It is a great tribute to Dr Matt Friedman that Psychiatry is republishing two of the foundational papers of the National PTSD Brain Bank. The initiative for the Brain Bank is a long-term investment that only starts to pay off after many years. It requires a vision of what may yield scientific discoveries in the field of PTSD. Throughout his career, Dr Friedman recognized that stress, especially the extreme stress of traumatic events, can alter both neurobiology and behavior. This motivated him to explore approaches to understanding brain–environment interactions and make a case for postmortem brain tissue research to advance our understanding of the effects of the environment on the brain. He was keen to advocate that such a resource would facilitate discoveries related to the psychiatric illnesses of acute stress disorder and PTSD, enabling scientists to relate structural and functional imaging findings with tissue abnormalities and essential to validate the results from other research domains, e.g., genetics.
Dr Friedman worked as a clinician and researcher for over 40 years, during which he published numerous papers on biological psychiatry with a particular focus on PTSD. He is a true pioneer in the field of traumatic studies, was involved in several large scale clinical trials on PTSD (Friedman & Harris, Citation2004; Schnurr et al., Citation2007), and has spent his professional life dedicated to facilitating the recovery of trauma survivors, as he did in his role as past president of the International Society for Traumatic Stress Studies (for which he received the ISTSS Lifetime Achievement Award in 1999), and past chair of the American Psychiatric Association’s DSM-5 and DSM-IV-TR PTSD Work Groups.
BRAIN BANKS
In his landmark paper toward a National PTSD Brain Bank published in 2004 with Dr William Harris, they describe the pressing need (“immediately”) to establish a Brain Bank dedicated to supporting research of PTSD (Friedman & Harris, Citation2004). For over a century, the early brain banks have been collecting postmortem tissue from patients who suffered from neurological and psychiatric disorders. In more recent years, there have been important links between clinicians, scientists, and neuropathologists mainly involved in aging and dementia research. Dr Friedman was favoring a national resource with rigorous scientific oversight. Its executive committee, consisting of eminent scientists, would be expected to adhere to the highest standards of scientific rigor, supported by public funding, and housed in the Department of Defense, Veterans Affairs, or Health and Human Services. He favored ensuring the strictest safeguards to protect human subjects and privacy concerns (Friedman et al., Citation2017).
He well understood that due to the large variability of the material, there are quite a few drawbacks in using postmortem brains. Therefore, collecting human brain tissue for research should include matching for several factors, both antemortem, and postmortem. Thus far, Brain Bank organizations for various neurological diseases have been an important clinicopathological link predominantly in aging and dementia research. Psychiatry, and most importantly, the field of PTSD, had been lagging behind. With the availability of postmortem human brain tissue, it would become possible to investigate those diseases for which no animal model is available or sufficient to explain the pathophysiology of the disorder of study. Dr Friedman recognized this to be the case for PTSD.
STRUCTURAL MRI STUDIES
There has been a debate for PTSD as a hippocampal-based disorder since volume loss was made visible with structural MRI techniques. Studies using structural magnetic resonance imaging (MRI) volumetrics showed smaller hippocampal volume in patients with post-traumatic stress disorder (PTSD; Bremner et al., Citation1995; Vermetten et al., Citation2003). These studies were cross-sectional and did not address whether the smaller volume is secondary to stress-induced damage or whether preexisting factors could account for the findings. A large-scale neuroimaging consortium study on PTSD conducted by the Psychiatric Genomics Consortium (PGC)-Enhancing Neuroimaging Genetics through Meta-Analysis (ENIGMA) PTSD Working Group has assessed the volumes of subcortical structures (nucleus accumbens, amygdala, caudate, hippocampus, pallidum, putamen, thalamus, and lateral ventricle) and used a standardized image-analysis and quality-control pipeline established by this consortium. In a recent meta-analysis of all samples, they also found significantly smaller hippocampi in subjects with current PTSD compared with trauma-exposed control subjects as well as smaller amygdalae (Logue et al., Citation2018). Also, they found that volumes of left and right lateral orbitofrontal gyri (LOFG), left superior temporal gyrus, right insular, lingual and superior parietal gyri were significantly smaller and were significantly negatively correlated with PTSS severity (Wang et al., Citation2021). In a co-twin case-control and structural MRI scans, Bremner et al. assessed the relative contribution of genetic and environmental factors to hippocampal volume in PTSD and also reported consistent findings with smaller hippocampal volume in PTSD and, more importantly, evidenced that the debated effects were primarily due to environmental effects such as the stress of combat (Bremner et al., Citation2021). This all sets the stage for postmortem brains of patients with PTSD. Also, this calls for corroboration by histopathological studies that can be backward translated to preclinical findings. Lastly, on this issue, as PTSD is associated with markers of accelerated aging, it is interesting to see how younger versus older males may indicate a critical window when PTSD impacts brain aging, followed by age-related brain changes that are consonant with individuals without PTSD. A brain bank may yield knowledge of how PTSD may affect brain aging across the lifespan.
GENETIC STUDIES
Since then, i.e., over the past 17 years, the research community has made significant advances in unraveling the (neuro)biology of stress susceptibility and PTSD. One of the significant developments in this effort includes the rise of genetic studies. While initially, small studies were researching a select number of candidate genes involved in the stress response, including BDNF and the serotonin transporter 5-HTTLPR, these past years have seen a rise in large-scale, agnostic genome-wide association studies (GWASs) and meta-analyses. Such well-powered efforts were made possible due to international consortia such as the Psychiatric Genomics Consortium (PGC), which initially included research groups on other psychiatric disorders such as schizophrenia (Schizophrenia Working Group of the Psychiatric Genomics, ca. Citation2014). In 2013, the PGC-PTSD was initiated, which encouraged international investigators to combine their datasets of military or civilian cohorts of PTSD to identify meaningful robust dysregulations (Logue et al., Citation2015). Since then, some of the most recent and most well-powered genome-wide findings in PTSD point to (i) substantial heritability, which appears to be higher in females as compared to males (Nievergelt et al., Citation2019), (ii) the polygenic architecture of PTSD and shared common variants with other psychiatric disorders (Lori et al., Citation2021), (iii) several ancestry-specific significant genetic risk loci (Nievergelt et al., Citation2019). However, most findings lack replication, partly due to the large sample sizes required for GWAS and the relatively limited (although increasing) sample sizes currently available for PTSD research. Continued efforts of the PGC PTSD, and their GWAS workgroup, in particular, will undoubtedly aid in expanding sample sizes in the future.
THE ENVIRONMENTAL COMPONENT IN PTSD DIAGNOSIS
One of the complexities in studying PTSD is the strong environmental, i.e., traumatic, component required for its diagnosis. Hence, next to studying the genetic architecture of PTSD, understanding whether and how a traumatic event can impact biological function through epigenetic modifications has become increasingly relevant. Most studies aiming to elucidate the epigenetic underpinnings of PTSD have focused on DNA methylation in blood-derived DNA and through standardized microarrays, of which the latest version can profile the DNA methylation status of ~850 K CpG sites across the genome. Differential methylation patterns have been reported in several genes involved in the immune and stress responses (Morrison et al., Citation2019). Noteworthy findings from some of the most extensive epigenome-wide association studies (EWAS) by PGC-PTSD and others to date point to blood-based DNA methylation alterations in the aryl-hydrocarbon receptor repressor (AHRR; (Smith et al., Citation2020)), which was replicated in an independent cohort along with another replicated hit in the gene GoS2 (Logue et al., Citation2020; Rutten et al., Citation2018), and longitudinal differences in DNA methylation upon trauma exposure (Rutten et al., Citation2018; Snijders et al., Citation2020).
A TRANSLATIONAL RESEARCH CYCLE
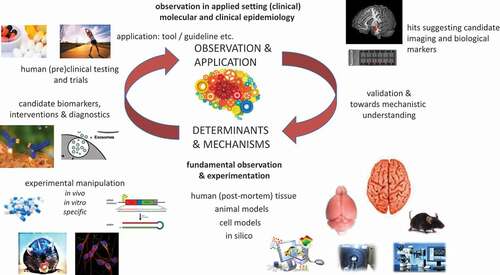
While observations in human cohort studies using deep phenotyping and molecular epidemiological approaches are an excellent starting point for the translational research cycle (see ), these observations need to be followed up, first by methodological validation and independent replications and second by biological validation and functional analyses. As the brain is mainly one of the central issues of interest in PTSD (besides the immune system), the capacity for doing postmortem brain investigations is of paramount importance for the biological validation and interpretation of biological findings found in other samples, such as blood. For example, most (epi)genetic studies have been conducted on blood samples, in part due to the inaccessibility of the human brain in living people and the so-far relative unavailability of postmortem brain tissue for PTSD. Although the question as to how well dysregulations identified in blood samples could reflect processes occurring within the brain remains unanswered, circadian, glucocorticoid, immune, and metabolic dysregulations have repeatedly been picked up using peripheral blood, which could potentially prove to be useful as biomarkers for disease (Baker et al., Citation2012; Daskalakis et al., Citation2016). While most gene expression and DNA methylation studies conducted in peripheral blood often report isolated findings, which lack replication, most studies consistently point to immune-related pathways (Nievergelt et al., Citation2019; Ratanatharathorn et al., Citation2017). Until recently, the only insight we had into the central nervous system came from neuroimaging studies of PTSD subjects. Imaging studies conducted in these past years have increasingly suggested that chronic stress is associated with structural differences in key regions, such as the hippocampus and the ventromedial prefrontal cortex (vmPFC). Similarly, on a functional level, several neuroimaging studies have shown altered activation patterns in the same regions as well as the amygdala and the dorsal anterior cingulate cortex (dACC; Holmes et al., Citation2018; Karl et al., Citation2006).
FINDINGS FROM STUDIES USING POSTMORTEM BRAIN TISSUE
Large-scale efforts that led to the establishment of the National PTSD Brain Bank (NPBB) in 2014, of which Friedman became the director, are now resulting in the first region- and cell-specific molecular studies of PTSD. Using postmortem brain tissue will be paramount in assessing the blood/brain correlations mentioned earlier and drive the discovery of novel treatment targets for PTSD. Since no new therapeutic target for PTSD has been identified in years, and effective medication is still lacking, these efforts will be crucial. The first findings from studies using postmortem brain tissue from subjects with PTSD emerged in the past few years. One study published in 2015 conducted the first whole-transcriptome analysis of dorsolateral prefrontal tissue from a select number of PTSD subjects and reported gene expression changes in PTSD, such as decreased levels of the glucocorticoid-responsive gene SGK1 (Licznerski et al., Citation2015). More recently, Girgenti et al. showed altered gene expression patterns in four regions of the prefrontal cortex, highlighting distinctive transcriptomic signatures in PTSD pointing to dysregulations in inhibitory neurons (Girgenti et al., Citation2021). This study was replicated and expanded in the vmPFC (Logue et al., Citation2021). Also, using human postmortem brain prefrontal cortex tissue, this time assessing DNA methylation profiles, Logue et al. suggested that PTSD associations identified in blood could reflect associations within the brain (Logue et al., Citation2020). In another study recently published on biorxiv, Jaffe and colleagues conducted RNA-sequencing of the postmortem prefrontal cortex and amygdala regions (Jaffe et al., Citation2021). They identified differentially expressed genes between individuals with PTSD, major depressive disorder (MDD), and neurotypical controls, pointing to dysregulated neuroinflammation in both MDD and PTSD. Similarly, Chatzinakos et al. conducted single-nucleus RNA-sequencing on postmortem dorsolateral prefrontal cortex (dlPFC) tissue from subjects with PTSD and MDD and identified cell-type-specific transcriptional changes in excitatory and inhibitory neurons in both disorders (Chatzinakos et al., Citation2021). These examples show the importance of establishing high-quality postmortem tissue banks for PTSD and other mental disorders, thereby establishing a solid basis for biological validation and interpretation.
BRAIN BANKS –MOVING FORWARD
In 2014, the National PTSD Brain Bank (NPBB) was established as a brain tissue biorepository to support research on the causes, progression, and treatment of PTSD. It was a six-part consortium led by VA’s National Center for PTSD with participating sites at VA Medical Centers in Boston, MA; Durham, NC; Miami, FL; West Haven, CT; White River Junction, VT, and the Uniformed Services University of Health Sciences. It was also well integrated with VA’s Boston-based brain banks that focus on Alzheimer’s disease, ALS, chronic traumatic encephalopathy, and other neurological disorders. The NPBB acquired and distributed brain tissue to support research on how PTSD affects brain structure and function.
Many brain banks operate as part of larger consortia that maintain virtual inventories of their combined collections, and they offer a centralized portal to match tissue requests with local supplies. They collect central nervous system tissue from various neurodegenerative diseases and normal aging controls. These banks share samples and attendant demographic and clinical information with qualified researchers worldwide, e.g., BrainNet Europe is an extensive network of 19 brain banks in 11 European countries. The National Alzheimer’s Coordinating Center holds the collections of the neuropathology cores at 27 NIA-funded Alzheimer’s Disease Research Centers into a single database. It contains records on over 13,000 brains, 3,000 of them with extensive clinical and cognitive data. The NeuroBioBank links six U.S. repositories through a standard web portal; its member banks store tissue primarily from neurodevelopmental and psychiatric disorders and neurodegenerative diseases other than Alzheimer’s disease. The Accelerating Medicines Partnership Program for Alzheimer’s Disease (AMP AD) and PsychENCODE Project have aggregated the most comprehensive molecular and other biological data from brain samples from neurodegenerative and neuropsychiatric (including PTSD) disorders. The data generation was funded by NIH and can be accessed through the synapse portal. Finally, the UK Brain Bank Network links 10 U.K. banks that contain samples from more than 10,000 brains into one centralized database.
Unfortunately, there is a worldwide serious shortage of human psychiatric brain tissue available for postmortem research. Therefore, among others, the Netherlands Brain Bank (NBB) launched a prospective donor program to recruit brain donors with psychiatric diseases in 2012. Since the early nineties of the last century, the NBB has performed rapid autopsies of donors who gave written informed consent during life to use their brain tissue and medical files for research (Ravid & Swaab, Citation1993). The NBB was initiated in the Netherlands as a Brain Bank for Psychiatry (NBB-Psy), a prospective donor program for psychiatric diseases. NBB-Psy is expanding the tissue collections to provide a solid incentive to increase research in psychiatry. The ultimate goal of NBB-Psy is to reduce the burden of psychiatric disorders for patients, their families, and society as a whole. NBB-Psy consists of an antemortem and postmortem donor program. In the design of NBB-Psy, much effort has to be put into the antemortem donor program, where patients and relatives are actively informed on the possibility of becoming a brain donor. Since the initiation of NBB-Psy in the Netherlands, the number of registered donors with a psychiatric diagnosis has increased significantly from 312 (most of which were patients with major depressive disorder) in the year 2010 to 1187 in 2017, of which most interesting that 146 are PTSD patients (De Lange, Citation2017; Rademaker et al., Citation2018). Moving forward in the brain bank project “will facilitate the understanding of the role of the environment in all psychiatric conditions, the effects of exposure to traumatic events in other brain disorders already under study, and clarify how the environment influences genetic expression to produce behavioral alterations” (Osuch et al., Citation2004). In response to Osuch, Friedman stated “We support their efforts to create a stand-alone PTSD brain bank that would support scientific efforts to understand the etiology of PTSD and related disorders. The ultimate goal is, of course, the discovery of better preventive and therapeutic strategies with which to combat the development of posttraumatic medical and psychiatric disorders” (Friedman & Harris, Citation2004). These words are still very accurate and relevant, and for that reason, it is highly commended that these papers are revisited in this issue.
DISCLOSURE STATEMENT
No potential conflict of interest was reported by the author(s).
Additional information
Funding
REFERENCES
- Baker, D. G., Nievergelt, C. M., & O’Connor, D. T. (2012). Biomarkers of PTSD: Neuropeptides and immune signaling. Neuropharmacology, 62(2), 663–673. https://doi.org/https://doi.org/10.1016/j.neuropharm.2011.02.027
- Bremner, J. D., Hoffman, M., Afzal, N., Cheema, F. A., Novik, O., Ashraf, A., Brummer, M., Nazeer, A., Goldberg, J., & Vaccarino, V. (2021). The environment contributes more than genetics to smaller hippocampal volume in posttraumatic stress disorder (PTSD). Journal of Psychiatric Research, 137, 579–588. https://doi.org/https://doi.org/10.1016/j.jpsychires.2020.10.042
- Bremner, J. D., Randall, P., Scott, T. M., Bronen, R. A., Seibyl, J. P., Southwick, S. M., Delaney, R. C., McCarthy, G., Charney, D. S., & Innis, R. B. (1995). MRI-based measurement of hippocampal volume in patients with combat-related posttraumatic stress disorder. The American Journal of Psychiatry, 152(7), 973–981. https://doi.org/https://doi.org/10.1176/ajp.152.7.973
- Chatzinakos, C., Morrison, F., McCullough, K., Pernia, C., Logue, M., Wolf, E., Carlezon, W., Kellis, M., Ressler, K., Miller, M., Huber, B., & Daskalakis, N. (2021). Single-nucleus transcriptomic dissection of PTSD and MDD in human post-mortem DLPFC reveals genetic and environmental regulation. Biological Psychiatry, 89(9), S71. https://doi.org/https://doi.org/10.1016/j.biopsych.2021.02.191
- Daskalakis, N. P., Cohen, H., Nievergelt, C. M., Baker, D. G., Buxbaum, J. D., Russo, S. J., & Yehuda, R. (2016). New translational perspectives for blood-based biomarkers of PTSD: From glucocorticoid to immune mediators of stress susceptibility. Experimental Neurology, 284(Pt B), 133–140. https://doi.org/https://doi.org/10.1016/j.expneurol.2016.07.024
- de Lange, G. M. (2017). Understanding the cellular and molecular alterations in PTSD brains: The necessity of post-mortem brain tissue. European Journal of Psychotraumatology, 8(1), 1341824. https://doi.org/https://doi.org/10.1080/20008198.2017.1341824
- Friedman, M. J., & Harris, W. W. (2004). Toward a national PTSD brain bank. Psychiatry: Interpersonal and Biological Processes, 67(4), 384–390. https://doi.org/https://doi.org/10.1521/psyc.67.4.384.56564
- Friedman, M. J., Huber, B. R., Brady, C. B., Ursano, R. J., Benedek, D. M., Kowall, N. W., McKee, A. C., & Traumatic Stress Brain Research, G. (2017). VA’s national PTSD brain bank: A national resource for research. Current Psychiatry Reports, 19(10), 73. https://doi.org/https://doi.org/10.1007/s11920-017-0822-6
- Girgenti, M. J., Wang, J., Ji, D., Cruz, D. A., Traumatic Stress Brain Research, G., Stein, M. B., Gelernter, J., Young, K. A., Huber, B. R., Williamson, D. E., Friedman, M. J., Krystal, J. H., Zhao, H., & Duman, R. S. (2021). Transcriptomic organization of the human brain in post-traumatic stress disorder. Nature Neuroscience, 24(1), 24–33.
- Holmes, S. E., Scheinost, D., DellaGioia, N., Davis, M. T., Matuskey, D., Pietrzak, R. H., Hampson, M., Krystal, J. H., & Esterlis, I. (2018). Cerebellar and prefrontal cortical alterations in PTSD: Structural and functional evidence. Chronic Stress (Thousand Oaks), 2. https://doi.org/https://doi.org/10.1177/2470547018786390
- Jaffe, A. E., Tao, R., Tran, M. N., Page, S. C., Maynard, K. R., Pattie, E. A., Nguyen, C. V., Deep-Soboslay, A., Bharadwaj, R., Young, K. A., Friedman, M. J., Williamson, D. E., Traumatic Stress Brain Research, G., Shin, J. H., Hyde, T. M., Martinowich, K., & Kleinman, J. E. (2021). Decoding shared versus divergent transcriptomic signatures across cortico-amygdala circuitry in PTSD and depressive disorders. bioRxiv. https://doi.org/https://doi.org/10.1101/2021.01.12.426438
- Karl, A., Schaefer, M., Malta, L. S., Dorfel, D., Rohleder, N., & Werner, A. (2006). A meta-analysis of structural brain abnormalities in PTSD. Neuroscience & Biobehavioral Reviews, 30(7), 1004–1031. https://doi.org/https://doi.org/10.1016/j.neubiorev.2006.03.004
- Licznerski, P., Duric, V., Banasr, M., Alavian, K. N., Ota, K. T., Kang, H. J., Jonas, E. A., Ursano, R., Krystal, J. H., Duman, R. S., & Traumatic Stress Brain Study, G. (2015). Decreased SGK1 expression and function contributes to behavioral deficits induced by traumatic stress. PLoS Biology, 13(10), e1002282. https://doi.org/https://doi.org/10.1371/journal.pbio.1002282
- Logue, M. W., Amstadter, A. B., Baker, D. G., Duncan, L., Koenen, K. C., Liberzon, I., Miller, M. W., Morey, R. A., Nievergelt, C. M., Ressler, K. J., Smith, A. K., Smoller, J. W., Stein, M. B., Sumner, J. A., & Uddin, M. (2015). The psychiatric genomics consortium posttraumatic stress disorder workgroup: Posttraumatic stress disorder enters the age of large-scale genomic collaboration. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology, 40(10), 2287–2297. https://doi.org/https://doi.org/10.1038/npp.2015.118
- Logue, M. W., Miller, M. W., Wolf, E. J., Huber, B. R., Morrison, F. G., Zhou, Z., Zheng, Y., Smith, A. K., Daskalakis, N. P., Ratanatharathorn, A., Uddin, M., Nievergelt, C. M., Ashley-Koch, A. E., Baker, D. G., Beckham, J. C., Garrett, M. E., Boks, M. P., Geuze, E., Grant, G. A., … Hauser, M. A. (2020). An epigenome-wide association study of posttraumatic stress disorder in US veterans implicates several new DNA methylation loci. Clinical Epigenetics, 12(1), 46. https://doi.org/https://doi.org/10.1186/s13148-020-0820-0
- Logue, M. W., van Rooij, S. J. H., Dennis, E. L., Davis, S. L., Hayes, J. P., Stevens, J. S., Densmore, M., Haswell, C. C., Ipser, J., Koch, S. B. J., Korgaonkar, M., Lebois, L. A. M., Peverill, M., Baker, J. T., Boedhoe, P. S. W., Frijling, J. L., Gruber, S. A., Harpaz-Rotem, I., Jahanshad, N., … Morey, R. A. (2018). Smaller hippocampal volume in posttraumatic stress disorder: A multisite ENIGMA-PGC study: Subcortical volumetry results from posttraumatic stress disorder consortia. Biological Psychiatry, 83(3), 244–253. https://doi.org/https://doi.org/10.1016/j.biopsych.2017.09.006
- Logue, M. W., Zhou, Z., Morrison, F. G., Wolf, E. J., Daskalakis, N. P., Chatzinakos, C., Georgiadis, F., Labadorf, A. T., Girgenti, M. J., Young, K. A., Williamson, D. E., Zhao, X., Grenier, J. G., Traumatic Stress Brain Research, G., Huber, B. R., & Miller, M. W. (2021). Gene expression in the dorsolateral and ventromedial prefrontal cortices implicates immune-related gene networks in PTSD. Neurobiology of Stress, 15, 100398.
- Lori, A., Coppede, F., & Pellegrini, S. (2021). Editorial: Shared genetic risk factors among psychiatric diseases and other medical diseases and traits. Frontiers in Neuroscience, 15, 802064. https://doi.org/https://doi.org/10.3389/fnins.2021.802064
- Morrison, F. G., Miller, M. W., Logue, M. W., Assef, M., & Wolf, E. J. (2019). DNA methylation correlates of PTSD: Recent findings and technical challenges. Progress in Neuro-Psychopharmacology and Biological Psychiatry, 90, 223–234. https://doi.org/https://doi.org/10.1016/j.pnpbp.2018.11.011
- Nievergelt, C. M., Maihofer, A. X., Klengel, T., Atkinson, E. G., Chen, C. Y., Choi, K. W., Coleman, J. R. I., Dalvie, S., Duncan, L. E., Gelernter, J., Levey, D. F., Logue, M. W., Polimanti, R., Provost, A. C., Ratanatharathorn, A., Stein, M. B., Torres, K., Aiello, A. E., Almli, L. M., … Koenen, K. C. (2019). International meta-analysis of PTSD genome-wide association studies identifies sex- and ancestry-specific genetic risk loci. Nature Communications, 10(1), 4558. https://doi.org/https://doi.org/10.1038/s41467-019-12576-w
- Osuch, E., Ursano, R., Li, H., Webster, M., Hough, C., Fullerton, C., & Leskin, G. (2004). Brain environment interactions: Stress, posttraumatic stress disorder, and the need for a postmortem brain collection. Psychiatry: Interpersonal and Biological Processes, 67(4), 353–383. https://doi.org/https://doi.org/10.1521/psyc.67.4.353.56565
- Rademaker, M. C., de Lange, G. M., & Palmen, S. (2018). The Netherlands brain bank for psychiatry. Handbook of Clinical Neurology, 150, 3–16. https://doi.org/https://doi.org/10.1016/B978-0-444-63639-3.00001-3
- Ratanatharathorn, A., Boks, M. P., Maihofer, A. X., Aiello, A. E., Amstadter, A. B., Ashley-Koch, A. E., Baker, D. G., Beckham, J. C., Bromet, E., Dennis, M., Garrett, M. E., Geuze, E., Guffanti, G., Hauser, M. A., Kilaru, V., Kimbrel, N. A., Koenen, K. C., Kuan, P. F., Logue, M. W., … Smith, A. K. (2017). Epigenome-wide association of PTSD from heterogeneous cohorts with a common multi-site analysis pipeline. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics, 174(6), 619–630. https://doi.org/https://doi.org/10.1002/ajmg.b.32568
- Ravid, R., & Swaab, D. F. (1993). The Netherlands brain bank–a clinico-pathological link in aging and dementia research. Journal of Neural Transmission. Supplementum, 39, 143–153. https://www.ncbi.nlm.nih.gov/pubmed/8360654
- Rutten, B. P. F., Vermetten, E., Vinkers, C. H., Ursini, G., Daskalakis, N. P., Pishva, E., de Nijs, L., Houtepen, L. C., Eijssen, L., Jaffe, A. E., Kenis, G., Viechtbauer, W., van den Hove, D., Schraut, K. G., Lesch, K. P., Kleinman, J. E., Hyde, T. M., Weinberger, D. R., Schalkwyk, L., … Boks, M. P. M. (2018). Longitudinal analyses of the DNA methylome in deployed military servicemen identify susceptibility loci for post-traumatic stress disorder. Molecular Psychiatry, 23(5), 1145–1156. https://doi.org/https://doi.org/10.1038/mp.2017.120
- Schizophrenia Working Group of the Psychiatric Genomics, C. (2014). Biological insights from 108 schizophrenia-associated genetic loci. Nature, 511(7510), 421–427. https://doi.org/https://doi.org/10.1038/nature13595
- Schnurr, P. P., Friedman, M. J., Engel, C. C., Foa, E. B., Shea, M. T., Chow, B. K., Resick, P. A., Thurston, V., Orsillo, S. M., Haug, R., Turner, C., & Bernardy, N. (2007). Cognitive behavioral therapy for posttraumatic stress disorder in women: A randomized controlled trial. JAMA, 297(8), 820–830. https://doi.org/https://doi.org/10.1001/jama.297.8.820
- Smith, A. K., Ratanatharathorn, A., Maihofer, A. X., Naviaux, R. K., Aiello, A. E., Amstadter, A. B., Ashley-Koch, A. E., Baker, D. G., Beckham, J. C., Boks, M. P., Bromet, E., Dennis, M., Galea, S., Garrett, M. E., Geuze, E., Guffanti, G., Hauser, M. A., Katrinli, S., Kilaru, V., … Nievergelt, C. M. (2020). Epigenome-wide meta-analysis of PTSD across 10 military and civilian cohorts identifies methylation changes in AHRR. Nature Communications, 11(1), 5965. https://doi.org/https://doi.org/10.1038/s41467-020-19615-x
- Snijders, C., Maihofer, A. X., Ratanatharathorn, A., Baker, D. G., Boks, M. P., Geuze, E., Jain, S., Kessler, R. C., Pishva, E., Risbrough, V. B., Stein, M. B., Ursano, R. J., Vermetten, E., Vinkers, C. H., Consortium, P. P. E., Smith, A. K., Uddin, M., Rutten, B. P. F., & Nievergelt, C. M. (2020). Longitudinal epigenome-wide association studies of three male military cohorts reveal multiple CpG sites associated with post-traumatic stress disorder. Clinical Epigenetics, 12(1), 11. https://doi.org/https://doi.org/10.1186/s13148-019-0798-7
- Vermetten, E., Vythilingam, M., Southwick, S. M., Charney, D. S., & Bremner, J. D. (2003). Long-term treatment with paroxetine increases verbal declarative memory and hippocampal volume in posttraumatic stress disorder. Biological Psychiatry, 54(7), 693–702. https://doi.org/https://doi.org/10.1016/s0006-3223(03)00634-6
- Wang, X., Xie, H., Chen, T., Cotton, A. S., Salminen, L. E., Logue, M. W., Clarke-Rubright, E. K., Wall, J., Dennis, E. L., O’Leary, B. M., Abdallah, C. G., Andrew, E., Baugh, L. A., Bomyea, J., Bruce, S. E., Bryant, R., Choi, K., Daniels, J. K., Davenport, N. D., … Liberzon, I. (2021). Cortical volume abnormalities in posttraumatic stress disorder: An ENIGMA-psychiatric genomics consortium PTSD workgroup mega-analysis. Molecular Psychiatry, 26(8), 4331–4343. https://doi.org/https://doi.org/10.1038/s41380-020-00967-1