Abstract
With a few exceptions, comprehensive lists of alien plants that invade natural ecosystems are lacking in sub-Saharan Africa. Some available lists are either preliminary or localised, or focus on agricultural weeds. This study set out to compile a list of alien plant species that are invading natural ecosystems and rangelands in five countries in eastern Africa, and to map the distribution of the species that threaten ecosystem integrity and productivity. The location of all alien plant species seen during surveys between 2008 and 2016 was recorded using a hand-held GPS device, as well as their status in terms of either being present and/or naturalised, or invasive and spreading. Individual occurrence records were summarised at the scale of half degree grid cells (∼55 km × 55 km). The survey covered almost half (522) of the 1063 grid cells in Ethiopia, Kenya, Tanzania, Uganda and Rwanda. We recorded 164 invasive alien species in 110 genera and 47 families. We provide further information on the distribution and impacts of 30 species considered to have the greatest impacts in terms of transforming natural ecosystems, as well as on a further 21 species with limited distributions that could potentially become ecosystem transformers. Invasive alien plants are clearly a widespread and growing problem in eastern Africa, and capacity to manage them effectively remains a problem. A great deal of work needs to be done to raise awareness of the problem, and to identify appropriate responses that will be effective in resource-poor countries.
INTRODUCTION
Invasive alien plant species pose substantial threats to agriculture, biodiversity and the delivery of ecosystem services globally. Good information on the occurrence and distribution is an essential starting point that would enable the quantification of these threats, and inform the development of appropriate responses. Comprehensive lists of invasive alien plants are generally lacking in most parts of sub-Saharan Africa with the notable exceptions of South Africa (Henderson, Citation2007; van Wilgen et al., Citation2014; Henderson & Wilson, Citation2017), Namibia (Brown et al., Citation1985; Bethune et al., Citation2004); Swaziland (SNTC, Citation2016), and Zimbabwe (Maroyi, Citation2006, Citation2012). Pyšek et al. (Citation2017) provides an overview of the naturalised alien flora of the world, including that of the eastern African countries under review in this paper. There is a database on some of the invasive alien plants in East Africa (Lusweti et al., Citation2011), together with global databases such as the Invasive Species Compendium (CABI, Citation2017), and the Global Invasive Species Database (GISD, Citation2017), which covers countries in the region. Other lists are either preliminary [e.g. Rejmánek et al. (Citation2017) for Angola] or localised [e.g. Dawson et al. (Citation2008) and Sheil (Citation2008) for the Usambara mountains, Tanzania; Rejmánek (Citation1996) and IUCN/PACO (Citation2013) for protected areas in Uganda and West Africa, respectively; Witt et al. (Citation2017) for the Serengeti-Mara ecosystem in Tanzania and Kenya] or focus mainly on agricultural weeds [e.g. Bogdan (Citation1950, Citation1965),Terry (Citation1984), Ivens (Citation1967) and Terry & Michieka (Citation1987) for East Africa; Germain (Citation1952), Mullenders (Citation1954), Schmitz (Citation1971), Mosango (Citation1983a, Citationb) and Lubini (Citation1986) for Central Africa; Wild (Citation1955) and Drummond (Citation1984) for Zimbabwe and El Hadidi et al. (Citation1996) for Egypt]. Despite the lack of detailed lists and distribution data, and a dearth of accurate data on the impacts of invasive alien plants, an appreciation of the imperative to deal with invasive alien plants in sub-Saharan Africa is growing (Boy & Witt, Citation2013).
In eastern Africa, invasive alien plant species are known to impact negatively on the conservation of biodiversity as well as the livelihoods of rural people that depend heavily on natural resources (Maundu et al., Citation2009; Tamado & Milberg, Citation2000; Kebede & Coppock, Citation2015; Shackleton et al., Citation2017a, Citation2017b, Citationc). Several of the species that are now problematic were deliberately introduced by governments and aid agencies to augment existing natural resources, precipitating impacts that far outweigh any benefits they may have brought (Mwangi & Swallow, Citation2008; Maundu et al., Citation2009). Invasions by alien plants have added a new dynamic to landscape management in the region, and they often lead to conflict over resources (Mwangi & Swallow, Citation2008). For example, reductions in the capacity of rangelands to support livestock, coupled with growing human populations and competing demands for the use of land, have led to the marginalisation of many rural pastoralists (Maundu et al., Citation2009). Further conflict arises because opinions can be divided about how to appropriately respond to the problem. Two pertinent examples in the case of invasive alien plants relate to whether or not to use biological control, and whether or not to encourage the use or utilisation of alien plant biomass in an attempt to reduce their impacts. These conflicts need to be resolved, and the development of appropriate policies and management interventions that would lead to such resolution must in turn be informed by the best possible understanding of the species that contribute to the problem, the area over which they are distributed, and the impacts that they generate.
In this paper, we report on a survey of alien plant species that are invading natural ecosystems and rangelands in five countries in eastern Africa. We provide a list of the species that threaten the integrity and productivity of these ecosystems, along with distribution data for the most important taxa, based on extensive roadside surveys. We also provide recommendations for strategies to manage invasive alien plants. We envisage that this information will be useful for policy development and strategic planning in the field of invasive alien plant management in the region.
METHODS
Surveys
Our surveys covered five countries in eastern Africa (Ethiopia, Kenya, Tanzania, Uganda and Rwanda, ). Alien plant species were recorded during roadside surveys, similar to those undertaken by Henderson (Citation2007), Rejmánek et al., (Citation2017) and Shackleton et al., (Citation2017a, b, c), over seven years, between 2008 and 2015. During this time, one of us (ABRW) drove considerable distances, covering tens of thousands of kilometres () and recorded the location (using a hand-held GPS device) and status (present and/or, naturalised, or invasive and spreading) of all alien plant species seen. A new locality for any particular exotic species was only recorded if it was seen at least 1 km from the previous record. Naturalised species are those that reproduce consistently, and have established self-sustaining populations but have not yet spread widely, whereas invasive species are those that produce large numbers of reproductive offspring that have spread often over substantial distances from where they have become naturalised (Richardson et al., Citation2000; Blackburn et al., Citation2011). Invasive species can also cause ecological or economic harm and/or have negative health impacts. When a species could not be immediately identified, specimens were collected or photographs were taken for later identification by taxonomists.
Figure 1. Location of five countries in East Africa in which surveys were undertaken to record the presence of alien plant species.
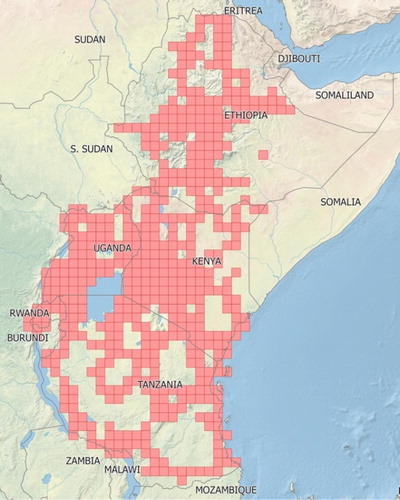
Figure 2. Grid cells (approximately 55 km × 55 km) within five countries in East Africa that were surveyed for the presence of alien plant species between 2008 and 2016.
Naturalised and invasive grass species, which can be very difficult to identify during roadside surveys, were not recorded. Information from other sources as to the presence or distribution of grasses was also not included. Many Eucalyptus (Myrtaceae) and Pinus (Pinaceae) species have been introduced into the region and cultivated, in some cases becoming naturalised or even locally abundant, but these too can be difficult to identify at species level, and were recorded at genus level with the exception of Pinus patula and P. caribaea. Many other species, of vines for example, especially those in the genus Ipomoea (Convolvulaceae), are difficult to identify when not in flower and may not have been seen and as such recorded at many localities. Other species, especially herbaceous plants, are often obscure and difficult to observe, while others cannot easily be differentiated from native plant species of similar appearance. So, where a plant species was not recorded as being present in a particular area, it did not necessarily mean that the species was not present there, only that it was just not seen there during surveys. Additional information on the presence and status of invasive alien plant species was sourced from the literature, especially the Flora of Tropical East Africa (FTEA) and Flora of Ethiopia and Eritrea (FEE). The islands of Pemba and Zanzibar were not surveyed, but information from published sources on invasive species present there was included in this analysis. Comparatively very little information on the distribution of invasive alien plant species in eastern Africa was sourced elsewhere with most distribution data obtained from our surveys.
We also attempted to establish the dates that each species was first introduced to the region, with the exception of Ethiopia, by examining a range of sources, including various editions of Government Gazette (Citation1899–1964) for the East African region dating back to 1899, the FTEA (Great Britain Colonial Office, Citation1952), herbarium specimens, and various other reports and publications (Dale, Citation1953; Engler Citation1895a, Citationb; Greenway, Citation1934, Citation1941; Hutchins, Citation1909; Williams, Citation1949). We also included other information of interest such as the species’ regions of origin, their life-forms, and the purpose for which they were introduced (a taxon was allocated to more than one use category if it had multiple uses).
RESULTS
Surveys
Individual records of plant occurrence from the surveys were entered into a database and the distributions mapped at the scale of half degree grid cells (∼55 km × 55 km). If a plant species was found to be present, naturalised, and invasive at various localities in the same cell then the latter took precedence in the species map, indicating that it was found to be invasive in at least one locality within that particular cell. We were only able to reach half of these grid cells in the region during our surveys (). In Ethiopia, we were only able to reach just over one third (35%) of the grid cells, with most of the arid east remaining un-surveyed; this was due to a combination of a lack of access roads, available accommodation, as well as concerns about safety. The inability to survey these and other areas in eastern Africa means that our distribution data is conservative, and that invasions may be significantly more widespread than indicated. However, we are fairly confident that no other invasive plant species are present in these areas that have not been recorded elsewhere in the region.
Table 1. The total number of grid cells (approximately 55 km × 55 km) in five eastern African countries, and the number of grid cells included in surveys of invasive alien plant species.
Invasive alien plant species
Of the 164 invasive alien species found in eastern Africa (Supplementary Table S1), four species, Brillantaisia lamium, Elaeis guineensis, Maesopsis eminii and Landolphia owariensis are considered to be native to parts of the region but have been introduced elsewhere within eastern Africa and have subsequently become invasive. Nine problematic plant species with uncertain or disputed origin () and some native species regarded as extra-limital or range extension species (origin given as “tropical Africa” in Supplementary Table S1) have been included in our species list but have not been included in any of the summary statistics presented below. All of the species we listed are invading natural vegetation or aquatic ecosystems in eastern Africa. We did not include several species in our list recorded as “invasive” by others such as Acacia leptocarpa (Fabaceae) and Pinus radiata (Pinaceae) (Haysom & Murphy, Citation2003); Cananga odorata (Annonaceae), Schinus molle (Anacardiaceae) and Jatropha multifida (Euphorbiaceae), which were reported to have “gone wild” by Beentje (Citation1994); Manihot carthaginensis (Euphorbiaceae) (previously M. glaziovii) recorded as “wild in parts of Uganda”(Birnie & Noad, Citation2011) and Cuscuta suaveolens (Convolvulaceae) (Agnew & Agnew, Citation1994) because we could not confirm their status. We have also not included those invasive alien species which are only having a known impact on agricultural crop production or those species that are largely confined to roadsides and other highly disturbed areas although all of these species could also be regarded as naturalised and/or invasive.
Table 2. Invasive species with an uncertain or disputed origin1 and problematic native species2 (sometimes referred to as extra-limital or range extension species) in Ethiopia (ET), Kenya (KE), Rwanda (RW), Tanzania (TZ) and Uganda (UG), with brief notes on habitat types invaded (Fo, forest; Sa, savanna; Gr, grassland; Tr, transformed; Rr, road/railside; Ha, around habitation; Pl, plantation; Ar, arable/ploughed land; Pa, pastoral; Ws, wasteland; Wc, watercourse; Wt, wetland; Dr, dryland/well drained; Kl, kloof/ravine; Ro, rocky site), and impacts. A full set of references to accounts of impact are contained in Witt and Luke (Citation2017).
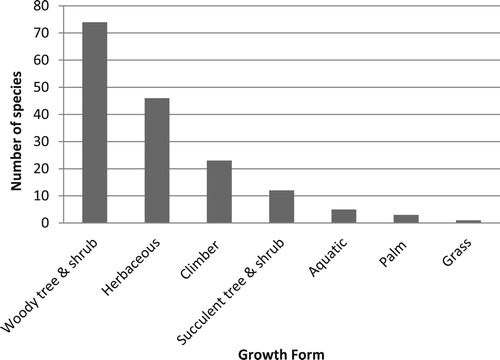
The 164 invasive alien species found in eastern Africa belong to 110 genera and 47 families. The family Fabaceae is best represented with 27 species, followed by the Asteraceae (17), Solanaceae (13) and Cactaceae (8) (). The genus Senna (Fabaceae) has the most number of species (8) followed by the genera Acacia (5) (Fabaceae), Opuntia (5) (Cactaceae) and Ipomoea (4) (Convolvulaceae). The dominant growth form among invading alien plants were woody trees and shrubs (74 species; 45%), followed by herbaceous plants (46 species; 28%) and climbers (23 species; 14%) (Supplementary Table S1; ). A large majority of the species (131; 80%) originated from the tropics, 25 (15%) from temperate regions and 8 (5%) from temperate and tropical regions. Most of the invasive alien species (116; 71%) present in eastern Africa have their origins in the America’s, the vast majority from the American tropics with a few serious invasive species such as Azadirachta indica (Meliaceae), Broussonetia papyrifera (Moraceae) and Rubus niveus (Rosaceae) from Asia. Most (128; 80%) of these invasive alien species were introduced between 1881 and 1960 (), some even prior to that. For example, the rose apple (Syzygium jambos; Myrtaceae) was introduced to Zanzibar as early as the 1300s, and guava trees (Psidium guajava; Myrtaceae) were introduced around 1520. Others are relatively recent introductions, including the aggressively invasive shrub Chromolaena odorata (Asteraceae), first recorded in about 2009. Most species were intentionally introduced and are now cultivated and utilised for various purposes (). Most species (146; 46%) are grown as ornamentals or used as barriers/hedges (48; 15%), and as agricultural crops ().
Figure 4. Date of first records of introduced plant species now considered to be invasive in eastern Africa.
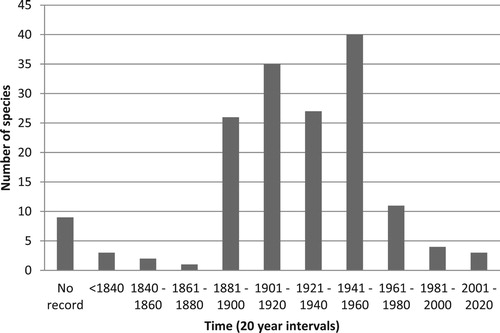
Table 3. Families with four or more invasive alien species in eastern Africa
Table 4. Cultivated uses of invasive alien species recorded in eastern Africa. Species may have been allocated to more than one category.
A number of the invasive alien plant species that occur in eastern Africa are particularly problematic, based on their distribution and/or impacts on biodiversity and livelihoods () and include Lantana camara, Parthenium hysterophorus, Prosopis juliflora, various Opuntia species, C. odorata, Leucaena leucocephala, Mimosa pigra, M. diplotricha, Azadirachta indica, Cestrum aurantiacum, Tithonia diversifolia, various Australian Acacia species, Caesalpinia decapetala, Psidium guajava, Solanum mauritianum, various Senna species and Eichhornia crassipes. Other widespread and abundant species include Ageratum conyzoides, species in the genus Datura and Xanthium strumarium. Many of these species could be considered to be “transformer species”, defined as “invasive species that change the character, condition, form or nature of ecosystems” (Richardson et al., Citation2011).
Table 5. The distribution of what we consider to be the 30 species with the greatest impact in terms of transforming natural vegetation in Ethiopia (ET), Kenya (KE), Rwanda (RW), Tanzania (TZ) and Uganda (UG), with brief notes on habitat types invaded (Fo, forest; Sa, savanna; Gr, grassland; Tr, transformed; Rr, road/rail side; Ha, around habitation; Pl, plantation; Ar, arable/ploughed land; Pa, pastoral; Ws, wasteland; Wc, watercourse; Wt, wetland; Dr, dryland/well drained; Kl, kloof/ravine; Ro, rocky site), and impacts. A full set of references to accounts of impact are contained in Witt and Luke (Citation2017).
Other than these “transformer species” there are a number of species that we would consider to be potential transformers (). These include species that are currently relatively localised but have the potential to substantially spread beyond their current distribution and impact negatively on biodiversity and rangeland production. Many of these species have been widely planted as ornamentals or agro-forestry species, increasing their propagule pressure. Such species include Dahlia imperialis, Tecoma stans, Anredera cordifolia, Cardiospermum grandiflorum, Pereskia aculeata, Antigonon leptopus, Acacia colei, various Cestrum and Ipomoea species, and Mirabilis jalapa, among others. These species are currently locally abundant, especially in urban open spaces, and in peri-urban areas, and have the potential to spread further. Evidence from elsewhere suggests that they have significant negative impacts on biodiversity and we are assuming that the situation will be no different in eastern Africa.
Table 6. Details on the invasive alien plant species considered to be potential transformers of natural vegetation in Ethiopia (ET), Kenya (KE), Rwanda (RW), Tanzania (TZ) and Uganda (UG), with brief notes on habitat types invaded (Fo, forest; Sa, savanna; Gr, grassland; Tr, transformed; Rr, road/railside; Ha, around habitation; Pl, plantation; Ar, arable/ploughed land; Pa, pastoral; Ws, wasteland; Wc, watercourse; Wt, wetland; Dr, dryland/well drained; Kl, kloof/ravine; Ro, rocky site), and impacts. A full set of references to accounts of impact are contained in Witt and Luke (Citation2017).
DISCUSSION
Features of invasive alien plants in eastern Africa
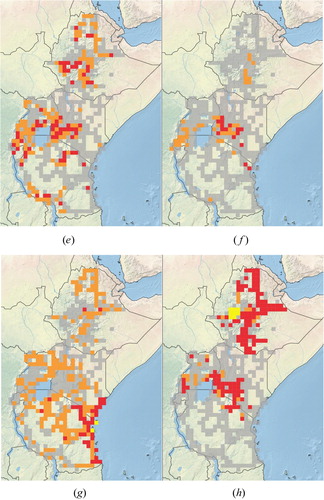
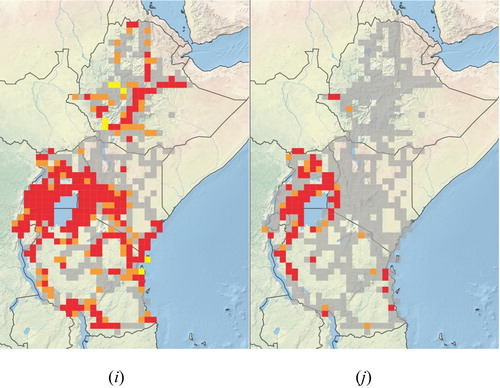
The distribution of these widely established and potential transformer plant species is determined by a number of factors with climate being the dominant factor at the continental scale with topography, land use and land cover being most important at the regional scale (Milbau et al., Citation2009). The wide range of ecosystems, ranging from deserts to forests, and climatic conditions found across eastern Africa (Nicholson, Citation1996) makes the region particularly prone to invasions by many introduced plant species. Such invasions are being facilitated by increased land degradation, especially through overgrazing and deforestation, and also by climate change. Large parts of the arid and semi-arid landscapes of eastern, northern and southern Ethiopia, and of northern and north-eastern Kenya, have been invaded by introduced trees and shrubs, adapted to these low rainfall areas, such as mesquite (Prosopis species) ( (a)), as well as by various succulents, including pest pear Opuntia stricta) ( (b)) and sweet prickly pear (O. ficus-indica). Many other succulents such as various Agave species (Agavaceae) are well adapted to growing in these areas, as are species in the family Crassulaceae, mainly Bryophyllum species, especially B. delagoense ( (c)), which were introduced as ornamentals but which have subsequently escaped cultivation. Calotropis species (Apocynaceae), including the native but spreading C. procera and the alien invasive C. gigantea are also well adapted to survive and to spread in these semi-arid environments.
Figure 5. Distributions of some invasive alien plant species in eastern Africa. Grey grid squares represent areas that were surveyed but where no naturalised, invasive, or potentially invasive plant species were seen; orange grid squares represent areas where a plant species was found to be present and/or naturalised; red grid squares represent areas where a plant species was considered to be invasive; and yellow grid squares where locality data were obtained from other sources with no reference being made to plant densities or invasiveness. (a) Invasive Prosopis species, (b) Opuntia stricta, (c) Bryophyllum delagoense, (d) Acacia mearnsii, (e) Caesalpinia decapetala, (f) Euryops chysanthemoides, (g) Azadirachta indica, (h) Parthenium hysterophorus, (i) Lantana camara, (j)Mimosa pigra.
The Eastern African highlands, especially those in Kenya, Tanzania and Rwanda, have largely been invaded by introduced Australian wattles such as black wattle (Acacia mearnsii; Fabaceae) ( (d)) and blackwood (A. melanoxylon; Fabaceae). Pinus patula (Pinaceae), introduced from Central America, has also escaped from cultivation in these areas, along with spiny shrubs such as various Rubus species (Rosaceae) and Mauritius thorn ( (e)). African bush daisy (Euryops chrysanthemoides; Asteraceae), which has been introduced from southern Africa as an ornamental, also tends to favour higher-lying areas, establishing dense stands, especially on roadsides ( (f)). Many introduced species recorded as invasive are only present in the East Usambaras, escapes from the Amani Botanical Gardens. This area has been well studied (Sheil, Citation1994; Dawson et al., Citation2008), so there are good records of the presence of alien species that have escaped cultivation, but which have not yet been recorded as being problematic elsewhere in the region, such as Maesopis eminii, Hura crepitans (Euphorbiaceae), Hevea brasiliensis (Euphorbiaceae), Arenga pinnata (Arecaceae), Castilla elastica (Moraceae), Cordia alliodora (Boraginaceae), Piper aduncum (Piperaceae) and others. Dawson et al. (Citation2008) recorded 38 and 16 naturalised species with a known and an unclear planting history in the Amani Botanic Garden (ABG), respectively, and 16 invasive or spreading alien species which had been planted in the ABG. Sheil (Citation1994) also recorded a large number of naturalised species in the East Usambaras (). Some of these species, and a host of others, are known to be naturalised elsewhere in eastern Africa, posing a significant potential threat to the Eastern African highlands.
Table 7. Naturalised species of plants in the East Usambara mountains, Tanzania, as recorded by Sheil (Citation1994).
The coastal region is dominated by species such as neem (Azadirachta indica; Meliaceae) ( (g)) and leucaena (Leucaena leucocephala; Fabaceae), which were intentionally introduced, and are particularly aggressive there, although localised invasions are occasionally present further inland. Antigonon leptopus, introduced from tropical America as an ornamental, is also invasive along the Kenyan and Tanzania coastline. Although C. odorata has the potential to establish along the eastern seaboard it is currently invasive in savanna habitats in northern Tanzania and Uganda (Shackleton et al., Citation2017a), while other species, such as famine weed (Parthenium hysterophorus) ( (h)), lantana (Lantana camara; Verbenaceae) (Shackleton et al., Citation2017b) ( (i)), goatweed (Ageratum conyzoides; Asteraceae) and common thorn apple (Datura stramonium; Solanaceae), are adapted to survive and to proliferate across a wider range of habitats, wherever there is sufficient rainfall or soil moisture. Datura spp. are especially prevalent on highly disturbed soils, such as on roadsides. Semi-aquatic species, such as the giant sensitive plant (Mimosa pigra; Fabaceae) ( (j)), thrive on floodplains and around the edges of swamps and other waterbodies. Other species, such as Brugmansia suaveolens (Solanaceae) are generally invasive only along streams and on riverbanks, often in highland areas, while spectacular cassia [Senna spectabilis; Fabaceae] and yellow cestrum (Cestrum aurantiacum; Solanaceae) are often problematic in forests and in woodlands. Many introduced vines or ‘climbers’, such as Anredera cordifolia, Cardiospermum grandiflorum and Pereskia aculeata, have escaped from gardens, and are now well established in urban open space, especially in Kenya.
Potential for future spread and impact
Land degradation or disturbances are among the main drivers of plant invasions. Unsuitable land uses and inappropriate land management practices such as slash and burn agriculture, timber and charcoal extraction, deforestation, overgrazing, cultivation on steep slopes, uncontrolled fires and pollution of water resources (Dregne, Citation2002) all cause significant disturbances which facilitate plant invasions. Currently land degradation hotspots cover about 51%, 23% and 22% of the terrestrial areas in Tanzania, Ethiopia and Kenya, respectively (Kirui & Mrzabaev, Citation2016), which together with climate change will facilitate the expansion of invasive plant species already present in eastern Africa. However, invasive plant species will only establish in those areas which are eco-climatically suitable for establishment, but they are more likely to do so if the area is already highly disturbed. There are very few eco-climatic studies for most species, so predicting future spread is problematic. Available studies include Lantana camara (Taylor et al., Citation2012), Chromolaena odorata (Kriticos et al., Citation2005), Parthenium hysterophorus (McConnachie et al., Citation2010), Prosopis juliflora (Maundu et al., Citation2009), Hyptis suaveolens (L.) Poit. (Lamiaceae) (Padalia et al., Citation2015) and Cryptostegia grandiflora Roxb. ex R.Br (Asclepiadaceae) (Kriticos et al., Citation2003). These studies are discussed briefly below.
Lantana camara is unlikely to invade northern Kenya and eastern Ethiopia, areas which receive less than 500 mm of rainfall per year, while parts of the Ethiopian highlands are probably too cold, with night-time temperatures dropping to 5 oC. However, based on the CLIMEX model developed by Taylor et al. (Citation2012) most of Tanzania and northern Uganda, which is currently uninvaded, is climatically suitable for Lantana camara. Protected areas such as Ruaha, Saadani and Selous, all in Tanzania, have climates suitable for lantana establishment and proliferation. Further invasions pose serious threats not only to biodiversity, but also livelihoods. Based on socio-economic surveys undertaken in Uganda, lantana can reduce the amount of forage available to livestock by more than 50% and reduce crop yields by 26–50% (Shackleton et al., Citation2017b).
C. odorata currently has a fairly localised distribution in eastern Africa with dense invasions in northern central Tanzania, localised stands in south-eastern Kenya, and some populations in eastern Uganda (Shackleton et al., Citation2017a). Despite its current limited distribution, bioclimatic models (McFadyen & Skarratt, Citation1996; Kriticos et al., Citation2005; Raimundo et al., Citation2007) have indicated that much of the region is climatically suitable for the species, especially areas along the Kenyan and Tanzanian coasts, large parts of Uganda and areas around Lake Victoria. Socio-economic surveys in northern central Tanzania found that C. odorata reduced the presence of native grasses, shrubs and trees, impacted negatively on livestock production and reduced crop yields by up to 50% (Shackleton et al., Citation2017b).
Parthenium hysterophorus is a widespread invader of rangelands and cropping fields in all the eastern African countries surveyed. Based on an eco-climatic model developed by McConnachie et al. (Citation2010) much of eastern Africa is climatically suitable for famine weed invasions. Most of Uganda is eco-climatically suitable as is the northeast of Tanzania, much of the Kenyan coastline and large parts of Ethiopia. Invasions are also likely to spread in Rwanda. The species is known to be allelopathic, which enables it to displace natural vegetation (Evans, Citation1997; Aggarwal & Kohli, Citation1992; McFadyen, Citation1992), reducing stocking rates by as much as 80% in heavy infestations (McFadyen, Citation1992). In addition, it can cause severe allergenic reactions in a large proportion of people who come into contact with it, as well as in livestock and wildlife (Towers & Mitchell, Citation1983; Patel, Citation2011). Most (90%) farmers in the lowlands of Ethiopia consider famine weed to be the most serious weed of croplands and grazing areas (Tamado & Milberg, Citation2000).
Invasive Prosopis spp. can reduce grazing capacity (Ndhlovu et al., Citation2011), displacing many species from invaded ecosystems (Steenkamp & Chown, Citation1996; Dean et al., Citation2002; Shackleton et al., Citation2015; Schachtschneider & February, Citation2013), and reducing water resources (Dzikiti et al., Citation2013). In some parts of Ethiopia, P. juliflora has reduced understorey cover for perennial grasses from 68% to 2% (Kebede & Coppock, Citation2015) and a relatively light infestation (15% cover) in South Africa led to a 34% reduction in grazing capacity (Ndhlovu et al., Citation2011). Communities in Kenya, Sudan, Eritrea, Malawi, South Africa and Pakistan have all reported negative impacts of mesquite invasions (Pasiecznik et al., Citation2001; Brown et al., Citation2004). In semi-arid parts of Africa, invasive Prosopis species and their hybrids have depleted the natural resources on which many people depend, often spawning conflict between communities over access to water and grazing land. These impacts are bound to increase as much of eastern Africa, especially Tanzania, is climatically suitable for P. juliflora and its hybrids (Maundu et al., Citation2009). Based on the model developed by Maundu et al. (Citation2009) the northwest, east and northeast of Kenya, with large areas in western and southeastern Tanzania are a good bioclimatic match.
The unpalatable and allelopathic shrub Hyptis suaveolens is now regarded as one of world’s most noxious weeds displacing native plant species (Padalia et al., Citation2014) and physically competing for space and nutrients in grain crops and peanuts (Parsons & Cuthbertson, Citation1992). In northern Australia it is considered to pose the greatest threat to “rangeland biodiversity”. Locally abundant in parts of eastern Africa large parts of the region, which are currently uninvaded, such as most of Tanzania, southwestern Kenya, and northern Uganda are climatically a good match, based on the model developed by Padalia et al. (Citation2015).
The large shrub or climber Cryptostegia grandiflora climbs into trees, smothering native vegetation and often causing canopy collapse, to the detriment of native plant and animal species. In 1990 C. grandiflora was estimated to occupy more than 30,000 km2 in tropical Australia, “being described as the single biggest threat to natural ecosystems” (McFadyen & Harvey, Citation1990). It is highly toxic to both humans and animals with dense invasions reducing livestock carrying capacities in Australia by as much as 100% (Cook et al., Citation1990; Parsons & Cuthbertson, Citation1992; Paman, Citation2008). It is locally abundant and spreading rapidly on the western edge of the Awash National Park, Ethiopia, where it is smothering native Acacia species, and displacing valuable forage species, posing a threat to pastoralism in the region. According to the model developed by Kriticos et al. (2003) southwestern Ethiopia, much of Uganda, southwest Kenya and the coasts of Tanzania and Kenya are a good bioclimatic match.
Comparative magnitude of the East African problem
Our finding that more than 150 alien plant species are currently invading natural vegetation in eastern Africa indicates that the problem is serious and growing. We have not included any ruderal invasive alien plants, that is, introduced weeds which are abundant on roadsides and in croplands, and are not considered to pose a significant threat to biodiversity and livestock production in eastern Africa. We are also cognisant of the fact that many areas were not surveyed and that some smaller and less conspicuous herbaceous invasive alien plants may have been overlooked and as such not recorded. However, we are confident that all of the most conspicuous widespread and abundant invasive alien plants have been documented. This is supported by a recent desk-top assessment of invasive alien plant species in eastern Africa (excluding Ethiopia) (Lusweti et al., Citation2011) that only found 109 introduced plants to be invasive, which is probably a slight overestimate considering that some native or cosmopolitan species such as, among others, Dodonaea viscosa (L.) Jacq. (Sapindaceae), Cymbopogon nardus (L.) Rendle (Poaceae), Imperata cylindrica (L.) Beauv. (Poaceae), Achyranthes aspera L. (Amaranthaceae), Striga asiatica (L.) Kuntze (Scrophulariaceae) and introduced ruderal weeds such as Bidens pilosa L. (Asteraceae), Avena fatua L. (Poaceae), A. sterilis L. (Poaceae), Lolium temulentum L. (Poaceae) were also included. Pyšek et al. (Citation2017) found 16, 39, 6, 28 and 25 introduced plants to be invasive in Ethiopia, Kenya, Rwanda, Tanzania and Uganda, respectively. These are all much lower when compared to our findings. A review of the Invasive Species Compendium (ISC) (CABI, Citation2017) revealed that significantly fewer species have been recorded in the region with only 35, 27, 17, 11 and 5 invasive alien plants recorded in Tanzania, Kenya, Uganda, Ethiopia and Rwanda, respectively. This excluded species which we considered to be naturalised only, native, of uncertain origin, and ruderal or agricultural weeds. Based on our review of the country databases in the ISC, there are only 46 invasive alien plant species in eastern Africa (excluding Burundi) which is a gross underestimate, a reflection of the lack of research capacity and awareness in this field. Our database can therefore be considered to be comprehensive, despite the fact that we did not survey for invasive alien grasses and did not record invasive Eucalyptus and Pinus species, with the exception of P. patula and P. caribaea.
As far as we are aware there are no other regional invasive alien plant databases available in Africa, making regional comparisons near impossible. However, our data is supported by comparisons to the meagre data available for other African countries where surveys have been undertaken. Based on a review of Zimbabwean herbarium records 153, 154 and 84 alien plant species are regarded as being casual aliens, naturalised, and invasive species, respectively (Maroyi, Citation2012). However, as with other databases a large number of ruderal weeds such as Gomphrena celosioides Mart. (Amaranthaceae), Bidens pilosa L. (Asteraceae), Acanthospermum hispidum DC. (Asteraceae), Conyza species, Tagetes minuta L. (Asteraceae), Richardia scabra L. (Rubiaceae) and others, have been listed as invasive alien species. Based on our criteria fewer than 20 introduced plants, of those listed by Maroyi (Citation2012), would be considered to be invasive in Zimbabwe. In our opinion this is an underestimate, which is to be expected, considering that it was only a review of herbarium specimens and not based on the results of an active survey. In Angola, Rejmánek et al., (Citation2017) recorded 44 naturalised plant species, of which 19 were considered to be invasive. They found dense infestations of Chromolaena odorata, Inga vera Willd. (Fabaceae) and O. stricta, which they suggested would pose the greatest environmental and economic threats. Brown et al. (Citation1985) identified approximately 40 species of invasive alien plants in Namibia, listing (in order of priority) Salvinia molesta, Prosopis species, Nicotiana glauca, Datura species and Opuntia species as being of the greatest concern. More recently the Namibian list was increased to 57 invasive or potentially invasive terrestrial plant species in addition to more than six aquatic invasive plant species (Bethune et al., Citation2004). However, as is the case with the Zimbabwe database the Namibian list of terrestrial invasive plants includes a number of ruderal weeds.
South Africa has the most comprehensive and up-to-date database of invasive alien plants in the whole of Africa. Henderson’s (Citation2007) list is based on the South African Plant Invaders Atlas, a project that has run for over 30 years, with inputs from scores of observers. Although the list has recently been updated and now includes close on 800 species (Henderson & Wilson, Citation2017) we base our comparisons on the previous list. In South Africa, Swaziland and Lesotho Henderson (Citation2007) recorded 548 naturalised and casual alien plant species. She noted that the fynbos shrubland vegetation type was the most extensively invaded in South Africa, but that grassland and savanna vegetation was also heavily invaded. Species in the family Fabaceae were found to be prominent in all biomes, followed by species in the Rosaceae, Solanaceae and Asteraceae. Species in the Fabaceae also dominated the list of invasive alien plants in eastern Africa followed by species in the Asteraceae, Solanaceae and Cactaceae. Acacia mearnsii was by far the most prominent invasive species in South Africa, followed by Acacia saligna, A. cyclops, A. dealbata, Lantana camara, Opuntia ficus-indica, Solanum mauritianum, Populus alba, P. canescens, Melia azedarach and species in the genus Prosopis. Some of these species, such as L. camara and P. juliflora, were also found to be widespread and abundant in eastern Africa. All of the worst invasive species in South Africa are woody trees or shrubs, with the exception of the succulent Opuntia ficus-indica, which supports the findings of Henderson (Citation2007) that woody weeds dominate the invasive flora in South Africa. However, it needs to be recognised that roadside surveys are biased towards the larger, more conspicuous species and that herbaceous species are generally underestimated. This is in agreement with our surveys where 45% of the invasive species recorded in eastern Africa were woody trees and shrubs, followed by herbaceous plants and then climbers. In eastern Africa most of these species were being cultivated as ornamentals, barrier plants and agricultural crops which was similar to what was found in South Africa. Despite these broad similarities there are differences in that 80% of all of the recorded invasive species in eastern Africa have a tropical origin, compared to only 51% in South Africa, and more significantly only 15% of invasive plants in eastern Africa are from temperate regions compared to 48% in South Africa. Of interest, in South Africa the savanna and forest biomes are dominated by invasive species of tropical origin whereas the fynbos, grassland, Nama-Karoo, and Succulent Karoo biomes are mainly invaded by species from temperate regions.
There are also differences between South Africa and eastern Africa in terms of the length of time that alien species have been present. This is often called residence time and is an important factor in plant invasions (Rejmánek, Citation2000; Pyšek et al., Citation2004; Castro et al., Citation2005; Hamilton et al., Citation2005). In South Africa, the arrival of the Dutch in 1652 resulted in the first introduction of plants from other continents that would go on to become major invasives (Henderson, Citation2006). Of the 298 declared invasive plants under the Conservation of Agricultural Resources Act in South Africa, which is only a subset of all of the invasive alien plant species, 115 species were introduced in the 1800s compared to 103 species in the 1900s (Henderson, Citation2006). Most of the exotic plant species (117) currently invasive in eastern Africa were introduced in the 1900s and only 32 species were introduced prior to that.
There are a large number of additional factors which may be contributing to differences between the number of invasive alien plants in South Africa compared to eastern Africa. Factors such as propagule pressure, the abiotic characteristics of the invaded ecosystem and the characteristics of the recipient community and invading species (Catford et al., Citation2009) all need to be considered. Low investments in research and data collation may result in fewer documented invasive alien species (IAS) in developing countries (McNeely et al., Citation2005), but they may also have fewer IAS because of lower volumes of international trade and transport (McGeoch Citation2010).
Ability to quantify impacts
We know from anecdotal evidence, personal observations and a few scattered studies that invasive alien plants can have serious impacts on the integrity of ecosystems, and their ability to deliver a range of services to rural people whose livelihoods depend on the utilisation of natural resources (Shackleton et al. Citation2017a, b, c). As a consequence, people’s ability to make a living is compromised, and their quality of life decreases. Despite this, there are very few studies that have sought to quantify and document these impacts. In part, this arises from the early formulation of a research agenda by the SCOPE project on invasive alien species (Mooney et al., Citation2005), in which three key questions were identified: (1) which species become invasive? (2) which ecosystems are prone to invasions? and (3) how can the invasions be managed? Remarkably, the impacts generated by invasive alien species were not identified as a key question. Today, the lack of clear evidence of impacts causes a number of problems, including not being able to adequately support proposals aimed at securing funding for research and management, and for convincing governments to take the problem seriously. Although there have been efforts to develop a generic system whereby the magnitude of different impacts can be directly, consistently and transparently compared, such as the Environmental Impact Classification for Alien Taxa (EICAT) (Blackburn et al., Citation2014; Hawkins et al., Citation2015), and more recently a classification scheme which is closely aligned to EICAT to also rank socio-economic impacts (SEICAT) (Bacher et al., Citation2018), little data is actually available in order to rank any of these impacts. The quantification of the impacts of invasive alien species should therefore be given priority, especially in areas where the problem is less well appreciated, such as the developing countries of eastern Africa.
Appropriate responses
The main barriers to effective management of invasive alien plants in eastern Africa are the lack of appropriate policies and/or the implementation thereof; insufficient capacity especially with regard to the identification and management of invasive plants; lack of awareness among government officials and other stakeholders as to the impacts of invasive plants on biodiversity, water resources, crop and pasture production, human and animal health, and economic development; and an absence of sufficient resources to tackle the issue at a national or regional level. Two of the countries in eastern Africa – Ethiopia and Uganda – have developed National Invasive Species Strategies and Action Plans (Boy & Witt, Citation2013), although implementation remains a challenge. This is supported by McGeoch (Citation2010) who found that many countries have inadequate IAS strategies, deficient IAS management plans, and ineffective implementation of those plans. A lack of capacity is one of the main reasons for these deficiencies (McGeoch et al., Citation2006).
Another major impediment to management, which needs to be resolved, is the practice of ascribing some useful properties to even the most highly invasive and damaging alien plant species. Often, such perceptions are based on arguments perpetuated by international development agencies and others, but which have long since been discredited. The persistence of positive attitudes towards some invasive plants continues to hamper implementation of much-needed management interventions Witt (Citation2017). Only where a plant invader is limited in distribution and is present in very low densities are communities of people anywhere likely to benefit. Any benefits the invader might once have provided will soon be outweighed by the increasingly destructive consequences of its spread (Nuñez et al., Citation2012).
The difficulties in “selling” invasive species as a serious issue which requires immediate action and possible solutions have been well summarised by Courchamp et al. (Citation2017). Many of these issues also apply to the situation in eastern Africa where IAS concepts are poorly understood, including the definition of the term IAS, and it has been difficult to show impacts, especially those on biodiversity because of a lack of baseline data. Solutions include better definitions and a new communication model. A major impediment in eastern Africa, and much of the developing world for that matter, is also the perception that IAS are only a biodiversity issue, despite the fact that IAS have a significant impact on livelihoods in Africa (Shackleton et al., Citation2017a, b, c). Biodiversity issues are not at the top of the development agenda for most countries in Africa, possibly as a result of the fact that the links between biodiversity protection and the broader socio-economic welfare of human societies remain poorly understood. As such, research should possibly focus more on the socio-economic impacts of IAS to demonstrate the significant impacts of IAS on livelihoods. Research should also focus on the costs and benefits of IAS management. There are very few studies which have clearly demonstrated the environmental benefits of IAS control, especially for those invasive plant species which have a negative impact on biodiversity or rangeland productivity (Barton et al., Citation2007; Baider & Florens, Citation2011; Ndhlovu et al., Citation2011; Fill et al., Citation2017).
Endorsement or support of management interventions also remains a challenge. Despite the immense scale of the problem and the continuing lack of available resources in most developing African countries, much more emphasis needs to be placed on cost-effective management practices, such as biological control. The costs of sustained physical and chemical control are prohibitive in many developing countries, particularly given the scale of the interventions that are required in cases where millions of hectares have been invaded (Witt & Luke, Citation2017; Shackleton et al., Citation2017a, b, c). This means that only the most cost-effective IAS control options are likely to stand any chance of real success in eastern Africa, if progress is to be made over the coming years in the battle to keep invasive plants and their impacts at bay.
Biological control, which has been practised widely around the world and found to be safe and cost-effective (van Wilgen et al., Citation2004; Moran et al., Citation2005; Winston et al., Citation2014) may have the added advantage of helping to resolve conflicts of interest, particularly over beneficial and commercially valuable agro-forestry species that are also invasive. Introduced bud-galling or seed-feeding insects that reduce the reproductive potential of invasive plants, but which otherwise have no impact on the growth of these useful species, help to ensure that control can be achieved without at the same time sacrificing economic prospects.
There is still a tendency, among donors and global development and aid agencies, either to ignore the problem or to underestimate the extent to which it is responsible for so much of the poverty and suffering in Africa. What is needed, to help galvanise awareness and spur action, is a concerted global marketing campaign along the lines of the hugely successful campaign that was waged in the early 1980s drawing attention to the scourge of HIV/Aids. The critical mass generated by such a campaign might ensure that IAS issues are incorporated, as they should be, as one of the central components in all donor-funded development programmes. The enormity and the growing severity of the IAS problem is such that nothing short of a concerted global campaign – eliciting massive long-term commitment and funding from all major donor nations, international development and aid agencies and NGOs – is needed, if this scourge is to be brought under control.
Development agencies such as the Centre for Agriculture and Bioscience International (CABI) have attempted to remove some of these barriers by hosting numerous regional workshops on the identification and management of invasive species, disseminating awareness material, and developing identification guides (Witt & Luke, Citation2017; Witt, Citation2017). A number of regional Global Environment Facility (GEF) supported IAS projects such as “Removing Barriers to Invasive Plant Management in Africa” have been implemented by United Nations Environment and executed by CABI, which has contributed to increased capacity and awareness. However, more needs to be done and new approaches developed and implemented.
Supplemental Material
Download PDF (482.8 KB)ACKNOWLEDGEMENTS
Thanks to the country partners for their logistical, and other support, during survey activities, and for the provision of additional data on the presence and distribution of invasive plants. Winnie Nunda who kindly entered much of the data which facilitated the production of the distribution maps.
DISCLOSURE STATEMENT
No potential conflict of interest was reported by the authors.
Additional information
Funding
REFERENCES
- Aggarwal, A. & Kohli, R.K. 1992. Screening of crops for seeding for seed germination against Parthenium hysterophorus L. leachates. In Tauro, P. & Narwal, S.S. (Eds), Proceedings of the 1st National Symposium on Allelopathy in Agroecosystems, February 1992, Haryana Agricultural University, Hisar, India.
- Agnew, A.D.Q. & Agnew, S. 1994. Upland Kenya Wild Flowers. A Flora of the Ferns and Herbaceous Flowering Plants of Upland Kenya, 2nd edn. Nairobi, East Africa Natural History Museum.
- Bacher, S., Blackburn, T.M., Essl, F., Genovesi, P., Heikkilä, J., Jeschke, J.M., Jones, G., Keller, R., Kenis, M., Kueffer, C., Martinou, A.F., Nentwig, W., Pergl, J., Pyšek, P., Rabitsch, W., Richardson, D.M., Roy, H.E., Saul, W-C., Scalera, R., Vilà, M., Wilson, J.R.U. & Kumschick, S. 2018. Socio-economic impact classification of alien taxa (SEICAT). Methods in Ecology and Evolution 9(1): 159–168 doi: 10.1111/2041-210X.12844
- Baider, C. & Florens, F.B.V. 2011 Control of invasive alien weeds averts imminent plant extinction. Biological Invasions 13(12): 2641–2646. DOI:10.1007/s10530-011-9980-3.
- Barton, J., Fowler, S.V., Gianotti, A.F., Winks, C.J., de Beurs, M., Arnold, G.C. & Forrester, G. 2007. Successful biological control of mist flower (Ageratina riparia) in New Zealand: agent establishment, impact and benefits to the native flora. Biological Control 40: 370–385. doi: 10.1016/j.biocontrol.2006.09.010
- Beentje, H.J. 1994. Kenya Trees, Shrubs and Lianas. Nairobi, National Museums of Kenya.
- Bethune, S., Griffin, M. & Joubert, D. 2004. National review of invasive alien species, Namibia. Southern Africa Biodiversity Support Programme, Directorate of Environmental Affairs, Ministry of Environment and Tourism, Windhoek, Namibia.
- Birnie, A. & Noad, T. 2011. Trees of Kenya – An Illustrated Field Guide (3rd edition). Koeltz Scientific Books, Nairobi, Kenya.
- Blackburn, T.M., Pyšek, P., Bacher, S., Carlton, J.T., Duncan, R.P., Jarošík, V., Wilson, J.R.U. & Richardson, D.M. 2011. A proposed unified framework for biological invasions. Trends in Ecology and Evolution 26: 333–339. doi: 10.1016/j.tree.2011.03.023
- Blackburn, T.M., Essl, F., Evans, T., Hulme, P.E., Jeschke, J.M., Kühn, I. & Bacher, S. (2014). A unified classification of alien taxa based on the magnitude of their environmental impacts. PLoS Biology, 12, e1001850. doi: 10.1371/journal.pbio.1001850
- Bogdan, A.V. 1950. A list of weeds of the Kenya Highlands. East African Agriculture and Forestry Journal 15(3): 118–123. doi: 10.1080/03670074.1950.11664723
- Bogdan, A.V. 1965. Weeds in Kenya wheat. Weed Research 5: 351–352. doi: 10.1111/j.1365-3180.1965.tb00364.x
- Boy, G. & Witt, A.B.R. 2013. Invasive Alien Plants and their Management in Africa. Nairobi, CABI Africa.
- Brown, C.J., MacDonald, I.A.W. & Brown, S.E. 1985. Invasive Alien Organisms in South West Africa/Namibia. South African National Scientific Programmes Report No. 119, CSIR, Pretoria, South Africa.
- Brown, L.S., Boudjelas, S. & De Poorter, M. 2004. 100 of the World's Worst Invasive Alien Species: A Selection from the Global Invasive Species Database. Auckland, The Invasive Species Specialist Group (ISSG), Species Survival Commission (SSC) of the World Conservation Union (IUCN).
- CABI 2017. Invasive Species Compendium. Wallingford, CAB International, www.cabi.org/isc (accessed 3 May 2017).
- Castro, S.A., Figueroa, J.A., Muñoz-Schick, M. & Jaksic, F.M. 2005. Minimum residence time, biogeographical origin, and life cycle as determinants of the geographical extent of naturalized plants in continental Chile. Diversity and Distributions 11: 183–191. doi: 10.1111/j.1366-9516.2005.00145.x
- Catford, J.A., Jansson, R. & Nilsson, C. 2009. Reducing redundancy in invasion ecology by integrating hypotheses into a single theoretical framework. Diversity and Distributions 15: 22–40. doi: 10.1111/j.1472-4642.2008.00521.x
- Cook, D.R., Campbell, G.W. & Meldrum, A.R. 1990. Suspected Cryptostegia grandiflora (rubber vine) poisoning in horses. Australian Veterinary Journal 67(9): 344. doi: 10.1111/j.1751-0813.1990.tb07825.x
- Courchamp, F., Fournier, A., Bellard, C., Bertelsmeier, C., Bonnaud, E., Jeschke, J.M. & Russell, J.C. 2017. Invasion biology: specific problems and possible solutions. Trends in Ecology and Evolution (Amsterdam). 32: 13–22. doi: 10.1016/j.tree.2016.11.001
- Dale, I.R. 1953. A Descriptive List of the Introduced Trees of Uganda Protectorate. Entebbe, Government Printer.
- Dawson, W., Mndolwa, A.S., Burslem, D.F.R.P. & Hulme, P.E. 2008. Assessing the risks of plant invasions arising from collections in tropical botanical gardens. Biodiversity and Conservation 17(8): 1979–1995. doi: 10.1007/s10531-008-9345-0
- Dean, W.R.J., Anderson, M.D., Milton, S.J. & Anderson, T.A. 2002. Avian assemblages in native Acacia and alien Prosopis drainage line woodland in the Kalahari. South African Journal of Arid Environments 51: 1–19. doi: 10.1006/jare.2001.0910
- Dregne, H.E. 2002. Land degradation in the drylands. Arid Land Research and Management 16: 99–132. doi: 10.1080/153249802317304422
- Drummond, R.B. 1984. Arable Weeds of Zimbabwe: A Guide to the Recognition of the more Important Weeds of Crops. Harare, Agricultural Research Trust of Zimbabwe.
- Dzikiti, S., Schachtschneider, K., Naiken, V., Gush, M., Moses, G. & Le Maitre, D.C. 2013. Water relations and the effects of clearing invasive Prosopis trees on groundwater in an arid environment in the Northern Cape. South African Journal of Arid Environments 90: 103–113. doi: 10.1016/j.jaridenv.2012.10.015
- El Hadidi, M.N., Hosny, A.I. & El Husseini, N. 1996. Some aspects of the biodiversity of the weed flora in the farmlands of Egypt. In van der Maesen, L.J.G., van der Burgt X.M. & van Medenbach de Rooy J.M. (Eds), The Biodiversity of African Plants. Dordrecht, Springer.
- Engler, H.G.A. 1895a. Grundzuge der Planzenverbreitung in Deutsch-Ost-Afrika und den Nachbargebieten. In Engler, A. (Ed.), Deutsch-Ost-Afrika – Die Pflanzenwelt Ost-Afrikas und der Nachbargebiete, Band V – Theil A. Berlin, Geographische Verlagshandlung Dietrich Reimer.
- Engler, H.G.A. 1895b. Verzeichnis der bis jetzt aus Ost-Afrika bekannt gewordenen Pflanzen. In Engler, A. (Ed.) Deutsch-Ost-Afrika – Die Pflanzenwelt Ost-Afrikas und der Nachbargebiete, Band V – Theil C. Berlin, Geographische Verlagshandlung Dietrich Reimer.
- Evans, H.C. 1997. Parthenium hysterophorus L.: A review of its weed status and possibilities for biological control. Biocontrol News and Information 18(3): 89–98.
- Fill, J.M., Forsyth, G.G., Kritzinger-Klopper, S., Le Maitre, D.C. & van Wilgen, B.W. 2017. An assessment of the effectiveness of a long-term ecosystem restoration project in a fynbos shrubland catchment in South Africa. Journal of Environmental Management 185: 1–10. doi: 10.1016/j.jenvman.2016.10.053
- Germain, R. 1952. Les associations végétales de la plaine de la Ruzizi (Congo Belge) en relation avec le milieu. Publications de l'Institut National Pour l'étude Agronomique du Congo Belge, Série Scientifique 52: 1–321.
- Global Invasive Species Database (GISD) (2017). www.issg.org/database (accessed 8 July 2017).
- Government Gazette (1899–1964) http://kenyalaw.org/kl/index.php?id=3386https://books.google.co.ke/books/about/Kenya_Gazette.html?id=SiZddRcP0BcC&hl=en (accessed 7 July 2016)
- Great Britain Colonial Office 1952. Flora of Tropical East Africa. London, Crown Agents for Overseas Governments.
- Greenway, P.J. 1934. Report of a botanical survey of the indigenous and exotic plants in cultivation at the East African Agricultural Research Station, Amani, Tanganyika Territory. East African Agricultural Research Station, Amani, Tanganyika.
- Greenway, P.J. 1941. Dyeing and tanning plants in East Africa. Bulletin of the Imperial Institute 35: 222–245.
- Hamilton, M.A., Murray, B.R., Cadotte, M.W., Hose, G.C., Baker, A.C., Harris, C.J. & Licari, D. 2005. Life-history correlates of plant invasiveness at regional and continental scales. Ecology Letters 8: 1066–1074. doi: 10.1111/j.1461-0248.2005.00809.x
- Hawkins, C.L., Bacher, S., Essl, F., Hulme, P.E., Jeschke, J.M., Kühn, I. & Blackburn, T.M. 2015. Framework and guidelines for implementing the proposed IUCN environmental impact classification for alien taxa (EICAT). Diversity and Distributions, 21: 1360–1363. doi: 10.1111/ddi.12379
- Haysom, K.A. & Murphy, S.T. 2003. The status of invasiveness of forest tree species outside their natural habitat: A global review and discussion paper. Forest Health and Biosecurity Working Paper FBS/3E. Forestry Department, FAO, Rome. http://www.fao.org/docrep/006/J1583E/J1583E00.HTM (accessed 6 July 2017).
- Henderson, L. 2006. Comparisons of invasive plants in southern Africa originating from southern temperate, northern temperate and tropical regions. Bothalia 36: 201–222. doi: 10.4102/abc.v36i2.362
- Henderson, L. 2007. Invasive, naturalized and casual alien plants in southern Africa: a summary based on the Southern African Plant Invaders Atlas (SAPIA). Bothalia 37: 215–248. doi: 10.4102/abc.v37i2.322
- Henderson, L. & Wilson, J.R.U. 2017. Changes in the composition and distribution of alien plants in South Africa: An update from the Southern African Plant Invaders Atlas. Bothalia 47(2): a2172. https://doi.org/10.4102/abc.v47i2.2172.
- Hutchins, D.E. 1909. Report on the Forests of British East Africa. London, Darling and Son.
- IUCN/PACO 2013. Invasive Plants Affecting Protected Areas of West Africa. Management for reduction of risk for biodiversity. Gland, IUCN and Burkino Faso, Ouagadougou.
- Ivens, G. 1967. East African Weeds and their Control. Nairobi, Oxford University Press.
- Kebede, T.A. & Coppock, L.D. 2015. Livestock-mediated dispersal of Prosopis juliflora imperils grasslands and the endangered Grevy's Zebra in Northeastern Ethiopia. Rangeland Ecology and Management 68(5): 402–407. doi: 10.1016/j.rama.2015.07.002
- Kirui, O. & Mrzabaev, A. 2016. Cost of land degradation and improvement in Eastern Africa. In Fifth International Conference of African Association of Agricultural Economics (AAAE), 23–26 September 2016 http://ageconsearch.umn.edu/record/249321/files/135.%20Cost%20of%20land%20improvement.pdf (accessed 7 July 2017).
- Kriticos, D.J., Sutherst, R.W., Brown, J.R., Adkins, S.W. & , Maywald, G.F. 2003. Climate changing and biotic invasions: a case history of a tropical weed vine. Biological Invasions 5(3): 147–165. doi: 10.1023/A:1026193424587
- Kriticos, D.J., Yonow, T. & McFadyen, R.E. 2005. The potential distribution of Chromolaena odorata (Siam weed) in relation to climate. Weed Research 45: 246–254. doi: 10.1111/j.1365-3180.2005.00458.x
- Lubini, A. 1986. Végétation adventice et postculturale de Kisangani et de la Tshopo (Haut-Zaïre). Bulletin du Jardin Botanique National de Belgique – Bulletin van de Nationale Plantentuin van België 56: 315–348. doi: 10.2307/3668196
- Lusweti, A., Wabuyele, E., Ssegawa, P. & Mauremootoo, J.R. 2011. Invasive plants of East Africa. www.keys.lucidcentral.org/keys/v3/plants.htm (accessed 7 July 2017).
- Maroyi, A. 2006. A preliminary checklist of naturalized and introduced plants in Zimbabwe. Kirkia 18: 77–247.
- Maroyi, A. 2012. The casual, naturalised and invasive alien flora of Zimbabwe based on herbarium and literature records. Koedoe 54(1), Art. #1054, 6 pages. http://doi.org/10.4102/koedoe. v54i1.1054. doi: 10.4102/koedoe.v54i1.1054
- Maundu, P., Kibet, S., Morimoto, Y., Imbumi, M. & Adekar, R. 2009. Impact of Prosopis juliflora on Kenya's semi-arid and arid ecosystems and local livelihoods. Biodiversity 10(23): 33–50. doi: 10.1080/14888386.2009.9712842
- McConnachie, A.J., Strathie, L.W., Mersie, W., Gebrehiwot, L., Zewdie, K., Abdurehim, A., Abrha, B., Araya, T., Asaregew, F., Assefa, F., Gebre-Tsadik, R., Nigatu, L., Tadesse, B. & Tana, T. 2010. Current and potential geographical distribution of the invasive plant Parthenium hysterophorus (Asteraceae) in eastern and southern Africa. Weed Research 51: 71–84. doi: 10.1111/j.1365-3180.2010.00820.x
- McFadyen, R.E. 1992. Biological control against parthenium weed in Australia. Crop Protection 24: 400–407. doi: 10.1016/0261-2194(92)90021-V
- McFadyen, R.E.C. & Harvey, G.J., 1990. Distribution and control of rubber vine, Cryptostegia grandiflora, a major weed in northern Queensland. Plant Protection Quarterly 5(4): 152–155.
- McFadyen, R.E.C. & Skarrat, B. 1996. Potential distribution of Chromolaena odorata (Siam weed) in Australia, Africa and Oceania. Agriculture, Ecosystems and Environment 59: 89–96. doi: 10.1016/0167-8809(96)01035-3
- McGeoch, M.A., Butchart, S.H.M., Spear, D., Marais, E., Kleynhans, E.J., Symes, A., Chanson, J. & Hoffmann, M. 2010. Global indicators of biological invasion: species numbers, biodiversity impact and policy responses. Diversity and Distributions 16: 95–108. doi: 10.1111/j.1472-4642.2009.00633.x
- McGeoch, M.A., Chown, S.L. & Kalwij, J.M. 2006. A global indicator for biological invasion. Conservation Biology 20: 1635–1646. doi: 10.1111/j.1523-1739.2006.00579.x
- McNeely, J.A., Mooney, H.A., Neville, L.E., Schei, P.J. & Waage, J. K. 2005. A global strategy on invasive alien species: synthesis and ten strategic elements. In H.A. Mooney, H.A., Mack, R.N., McNeely, J.A., Neville, L.E., Schei, P.J. & Waage, J.K. (Eds), Invasive Alien Species: A new synthesis. Washington, DC, Island Press.
- Milbau, A., Jessen, B., Shevtsova, A.G. & Nijs, I. 2009. Effects of a warmer climate on seed germination in the subarctic. Annals of Botany 104(1): 287–296. doi: 10.1093/aob/mcp117
- Mooney, H.A., Mack, R.N., McNeely, J.A., Neville, L.E., Schei, P.J. & Waage, J.K. (Eds) 2005. Invasive Alien Species: A New Synthesis, Vol. 63 of SCOPE. Washington, DC, Island Press.
- Moran, V.C., Hoffmann, J.H. & Zimmermann, H.G. 2005. Biological control of invasive alien plants in South Africa: Necessity, circumspection, and success. Frontiers in Ecology and the Environment, 3(2): 77–83 doi: 10.2307/3868513
- Mosango, M. 1983a. Une estimation du niveau d’accumulation des diaspores dans les sols des groupements herbacés à Portulaca quadrifida L. et à Talinum triangulare Wild. des environs de Kisangani (Haut-Zaïre). Bulletin de la Société Royale de Botanique de Belgique, 116: 55–61.
- Mosango, M. 1983b. Influence des plantes adventices sur les plantes de culture: quelques résultats. Journal d'agriculture Traditionnelle et de Botanique Appliquée, 30(1): 35–48. doi: 10.3406/jatba.1983.3886
- Mullenders, W. 1954. La végétation de Kaniama (entre – Lubishi – Lubilash, Congo). Brussels, Publications de l'Institut National pour l'étude Agronomique du Congo Belge. Série Scientifique, Vol. 61, 499 pp.
- Mwangi, E. & Swallow, B. 2008. Prosopis juliflora invasion and rural livelihoods in the Lake Baringo area of Kenya. Conservation and Society 6: 130–140. doi: 10.4103/0972-4923.49207
- Ndhlovu, T., Milton-Dean, S.J. & Esler, K.J. 2011. Impact of Prosopis (mesquite) invasion and clearing on the grazing capacity of semiarid Nama Karoo rangeland, South Africa. African Journal of Range and Forage Science 28: 129–137. doi: 10.2989/10220119.2011.642095
- Nicholson, S. 1996. A review of climate dynamics and climate variability in eastern Africa, the limnology, climatology, and paleoclimatology of the East African Lakes. In Johnson, T.C. & Odada, E.O. (Eds), The Limnology, Climatology, and Paleoclimatology of the East African Lakes. New York, Gordon and Breach.
- Nuñez, M.A., Kuebbing, S., Dimarco, R.D. & Simberloff, D. 2012. Invasive species: to eat or not to eat, that is the question. Conservation Letters 5: 334–341. doi: 10.1111/j.1755-263X.2012.00250.x
- Padalia, I., Srivastava, V. & Kushwaha, S.P.S. 2014. Modelling potential invasion range of alien invasive species, Hyptis suaveolens (L.) Poit. in India: Comparison of MaxEnt and GARP. Ecological Informatics 22: 36–43. doi: 10.1016/j.ecoinf.2014.04.002
- Padalia, I., Srivastava, V. & Kushwaha, S.P.S. (2015) How climate change might influence the potential distribution of weed, bushmint (Hyptis suaveolens)? Environmental Monitoring and Assessment 187(210): 1–14.
- Paman, J. 2008. Poisonous rubber vine needs to be controlled. http://mauiinvasive.org/2011/12/08/poisonous-rubber-vine-needs-to-be-controlled/ (accessed 7 July 2017).
- Parsons, W.T. & Cuthbertson, E.G. 1992. Noxious Weeds of Australia. Melbourne, Inkata Press.
- Pasiecznik, N.M., Felker, P., Harris, P.J.C., Harsh, L.N., Cruz, G., Tewari, J.C., Cadoret, K. & Maldonado, L.J. 2001. The Prosopis juliflora – Prosopis pallida Complex: A Monograph. Coventry, HDRA.
- Patel, S. 2011. Harmful and beneficial aspects of Parthenium hysterophorus: an update. Biotechnology 1: 1–9.
- Pyšek, P., Pergl, J., Ess, F., Lenzner, B., Dawson, W., Kreft, H. & vanKluenen, M. 2017. Naturalized alien flora of the world: species diversity, taxonomic and phylogenetic patterns, geographic distribution and global hotspots of plant invasion. Preslia 89: 203–274. doi: 10.23855/preslia.2017.203
- Pyšek, P., Richardson, D.M., Rejmánek, M., Webster, G.L., Williamson, M. & Kirschner, J. 2004. Alien plants in checklists and floras: Towards better communication between taxonomists and ecologists. Taxon 53: 131–143. doi: 10.2307/4135498
- Raimundo, R.L.G., Fonseca, R.L., Schachetti-Pereira, R., Townsend Peterson, A. & Lewinsohn, T.M. 2007. Native and exotic distributions of Siam weed (Chromolaena odorata) modelled using the genetic algorithm for rule-set production. Weed Science 55(1): 41–48. doi: 10.1614/WS-06-083.1
- Rejmánek, M. 1996. Species richness and resistance to invasions. In Orians, G.H., Dirzo, R. & Cushman, J.H. (Eds), Biodiversity and Ecosystem Processes in Tropical Forests. Ecological Studies (Analysis and Synthesis), Vol. 122. Berlin/Heidelberg, Springer.
- Rejmánek, M. 2000. Invasive plants: approaches and predictions. Austral Ecology 25(5): 497–506.
- Rejmánek, M., Le Roux, J.J., Huntley, B.J. & Richardson, D.M. 2017. Rapid assessment survey of the invasive plant species in western Angola. African Journal of Ecology 55(1): 56–69. doi: 10.1111/aje.12315
- Richardson, D.M., Pyšek, P. & Carlton, J.T. 2011. A compendium of essential concepts and terminology in invasion ecology. In Richardson, D.M. (Ed.), Fifty Years of Invasion Ecology: The legacy of Charles Elton. Oxford, Wiley-Blackwell. pp. 409–420.
- Richardson, D.M., Pyšek, P., Rejmánek, M., Barbour, M.G., Panetta, F.D. & West, C.J. 2000. Naturalization and invasion of alien plants: concepts and definitions. Diversity & Distributions 6: 93–107. doi: 10.1046/j.1472-4642.2000.00083.x
- Schachtschneider, K. & February, E.C. 2013. Impact of prosopis invasion on a keystone tree species in the Kalahari Desert. Plant Ecology 214: 597–605. doi: 10.1007/s11258-013-0192-z
- Schmitz, A. 1971. La végétation de la plaine de Lubumbashi (Haut-Katanga). Publications de l'Institut National pour l'étude Agronomique du Congo Belge, Série Scientifique 113: 1–406.
- Shackleton, R.T., Le Maitre, D.C. & Richardson, D.M. 2015. Stakeholder perceptions and practices regarding prosopis (mesquite) invasions and management in South Africa. Ambio 44: 569–581. doi: 10.1007/s13280-014-0597-5
- Shackleton, R.T., Witt, A.B.R., Nunda, W. & Richardson, D.M. 2017a. Chromolaena odorata (Siam weed) in eastern Africa: distribution and socio-ecological impacts. Biological Invasions 19: 1285–1298. doi: 10.1007/s10530-016-1338-4
- Shackleton, R.T., Witt, A.B.R., Aool, W. & Pratt, C. 2017b. Distribution of the invasive alien weed, Lantana camara, and its ecological and livelihood impacts in eastern Africa. African Journal of Range and Forage Science 34(1):1–11. doi: 10.2989/10220119.2017.1301551
- Shackleton, R.T., Witt, A.B.R., Piroris, F.M. & van Wilgen, B.W. 2017c. A survey of the distribution and perceptions of the socio-economic and ecological impacts of the invasive alien cactus Opuntia stricta in East Africa. Biologcal Invasions 19(8): 2427–2441. doi: 10.1007/s10530-017-1453-x
- Sheil, D. 1994. Invasive plants in tropical forests: Warnings from the Amani Botanic Gardens, Tanzania. Botanical Gardens Conservation News 2(3): 23–24.
- Sheil, D. 2008. Naturalized and invasive plant species in the evergreen forests of the East Usambara Mountains, Tanzania. African Journal of Ecology 32(1): 66–71. doi: 10.1111/j.1365-2028.1994.tb00556.x
- Steenkamp, H.E. & Chown, S.L. 1996. Influence of dense stands of an exotic tree Prosopis glandulosa Benson, on a savanna dung beetle (Coleoptera: Scarabeidae) assemblage in southern Africa. Biological Conservation 78: 305–311. doi: 10.1016/S0006-3207(96)00047-X
- Swaziland National Trust Commission (SNTC) 2016. Swaziland’s Alien Plants Database. http://www.sntc.org.sz/alienplants/speciesstatus.asp (accessed 7 July 2017).
- Tamado, T. & Milberg, P. 2000. Weed flora in arable fields of eastern Ethiopia with emphasis on the occurrence of Parthenium hysterophorus. Weed Research 40: 507–521. doi: 10.1046/j.1365-3180.2000.00208.x
- Taylor, S., Kumar, L. & Reid, N. 2012. Impacts of climate change and land-use on the potential distribution of an invasive weed: a case study of Lantana camara in Australia. Weed Research. 52: 391–401. doi: 10.1111/j.1365-3180.2012.00930.x
- Terry, P.J. 1984. A Guide to Weed Control in East African Crops. Kenya Literature Bureau, Nairobi, Kenya.
- Terry, P. J. & Michieka, R.W. 1987. Common Weeds of East Africa. Rome, FAO.
- Towers, G.H.N. & Mitchell, J.C. 1983. The current status of the weed Parthenium hysterophorus L. as a cause of allergic contact dermatitis. Contact Dermatitis 9: 465–469. doi: 10.1111/j.1600-0536.1983.tb04465.x
- Van Wilgen, B.W., de Witt, M.P., Anderson, H.J., Le Maitre, D.C., Kotze, I.M., Ndala, S., Brown, B. & Rapholo, M.B. 2004. Costs and benefits of biological control of invasive alien plants. Case studies from South Africa. South African Journal of Science 100: 113–122.
- van Wilgen, B.W., Davies, S.J. & Richardson, D.M. 2014. Invasion science for society: a decade of contributions from the Centre for Invasion Biology. South African Journal of Science 110(7/8), Art. A0074. doi: 10.1590/sajs.2014/a0074
- Wild, H. 1955. Common Rhodesian Weeds. Salisbury, Rhodesia, Government Printer.
- Williams, R.O. 1949. The Useful and Ornamental Plants in Zanzibar and Pemba, 1st edn. Zanzibar Protectorate.
- Winston, R.L., Shwarzländer, M., Hinz, H.L., Day, M.D., Cock, M.J.W. & Julien, M.H. (Eds) 2014. Biological Control of Weeds: A World Catalogue of Agents and Their Target Weeds, 5th edn. FHTET-2014-04. Morgantown, VA, USDA Forest Service, Forest Health Technology Enterprise Team.
- Witt, A.B.R. 2017. Use of non-native species for poverty alleviation in developing economies. In Vilá, M. & Hulme, P.E. (Eds), Impact of Biological Invasions on Ecosystem Services. Invading Nature-Springer Series in Invasion Ecology 12. Cham, Springer.
- Witt, A.B.R., Kiambi, S., Beale, T. & van Wilgen, B.W. 2017. A preliminary assessment of the extent and potential impacts of alien plant invasions in the Serengeti- Mara ecosystem, East Africa. Koedoe 59(1): a1426. https://doi.org/10.4102/koedoe. v59i1.1426.
- Witt, A.B.R. & Luke, Q. 2017. Guide to the Naturalized and Invasive Plants of Eastern Africa. Wallingford, CAB International.