ABSTRACT
In the upcoming years, a reduction in the use of critical elements, such as Ni and Cu, with unstable prices and high demand from the electromobility sector will become increasingly important for the PM-industry. Cr-alloyed sintered steels offer attractive properties at a moderate cost, but so far mostly Cr-prealloyed grades have been used. This work analyses the microstructural homogenisation process when Cr is introduced as admixed elemental powder. It is shown how – due to its high carbon affinity – Cr particles act as ‘internal carbon-getters’. There is an intermediate ‘heterogenization’ of the microstructure, i.e. the iron matrix is decarburised due to the formation of (Cr, Fe)-carbides. Final homogenisation depends on the formation of a transient liquid phase through the eutectic reaction between carbides and the iron matrix. Thus, the microstructure is not only sensitive to aspects such as sintering temperature or Cr-particle size but also to the heating rate and small variations in nominal carbon.
1. Introduction
Cr is among those alloying elements that positively affect the hardenability and temper resistance of steels at a moderate alloying cost [Citation1]. Therefore, it is the main alloy element in wrought low alloy structural steels such as e.g. AISI 41xx grades (e.g. [Citation2]). In ferrous powder metallurgy, Cr has not been too popular in the past, mainly due to its high affinity for oxygen. On the other hand, regarding increasing demand for the classical alloy elements Ni and Cu e.g. from electro mobility – which means increased prices and possibly supply problems – renders alloying of sintered steels with Cr more attractive. So far, mostly prealloying has been used as the main route to introduce Cr [Citation3–7] because of the lower oxygen affinity of prealloyed powders as compared to plain Cr (this is due to the lower chemical activity of Cr in the prealloyed form). Recently, Cr alloyed PM steel gears have been introduced in large-scale production, but once more prepared from prealloyed powders. Also, the masteralloy route, e.g. as ferroalloys or as complex carbidic masteralloys has been regarded as an option [Citation8–10], in particular, since new atomising techniques can yield high output of fine, rounded particles [Citation11–14]. On the other hand, adding Cr as elemental alloying particles has not been studied too frequently, although the mechanical properties reported are quite attractive [Citation15]. This has surely in part been due to the necessity to sinter at high temperatures (i.e. above 1200°C) in order to attain acceptable Cr homogenisation. Here, however, it should be remembered that also prealloyed Cr–Mo steels benefit from high-temperature sintering, as shown by Kremel et al. [Citation16], i.e. there is not so much difference between prealloyed and mixed grades in that respect. Furthermore, the risk of oxygen pickup during sintering – which has been reported to be critical [Citation17] – should not be overestimated. Cr forms passivating layers, and even if some oxygen is picked up by the Cr particles, either from the atmosphere or from the base iron powder through the ‘internal getter effect’ [Citation18–20], high-temperature sintering will result in quite efficient reduction. In [Citation21–23], the homogenisation of Cr when sintering steels containing admixed Cr particles is described, and it is shown that Cr-containing carbides are formed on the interface between Fe-C matrix and Cr particles. Melting of such carbides by the formation of a transient liquid phase from the eutectic reaction with the austenitic matrix accelerates the homogenisation of Cr, leading to improved mechanical properties at sintering temperatures above 1250°C. The formation of a transient liquid phase during the melting of the carbides leads to a significant swelling effect at the melting temperature, preceded by a gradual expansion starting already at 1100°C. The pronounced expansion causes a decrease in the density, but its adverse effect on the mechanical properties is more than offset by the beneficial effect of homogenisation. On the other hand, mechanical properties (particularly fatigue properties) can be significantly lowered due to secondary porosity, esp. if coarser Cr powder grades are used (see e.g. [Citation22]).
In this work, the underlying phenomena taking place when sintering PM steels containing admixed Cr particles are studied by analysing microstructural evolution, dimensional changes and reduction/oxidation reactions. The phenomena observed with admixed Cr particles can be particularly interesting when assessing the study of Cr-containing master alloy compositions, as it can more clearly show the consequences of the interaction between Fe particles and Cr.
2. Experimental procedure
Powder mixes containing Fe (plain iron grade ASC 100.29, Höganäs AB, Sweden) with graphite (grade UF4, Kropfmühl) and Cr (electrolytic grade, fraction 45–63 µm, d50∼50 µm) were pressed at 600 MPa to the shape of full-size impact test bars (ISO 5754). The coarse Cr powder used here is not suited for structural applications, however, the homogenisation reactions during sintering can be observed more clearly.
In the first series, specimens with composition Fe-2%Cr-0.7%C (all concentrations in mass %) were prepared. From the compacts, pieces about 10 mm long were dry cut. Using these small specimens, interrupted sintering runs were done in a pushrod dilatometer Bähr 801 in rotary pump vacuum by heating the specimens at a constant rate of 10 K.min−1 and stopping the heating at different temperatures with immediate rapid cooling at a rate of 2–3 K/s (linearised between maximum temperature and 500°C). Thus, the microstructure could be ‘frozen’ at different stages of the sintering / homogenisation process (see e.g. [Citation24]).
In a second test series, full size samples Fe-4%Cr-0.5%C were prepared. The higher Cr content was chosen to better illustrate the effects of Cr homogenisation. Sintering was done for 1 h in Ar (99.999% purity) at 900, 1120 and 1250°C, respectively, in a laboratory SiC rod-heated furnace with superalloy retort. After sintering, the samples were cooled at approximately 0.75 K.s−1, in a water-jacketed exit zone under the same protective atmosphere. These pressed and sintered samples were then prepared for metallographic examination. Both the cross sections and the fracture surfaces obtained after breaking the samples with a 50 Joule – Charpy pendulum were examined by SEM (FEI Quanta 200). Qualitative analysis of the chemical composition of the sample in specific points or areas of the examined surfaces was obtained with an integrated Energy Dispersive X-ray Spectrometry (EDS) detector.
Thermoanalytical studies combined with chemical analysis were carried out using a pushrod dilatometer Netzsch 402 with alumina measuring system and a high-performance modular Simultaneous Thermal Analyzer (STA) Netzsch STA 449 C. Full-size impact test bars were used for these dilatometry studies, while loose powder mixes (not pressed) were used in STA experiments. Experiments with loose powders and green compacts provide complementary information. While in loose powder mixes the evacuation and subsequent detection of gases in the mass spectrometer is facilitated, the experiments in green compacts provide a better understanding of the consequences of eliminating the open porosity in the specimen as the sintering progresses. Both dilatometer and STA devices were coupled with a quadrupol mass spectrometer (Netzsch Aeolos) by a heated quartz capillary. Mass spectrometry (MS) was used to analyse the gaseous species that evolved during the thermal treatment. The masses registered in these experiments were m12 (C), m14 (N), m15 (CH3*), m16 (CH4, O), m17 (OH), m18 (H2O), m28 (CO, N2), m32 (O2) and m44 (CO2).
3. Results and discussion
3.1 Microstructural studies
Interrupted sintering runs
In , the microstructures after heating up specimens with composition Fe-2%Cr-0.7%C to different temperatures are shown (after heating the samples were rapidly quenched). As can be clearly seen in (a–d), there is a rim formed around the Cr particles that can be distinguished from the particle core by a slightly different colour (see details in (b,d)). As already shown in [Citation21,Citation22], this rim contains Fe, Cr and C and has been analysed to be a carbide, supposed to be probably (Cr,Fe)7C3 (see in ref [Citation21]). As the temperature increases, the core becomes porous and contains less and less plain Cr. At 1250°C ((c,d), a hollow shell is formed that consists entirely of the carbide (see detail in (d)). Such microstructure suggests that there is rapid diffusion of Cr into the Fe matrix with much lower diffusion of Fe into Cr (and into the carbide) and that the cavity formed within the cores is actually diffusion porosity. This agrees with diffusion data given in [Citation25], in particular, if at least partial transformation of the austenite matrix into ferrite by Cr uptake is considered (although for a precise assessment of the diffusion processes also diffusion data for Cr and Fe within the carbide would be required).
Figure 1. Microstructures of Fe-2%Cr-0.7%C (Cr powder 45–63 µm) heated up to different temperatures and then rapidly cooled. Dilatometer Bähr, 10 K.min-1, vacuum, Nital etched.
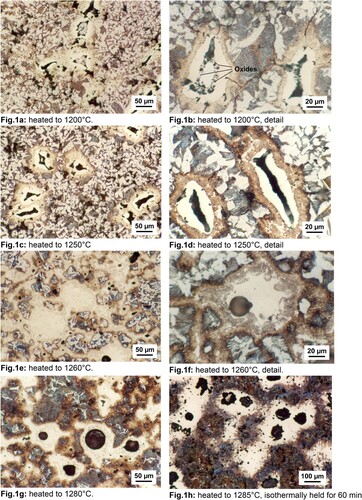
At 1260°C, a transient liquid phase is formed, and the Cr-rich carbides melt through eutectic reaction with the matrix, forming melt pools ((e)). By transfer of the melt into the matrix pore network, secondary pores are generated ((f,g)). The size of these secondary pores is about double the size of the Cr particles they originate from. This is due to the fact that the liquid phase is formed by the reaction with the iron matrix, therefore containing mostly Fe and only a minor fraction is Cr. This behaviour is typical for liquid phases that are formed by eutectic reactions [Citation26]. In contrast, in systems containing alloying elements with congruent melting points (as e.g. in the case of Fe-Cu compacts) secondary porosity presents the same size as the particles from which the liquid phase originates.
If not only the Cr particles are studied but the entire microstructure, it becomes evident that there is a redistribution of carbon in the microstructure. The admixed graphite is dissolved in the iron matrix in a fairly early stage of sintering [Citation27]. Considering that the carbon content in the specimens is 0.7 wt. %C, an almost fully pearlitic microstructure would be expected. However, the microstructures presented in suggest that, before the formation of the liquid phase, the matrix is depleted of carbon, as indicated by the large proportion of ferrite visible (see (a,c)). Apparently, the Cr particles act as ‘getter’ for the carbon, forming the carbide shells. Only after dissolution of Cr in the matrix through transient liquid phase, carbon is released into the matrix, and a fully pearlitic microstructure is obtained outside of the Cr-rich martensitic areas ((h)), as is to be expected from the combined carbon content of the material. Around the carburised Cr particles, a fully pearlitic rim is observed, which indicates that these areas are already alloyed with Cr, which shifts the C content of the pearlite eutectic to much lower levels. This effect is further supported by the lowering of the C activity by the presence of Cr in austenite [Citation28], which promotes C diffusion towards the Cr particles.
Isothermal sintering at different temperatures
Further experiments were done by isothermal sintering at different temperatures, followed by a moderate cooling rate (∼0.75 K.s−1). As stated above, in this second round of experiments, the Cr content was increased to 4 wt.%, in order to reveal more clearly the effects of the alloy elements. In addition, the carbon content was reduced to enhance the optical effects of carbon redistribution. One fairly low sintering temperature was chosen (900°C). shows metallographic cross sections of Fe-4.0%Cr-0.5%C samples sintered at 900, 1120 and 1250°C, respectively, on which also EDS line analyses were performed.
Figure 2. Microstructures of Fe-4%Cr-0.5%C (Cr powder d50∼45 µm) sintered in Ar at 900°C (a), 1120°C (b) and 1250°C (c). (Left) LOM Images of the cross sections. (Right) SEM image with EDS line scanning in the Cr-Fe interface.
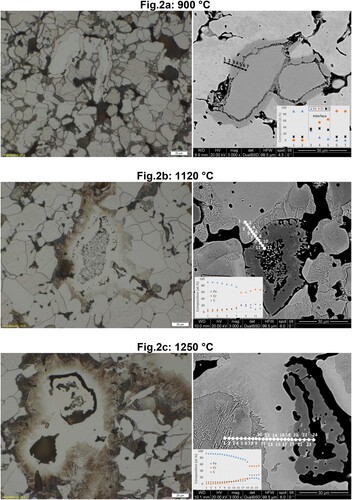
At 900°C ((a)) the metallic Cr particles are still clearly discernible (points 6 and 7 in the line scan). Towards the surrounding Fe particles, the diffusion of Cr has formed a layer with a distinctive contrast, which can be interpreted as an early stage of the formation of the carbide layer described in the previous section. EDS scanning suggests that the Cr and Fe contents progressively change within this layer (see points 3, 4 and 5 in the line scan). Besides, even though EDS is not the appropriate technique to quantify the C content, it can be at least qualitatively interpreted that the C content is markedly higher in this layer, which agrees with the WDS analyses presented in ref [Citation21].
At 1120°C, the morphology of the Cr particles has changed significantly, being now quite similar to that shown in (b). The interior of the particles exhibits a porous sponge-like structure surrounded by the Cr-rich rim which contains some Fe and is enriched in C (see line scan at 1120°C – (b)). This suggests, once more, that the rim is carbidic. The image obtained from the optical microscope exhibits pearlitic areas preferentially concentrated in the surrounding of the Cr particles (in which the Cr contained shifts the pearlite eutectoid to lower C levels, see (d)), while the remaining matrix is almost completely ferritic. Again, this indicates an ‘internal decarburization’ of the ferrous matrix due to the formation of Cr-rich carbides. ‘Internal getter effects’ are thus shown to occur not only by redistribution of oxygen in a PM-component, but also by redistribution of carbon.
At 1250°C, the carbidic structures are now completely hollow (as also observed in (d)) and show a relatively stable Cr/Fe ratio (see points 20–24 in (c)) which is lower than the Cr/Fe ratio observed at 1120°C (see points 8–12 in (c)). This means that, at 1250°C, the Fe content is higher in the carbide, which further confirms that Cr is now completely distributed between the carbide and the Fe matrix. The microstructure of the Fe matrix presents different areas. The region close to the carbide is richer in Cr (see points 19 to approximately 11 in (c)). As the distance to the carbide increases, the Cr content in the matrix decreases and becomes almost negligible (see points 10 to 1 in (c)). In the vicinity of the carbides (where the Cr content in the Fe matrix is at its maximum), no pearlite is observed, which might indicate that it consist of ferrite with high Cr content. Surrounding this region the pearlitic areas are found. From the micrographs shown in (c), it is clear that after sintering at 1250°C the microstructure consists of (Fe, Cr)-carbides embedded in an iron matrix with a heterogeneous distribution of Cr (i.e. enriched in Cr in the vicinity of the carbides).
The evolution in the morphology of the Cr particles at increasing temperatures was also observed on the fracture surfaces (see ). At 900°C, the Cr particles (dark contrast) are surrounded by oxides that seem to be enclosed by a (Cr-Fe)-carbide layer. At 1120°C, the morphology of the Cr particles has changed considerably, exhibiting now a porous sponge-like structure. Such structures cannot be found anymore when sintering at 1250°C. At this temperature, only a dense hollow shell is observed, in good agreement with the findings from the interrupted sintering runs (see (c,d)). The morphology of the surface of the carbide shells at 1250°C ( – 1250°C) also suggests that a liquid phase might have been formed on the interface between the Fe-matrix and the carbide.
Figure 3. SEM images of the fracture surface of Fe-4.0Cr-0.5C compacts sintered in Ar at 900, 1120 and 1250°C for 1 hr.
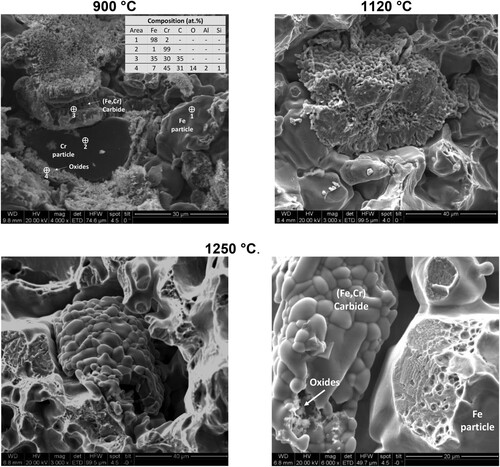
Another feature observed in these experiments is the formation of oxides in the vicinity of the Cr particles. At lower temperatures (900°C ), they form virtually a separating layer between the Cr core and the carbide. At higher temperatures (see 1200°C – (b)), these oxides are spheroidised and are located in the porous zone between the solid carbide shell and the remaining Cr cores. It can be assumed that these oxides are formed by the ‘internal getter’ effect, i.e. the reduction of the Fe oxides covering the base powder particles (which in this case takes place by carbothermal reactions) results in oxidation of the Cr particles, because the atmosphere that is reducing for iron is heavily oxidising for Cr at these temperatures (see [Citation18]). Apparently, these oxides are however sufficiently isolated not to inhibit inter-diffusion between Cr and Fe. At higher temperatures (above 1250°C), the oxides are not observed in the metallographic sections, but only the fracture surface forming loose particles in the central cavity of the carbide (see – 1250°C).
3.2 Thermodynamic calculations
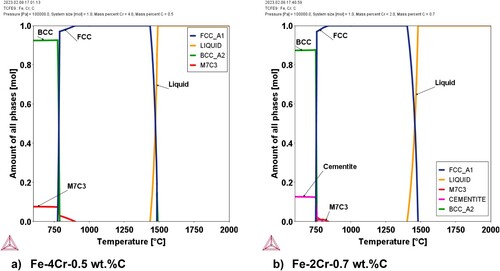
Thermodynamic calculations were carried out to provide a further understanding of phase formation in Fe-Cr-C sintered materials prepared by admixing elemental Cr. The evolution of phases at increasing temperatures, calculated for the two systems analysed in this study (Fe-4Cr-0.5C and Fe-2Cr-0.7C), is presented in . These calculations indicate that, if all the elements were homogeneously distributed in the material, the (Fe, Cr)-carbide phases would only be stable at low temperatures (below approximately 850°C, depending on the system), and at higher temperatures both Cr and C should be dissolved in the iron matrix. The carbide phases observed in this study are thus metastable (as a consequence of the inherent heterogeneity of admixed powders) and, at moderate temperatures, should theoretically be dissolved in the iron matrix if sufficient time would be given for diffusion. In any case, these graphs show that the admixed Cr is fully soluble in the austenitic matrix, the admixing route thus being able to yield sintered steels with homogeneous Cr distribution if the sintering conditions are selected accordingly.
Figure 4. Evolution of phases at different temperatures for the two systems analysed experimentally in this study: Fe-4Cr-0.5C (a) and Fe-2Cr-0.7C (b).
In order to provide a better understanding of the temperatures at which the formation of a liquid phase is possible in this system, two different representations of the Fe-Cr-C phase diagram are depicted in .
Figure 5. ThermoCalc calculations using the database TCFE9: (a) Isopleth Fe-Cr calculated for 3 wt.% C, (b) Isothermal section of the Fe-Cr-C system at 1260°C.
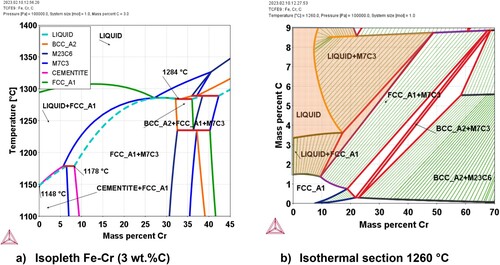
(a) shows an isopleth on the system for a constant carbon content of 3 wt.% C. A relatively high carbon content was selected in order to have a better overview of the reactions forming liquid phases (which at low temperatures occur mainly at carbon contents above 1.5 wt. %C). The light blue dashed line in the diagram in (a) represents the limit of the stability region of the liquid phase (i.e. liquid is stable above this line). The minimum temperatures for the formation of a liquid phase can be predicted from this diagram by the evaluation of two relevant temperatures. 1148°C is the minimum temperature at which the liquid is formed and corresponds with the predicted temperature for the reaction FCC (austenite) + Cementite = Liquid, i.e. the eutectic on the Fe-C system. The next temperature indicated in the diagram is 1178°C for which the software predicts the overall reaction Cementite + FCC + M7C3 = Liquid + M7C3. It can be interpreted that, for temperatures above 1178°C the eutectic reaction between FCC (austenite) and M7C3 is possible, giving rise to the formation of a liquid phase, as long as the composition of the austenite in contact with the M7C3 phase would allow it. From the graph, it is also visible that, in contrast to e.g. the systems Fe-Mo-C and Fe-W-C, there is no ternary eutectic but the temperature of the eutectic austenite-carbide increases with higher Cr content, which agrees with the experimentally obtained phase diagrams (e.g. [Citation29]).
(b) shows an isothermal section of the Fe-Cr-C diagram (for the Fe-rich region) at 1260°C. The shaded area indicates the region in which the liquid phase is stable, which is observed at total carbon contents above approximately 1 wt.% in the Fe-rich regions. When considering the Liquid + M7C3 region it can be observed that, at a certain carbon content, the lower the amount of Cr in the liquid phase, the higher the amount of liquid that could be formed at this temperature.
None of these diagrams can represent the real situation in a PM component during the process of sintering. In powder mixes, the system evolves under non-equilibrium conditions, being in a ‘transient state’ from heterogeneous to homogeneous. Still, some conclusions can be drawn from the diagrams in . If the carbon activity is high enough in the austenite areas located close to the carbide, the formation of a liquid phase by eutectic reaction between the (Cr,Fe)-carbides (as indicated by (b), most likely M7C3) and the metallic matrix, can in principle occur at temperatures above 1179°C. In real experimental conditions, the exact temperature at which the eutectic liquid is formed will depend on the diffusion of Cr and C in the metallic matrix. This, in turn, means that aspects such as e.g. the particle size and the heating rate (both affecting the diffusion) will have an effect on the temperature at which the liquid phase is formed in Fe-Cr-C sintered steels produced by the admixing route.
3.3 Thermal analysis
Melting temperatures
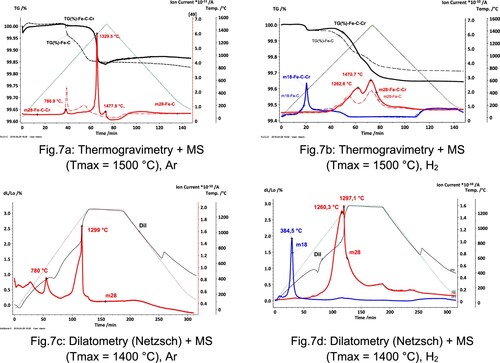
Differential thermoanalytical measurements were carried out to determine the critical temperatures for the formation of liquid phases in the system Fe-4%Cr-C. (a) shows DTA graphs for mixes with and without Cr addition. In comparison to the powder mixes without Cr, the mixes containing 4 wt.% Cr present an endothermic peak with an onset at around 1280°C–1290°C and a peak at 1326°C. This peak is most likely related to the eutectic reaction between the carbides and the austenitic matrix.
Figure 6. DTA analysis of different Fe-Cr-C mixes. (a) Fe-0.5C and Fe-4Cr-0.5C (Cr powder d50∼45 μm), (b) Mixes with different C contents (0.5 and 0.7 wt.%) and different particle sizes (fine-sieved below 25μm- and coarse-sieved above 63 μm-).
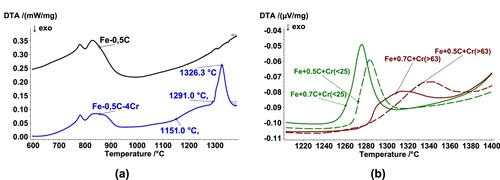
(b) shows a detail of the endothermic peak for mixes containing identical Cr contents (4 wt.%) but with variations in the particle size of the Cr particles (fine – below 25μm- and coarse – above 63 μm-) as well as variations in the total carbon content in the mix (0.5 wt.% or 0.7 wt.%). The results indicate a significant correlation between the temperature at which the transient liquid phase is formed and the Cr particle size. Mixes containing fine Cr powders present an endothermic peak at markedly lower temperatures, and the peaks observed are narrower and of higher intensity. In this ternary system, the alloying particles (Cr particles) are not melting spontaneously, as would Cu, but instead, the liquid phase is formed by a reaction between the iron matrix and Cr-rich carbides, i.e. inter-diffusion is needed. Thus, finer particle sizes can give rise to the formation of higher amounts of the liquid phase in a narrower temperature interval (see also [Citation22]), because of the larger contact area between the two phases. Besides, with smaller particle sizes also shorter diffusion distances are required, which also favours the formation of the transient liquid phase at lower temperatures.
It is also clear from (b) that, for both particle sizes, higher carbon contents decrease both the onset and the peak temperatures, suggesting that the liquid phase formation is enhanced at higher carbon contents, which agrees with studies done on Mo and W alloyed steels (e.g. [Citation15,Citation23]) and is also supported by the thermodynamic calculations.
Oxidation–reduction reactions
The peculiar microstructural evolution in Fe-Cr-C steels has consequences not only for the distribution of the alloying elements but also regarding the dimensional behaviour and the reduction/oxidation reactions. The results from STA + MS runs in Ar presented in show how the reduction processes observed in Fe-C powder mixes (broken lines) are considerably modified by the addition of Cr (solid lines). The mass loss observed at ∼800°C as well as the m28 (CO) degassing peak, which are typical for Fe-C, are almost negligible in Fe-Cr-C. This means that the reduction of the Fe oxides present on the surface of the Fe base particles is not detected, neither as mass loss nor as m28 (CO) degassing peak, because the reaction product (CO) is immediately ‘gettered’, oxidising the Cr particles, which act as oxygen traps. Therefore the oxygen is just transferred from the iron base powder to the surface of the Cr particles (‘internal oxygen getter effect’ [Citation18]). As the temperature increases, the oxides present on the surface of the Cr particles become progressively enclosed by the carbide layer formed on the Fe-Cr interface. Only at high temperatures (∼1300°C), a sharp mass loss and a simultaneous pronounced degassing peak are observed, which suggests that the reduction of the oxides has been enhanced by the formation of a transient liquid phase. Also, the dilatometry curve in Ar shows rather intense swelling upon transient liquid phase formation at ∼1300°C, with a simultaneous sharp peak in the m28 (CO) curve.
Sintering in H2 reduces considerably the effect of ‘oxide enclosure’, since part of the oxides present in the base powder is reduced already at ∼400°C, forming m18 (H2O) – see (b) and (d). The degassing peaks in STA + MS experiments (with loose powder mixes) are therefore more similar to those of Fe-0.5C compacts. In Dil + MS experiments done in H2 (on green IE bars), still some oxide enclosure effect is hinted by the sharp m28 (CO) peak at ∼1300°C which also here coincides with the expansion of the compact. In any case, however, oxygen removal occurs mainly at T > 1000°C, through carbothermal reduction, even in H2 as atmosphere.
Summary and conclusions
Microstructural evolution in steels containing admixed elemental Cr particles is characterised by the formation of Fe-Cr carbides, which agrees with observations in the systems Fe-Mo-C, Fe-W-C and Fe-V-C (e.g. [Citation21,Citation30,Citation31]) in which intermediate carbides are also formed. In contrast to other typical alloying additions in sintered steels – such as e.g. Cu or Ni – elements like Mo, Cr, V and W are stronger carbide formers. Due to the inherent heterogeneity of a powder mix, the formation of carbides of these elements is more likely to occur than the dissolution of the elements in the iron matrix, even if the carbides are – from a thermodynamic point of view – only stable at low temperatures (if considering a homogeneous distribution of the elements in the material).
The first consequence of the carbide formation is an intermediate decarburisation of the matrix. The Cr alloying particles act as ‘internal carbon getters’. Part of the carbon added to the green component, through admixed graphite, and dissolved in Fe in an earlier stage of heating diffuses towards the Cr particles at a higher temperature range and is bonded in the form of Cr-rich carbides up to rather high temperatures. As a consequence, the microstructures observed in steels with 0.5 wt.%C and 0.7 wt.%C, sintered at different temperatures, are mostly ferritic-pearlitic, with only some small fully pearlitic areas located around carbide shells. The formation of pearlite in these areas is most likely due to the decrease of the eutectoid C content caused by the presence of locally higher amounts of Cr. Only after the eutectic melting of the carbides – by reaction with the iron matrix – carbon is re-distributed in the metallic matrix, in parallel with the distribution of Cr.
According to thermodynamic calculations, Cr-rich carbides present a eutectic reaction with the iron matrix at temperatures above 1170°C (depending on the composition of the austenite phase in contact with the carbide). In contrast to Fe-Cu-C systems – in which the liquid phase is formed by congruent melting of the Cu particles – in the present case the formation of the liquid necessarily requires a reaction between the carbides and the Fe-matrix. As a consequence, the secondary pores observed after liquid phase formation are significantly larger than the size of the admixed Cr-particles.
The temperature at which the liquid phase is formed by the eutectic reaction is strongly dependent on the composition of the iron matrix in contact with the carbides, which in turn depends on the diffusion processes. This leads to a complex situation in which Cr homogenisation, and thus the effective use of Cr as alloying element, becomes very much dependent on parameters such as the sintering temperature and time, the heating/cooling rates, the Cr particle size and the carbon content.
High sintering temperatures ensure the formation of transient liquid phases, which results in dissolution of the carbides and homogenisation of Cr. Such high temperatures are not attractive for industrial production but on the other hand yield attractive mechanical properties, as shown also for prealloyed Cr steels. Another suitable option would be the use very fine Cr powders in which case solid state diffusion should improve homogenisation and facilitate the formation of the liquid at lower temperatures.
The use of Cr-containing masteralloys can also be an attractive alternative for introducing Cr in sintered steels. In this case, the composition of the masteralloys should be properly tailored. High Cr and C contents in the masteralloy powder tend to show homogenisation problems due to carbide formation. Studies with Fe-40Mn-15Cr-10Si-1C [Citation18] have shown that carbide formation on the interface was considerably inhibited, providing a more efficient use of Cr as alloying element at sintering temperatures below 1250°C. Furthermore, designing the composition of masteralloys towards low melting intervals also enhanced homogenisation during sintering [Citation32,Citation33]. In any case, both with Cr admixed particles and with Cr-containing masteralloys, it is to be expected that Cr homogenisation in the matrix will be somewhat sensitive to the overall carbon content in the material since in both cases the Cr interacting with the steel matrix is in fact carbidic.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Šalak A. Ferrous powder metallurgy. Cambridge: Cambridge Int. Sci. Publ.; 1995.
- ASM International. Handbook Committee, and American Society for Metals. Metals Handbook. 1990. Vol. 1, Properties and Selection : Irons, Steels, and High-performance Alloys. 10th ed. Materials Park, OH: ASM International
- Karasuno I, Koshiro K, Umino M, et al. Horizons of powder metallurgy (proc, PM'86 düsseldorf). Vol. 1, Freiburg: Verlag Schmid; 1986. p. 53–56
- Ichidate M, Karasuno I, Kubo T, et al. Horizons of powder metallurgy (proc, PM'86 düsseldorf). Vol. 1, Freiburg: Verlag Schmid; 1986. p. 57–60
- Unami S, Ogura K, Uenosono S. Proc. PM*98 world congress, granada. Vol. 3, Shrewsbury (UK): EPMA; 1998. p. 173–178
- Lindqvist B. Proc. EuroPM2001 nice. Vol. 1, Shrewsbury: EPMA; 2001. p. 13–21
- Berg S, Maroli B. Advances in Powder Metall. & Partic. Mater. -2002, V. Arnhold, C. -L. Chu, W. F. Jandeska, H. I. Sanderow eds., MPIF, Princeton NJ (2002), Part 8, 1–14.
- Hoffmann G, Dalal K. Powder Metall Int. 1979;11:177–180.
- Šalak A. Proc. 8th Int. PM Conf. in CSFR, Piestany 1992, Institute for Materials Research of the Slovak Academy of Sciences (IMR SAS), Kosice, Vol.1, p. 139-148.
- Banerjee S, Schlieper G, Thümmler F, et al. Mod Dev Powder Metall. 1981;13:143–156.
- Calderon R DO, Gierl-Mayer C, Danninger H. New chances for the masteralloy approach. Powder Metall Progr. 2018;18(No. 2):121–127.
- De Oro Calderon R, Jaliliziyaeian M, Dunkley J, et al. New masteralloys for sintered high strength steels – the attractive route between mixing and prealloying. Powder Metall Funct Coat. 2018;4:15–27. doi:10.17073/1997-308X-2018-4-15-27.
- Geroldinger S, de Oro Calderon R, Gierl-Mayer C, et al. Applying the masteralloy concept for manufacturing of sinter hardening PM steel grades. Adv Eng Forum. 2021;42:17–23.
- Geroldinger S, de Oro Calderon R, Gierl-Mayer C, et al. Sinter hardening PM steels prepared through hybrid alloying / Herstellung von sinterhärtenden pulvermetallurgischen Stählen durch Hybridlegierungstechnik. HTM J Heat Treat Mater. 2021;76(2):105–119.
- Danninger H, Zengin OZ. Mod Dev Powder Metall. 1988;21:171–182.
- Kremel S, Danninger H, Yu Y. Powder Metall. Progress. 2002;2(No.4):211–221.
- Tengzelius J, Grek SE, Blände CA. Modern Dev Powder Metall. 1981;13:159–182.
- De Oro Calderon R, Gierl-Mayer C, Danninger H. J Therm Anal Calorim. 2016.
- Gierl-Mayer C, De Oro Calderon R, Danninger H. JOM. 2016;68(3):920–927.
- Danninger H, De Oro Calderon R, Gierl-Mayer C. Powder Metall. 2018;61(3):241–250.
- Danninger H, Kara T, Ruhnow M, et al. Microchim Acta. 1990;101(1-6):219–229.
- Danninger H. Proc. 8th Int. PM Conf. in CSFR, Piestany, 1992, Institute for Materials Research of the Slovak Academy of Sciences (IMR SAS), Kosice, Vol.1, p. 81-90.
- Danninger H, Pöttschacher R, Bradac S, et al. Powder Metall. 2005;48(1):23–32.
- Danninger H. Powder Metall Int. 1992;24(No.3):163–168.
- Handbook of chemistry and physics. 57th Ed. Boca Raton (FL): CRC Press; 1987.
- Danninger H. Powder Metall Int 1988;20 (No.1):21–25.
- Danninger H, Frauendienst G, Streb K-D, et al. Dissolution of different graphite grades during sintering of PM steels. Mater Chem Phys. 2001;67(3-6):72–77.
- Lundberg R, Waldenström M, Uhrenius B. CALPHAD. 1977;1(2):159–199.
- Raynor GV, Rivlin VG. Phase equilibria in iron ternary alloys. London: The Institute of Metals; 1988. p. 143–156.
- Danninger H, Kara T, Ruhnow M, et al. Proc. IX. Int. PM conference. Dresden: Academy of Sciences of GDR; 1989. Vol. 2, p. 163–176.
- Danninger H, Gierl C, Vassileva V, et al. Preprints PM2008 powder metallurgy world congress. Washington D.C., Princeton N.J: MPIF; 2008. p. 336-344 (on CD).
- De Oro Calderon R, Bernardo Quejido E, Campos Gomez M, et al. Powder Metall. 2016;59(No.1):31–40.
- De Oro Calderon R, Gierl-Mayer C, Danninger H. Pulvermetallurgie in Wissenschaft und Praxis No. 33 “Pulvermetallurgie - Schlüssel zur Mobilität”, H. Kolaska, H. Danninger, B. Kieback editor, Hagen: Fachverband Pulvermetallurgie, 2017. p. 159–184.