1):95–95.
- Enneti RK, Onbattuvelli VP, Gulsoy O, et al. Powder-binder formulation and compound manufacture in metal injection molding (MIM). In Handbook of metal injection molding. 2019;4:57–88.
- Subuki I, Jai J, Ismail MH, et al. Interaction between binder and powder in injection moulding of 316L stainless steel. In: R Ibrahim, MH Saleh, K Norsal, MZA Malek, MA Sulaiman, MA Baharom, editors. Advanced materials research. Malaysia: Trans Tech Publications Ltd; 2014. Vol. 879, p. 7–11.
- Hausnerova B, Bleyan D, Kasparkova V, et al. Surface adhesion between ceramic injection molding feedstocks and processing tools. Ceram Int. 2016;42(1):460–465.
- Dehghan-Manshadi A, Bermingham MJ, Dargusch MS, et al. Metal injection moulding of titanium and titanium alloys: challenges and recent development. Powder Technol. 2017;319:289–301.
- German RM. Powder Injection Molding;.Metal Powder Industries Federation; 1990.
- Cao P, Hayat MD. Feedstock technology for reactive metal injection molding: process, design, and application. Cambridge (US): Elsevier; 2020.
- Patti A, Lecocq H, Serghei A, et al. The universal usefulness of stearic acid as surface modifier: applications to the polymer formulations and composite processing. J Ind Eng Chem. 2021;96:1–33.
- Chiou YH, Liu SJ, Lin ST. Superplastic behaviour of a zirconia powder-binder blend. Ceram Int. 1996;22(3):211–217.
- Wongpanit P, Khanthsri S, Puengboonsri S, et al. Effects of acrylic acid-grafted HDPE in HDPE-based binder on properties after injection and debinding in metal injection molding. Mater Chem Phys. 2014;147: 238–246.
- Ghasemi-Mobarakeh L, Cano S, Momeni V, et al. Effect of increased powder–binder adhesion by backbone grafting on the properties of feedstocks for ceramic injection molding. Polymers (Basel). 2022;14(17):3653.
- Hayat MD, Jadhav PP, Zhang H, et al. Improving titanium injection moulding feedstock based on PEG/PPC based binder system. Powder Technol. 2018;330: 304–309.
- Ye S, Mo W, Lv Y, et al. The technological design of geometrically complex Ti-6Al-4 V parts by metal injection molding. Appl Sci. 2019;997:1339–1349.
- Jiang X, Li D, Lu R, et al. Study of hyperbranched polymer on POM-based binder in metal injection molding. Mater Res Express. 2019;6(12);125377–125392.
- Bleyan D, Hausnerova B, Svoboda P. The development of powder injection moulding binders: a quantification of individual components’ interactions. Powder Technol. 2015;286: 84–89.
- Huang J, Cheng H, Wu J, et al. Blends of poly (propylene) and polyacetal compatibilized by ethylene vinyl alcohol copolymers. J Appl Polym Sci. 2003;89(6):1471–1477.
- Wacharawichanant S, Siripattanasak T. Mechanical and morphological properties of polypropylene/polyoxymethylene blends. Adv Chem Eng Sci. 2013;3(3): 202–205.
- Lin XD, Cheung WL. Effect of single screw extrusion through a round die on the morphology of POM/PP blends. J Mater Process Technol. 1997;63(1–3):476–480.
- Owens DK, Wendt RC. Estimation of the surface free energy of polymers. J Appl Polym Sci. 1969;13(8): 1741–1747.
- Rabel W. Wetting theory and its application to the study and use of the surface properties of polymers. Farbe und Lack. 1971;77:997–1006.
- Kaelble DH. Dispersion-Polar surface tension properties of organic solids. J Adhes. 1970;2(2):66–81.
- Sanford R, Sa TCKF, Banerjee S. Using a helium pycnometer as a quality tool in powder metal injection molding; 2009.
- Tadmor Z, Gogos CG. Principles of polymer processing. Hoboken (NJ): John Wiley & Sons; 1986.
- Weir FE. Moldability of plastics based on melt rheology. Part 1—theoretical development. Polym Eng Sci. 1963;3(1):32–36.
- Park SJ, Wu Y, Heaney DF, et al. Rheological and thermal debinding behaviors in titanium powder injection molding. Metall Mater Trans A Phys Metall Mater Sci. 2009;40:215–222.
- Lin ST, German RM. Interaction between binder and powder in injection moulding of alumina. J Mater Sci. 1994;29:5207–5212.
- Yang W, Wang XL, Li J, et al. Polyoxymethylene/ethylene butylacrylate copolymer/ethylene-methyl acrylate-glycidyl methacrylate ternary blends. Polym Eng Sci. 2018;58(7):1127–1134.
- Wu H, Hou A, Qu JP. Phase morphology and performance of supertough PLA/EMA-GMA/ZrP nanocomposites prepared through reactive melt-blending. ACS Omega. 2019;4(21):19046–19053.
- Rydz J, Wolna-Stypka K, Adamus G, et al. Forensic engineering of advanced polymeric materials. part 1 - degradation studies of polylactide blends with atactic poly[(R,S)-3-hydroxybutyrate] in paraffin. Chem Biochem Eng Q. 2015;29(2): 247–259.
- Gutmann JS, Müller-Buschbaum P, Schubert DW, et al. Influence of the blend compatibility on the morphology of thin polymer blend films. J Macromol Sci Phys. 1999;38(5–6):563–576.
- Hayat MD, Zhang H, Karumbaiah KM, et al. A novel PEG/PMMA based binder composition for void-free metal injection moulding of Ti components. Powder Technol. 2021;382:431–440:
- Shafrin EG, Zisman WA. Constitutive relations in the wetting of low energy surfaces and the theory of the retraction method of preparing monolayers. J Phys Chem. 1960;64(5): 519–524.
- Hausnerova B. Binder systems for powder injection molding: a review. Adv Mater Proc. 2021;2(12):761–768.
- Ani SM, Muchtar A, Muhamad N, et al. Effects of injection temperature and pressure on green part density for ceramic injection molding. Adv Mat Res. 2013;622:429–432.
- Li Y, Qu X, Huang B, et al. Rheological properties of metal injection molding binder and feedstock. Trans Nonferrous Metals Soc China. 1997;7(3):1–5.
- Bilovol VV, Kowalski L, Duszczyk J, et al. The effect of constitutive description of PIM feedstock viscosity in numerical analysis of the powder injection moulding process. J Mater Process Technol. 2006;178(1–3): 194–199.
- Zhang C, Pan Y, Sun J, et al. A net-shape forming process of Ti–6Al–4 V sphere joints. Powder Metall. 2021;64(5):404–411.