Abstract
Spider species distribution in the Cape Floristic Kingdom (CFK) was compiled as part of the South African National Survey of Arachnida (SANSA), whose main aim was to create a relational database for the country’s arachnid fauna. Data from the CFK was extracted from taxonomic and faunistic published papers, as well as unpublished faunistic survey data in national collections. A total of 11 500 records from 130 localities were recorded in the CFK until the end of 2023, representing 62 families, 334 genera and 960 species, with two further families (Synotaxidae and Theridiosomatidae) only known from undescribed species. This represents 42.4% of the total spider fauna of South Africa. For each species, the global and CFK distribution, as well as the level of endemicity and a conservation assessment using the IUCN Red List criteria, are provided. A total of 269 spp. (28.0%) are endemic to the CFK, 49 spp. (5.1%) are of special concern, and 229 spp. (23.9%) are Data Deficient. However, most of the species (682 spp., 71.0%) have a wide distribution with no known threats and are categorised as Least Concern. Salticidae is the most species-rich family (128 spp.), with 30 spp. endemic to the CFK, followed by the Gnaphosidae (107 spp.), Thomisidae (86 spp.) and Lycosidae (49 spp.), while six families are represented by a single species. The last decade has seen an exponential growth in the knowledge of spiders in South Africa, and there are certainly many more species that must still be discovered and described.
INTRODUCTION
To meet the requirements of the Convention on Biological Diversity (CBD), of which South Africa was one of the signatories, the South African National Survey of Arachnida (SANSA) was initiated in 1997, with the main aim to document the arachnid fauna of South Africa and collate all the data into a relational database (Dippenaar-Schoeman et al., Citation2015). The second phase of SANSA (2006–2010) was especially important, as it was funded by the Royal Norwegian Ministry through the Threatened Species Programme of the South African National Biodiversity Institute (SANBI) (Dippenaar-Schoeman et al., Citation2015) to do inventories inlarge parts of South Africa. Biodiversity inventories are essential to collect, identify and document species to determine their range in an area. This is to recognise key areas for conservation and to monitor the effects of threats on species (Balmford and Gaston, Citation1999). With the availability of this data, one of the first results of SANSA was the release of the First Atlas of the Spiders of South Africa in 2010 (Dippenaar-Schoeman et al., Citation2010), listing 2003 species with georeferenced records and distributional maps. The last decade has, however, seen considerable growth in the knowledge of spiders in South Africa, and the number of species known from South Africa has increased to 2265 species (Dippenaar-Schoeman et al., Citation2023a, Citation2023b).
The recently emerged field of conservation biogeography is concerned with the distributional dynamics of species and how they relate to the conservation of biodiversity (Robertson et al., Citation2010). Specimens in natural history collections play a vital role in achieving the CBD goals, i.e., to discover and make an inventory of a group, and to database the primary data to manage the information and make it available in an efficiently retrievable form. Primary data collected with specimens housed in collections provide extremely valuable biodiversity information to science (Robertson et al., Citation2010; Smith et al., Citation2003), and most of the information about South African biodiversity originates from the country’s natural history collections (Drinkrow et al., Citation1994; Hamer, Citation2012; Jacobs, Citation2020; Scoble, Citation2010). To document and conserve the biodiversity of a group, correct species identification and distribution data are of central importance (Bengtsson et al., Citation1997) to document their conservation status (Foord et al., Citation2020). Most spider taxonomic research in South Africa was undertaken during the period from 1700 to 1899, focusing largely on the fauna of the coastal provinces, as most of the practicing arachnologists were stationed there. From 1960 to 1980 there was a considerable decline in the description of new species, but during the last three decades there has been a resurgence; more than 500 species have been described since 1980, mainly due to modern taxonomic revisions, the training of South African taxonomists and the efforts of overseas taxonomists.
The Cape Floristic Region (CFK) is a region of exceptional floral diversity and recognised as one of the six floral kingdoms of the world (Goldblatt, Citation1997). The Fynbos Biome, which is part of the CFK, is a botanical hotspot and is renowned globally for its exceptionally high plant diversity and endemism (Schnitzler et al., Citation2011). It also has one of the highest known incidences of local floral endemism in the world (Cowling et al., Citation1998), but faces numerous threats from anthropogenic sources and invasive alien plants, which affect arthropods such as spiders (Maoela et al., Citation2016; Van der Colff et al., Citation2015). The CFK flora, and insect abundance and diversity, have been well studied (e.g., Pryke and Samways, Citation2008, Citation2009a, Citation2009b), but few studies have focused on spiders (Coetzee et al., Citation1990; Foord and Dippenaar-Schoeman, Citation2016; Haddad and Dippenaar-Schoeman, Citation2009; Tucker, Citation1920a; Visser et al., Citation1999; Yekwayo et al., Citation2019). Based on the distribution records generated through SANSA, the CFK has been highlighted as a region of exceptionally high South African endemics and species of conservation concern, particularly those in the Rare and Endangered category (Foord et al., Citation2020).
The aim of this study is to provide an overview of the diversity, endemicity and present conservation status of the spider fauna of the CFK, and to review the past research conducted within the CFK from conserved areas and agroecosystems that included spiders as a target taxon. It follows on from papers dealing with the fauna of the Savanna (Foord et al., Citation2011) and Grassland biomes (Haddad et al., Citation2013).
MATERIAL AND METHODS
Study area
The CFK is situated in the southern parts of South Africa and stretches from Niewoudville in the north of the Western Cape south- and eastwards along the coast and interior to Gqeberha (Port Elizabeth) in the east of the Eastern Cape (). The oceans bound the CFK to the south and west and the arid Succulent Karoo and Nama Karoo to the north and east, respectively. It only covers approximately 90 000 km2. While the Fynbos Biome comprises three vegetation types, i.e., fynbos, renosterveld and strandveld, the CFK includes more vegetation types, such as Fynbos as well as inclusions of the Forest, Nama Karoo, Succulent Karoo and Thicket biomes (). Agriculture, especially dairy, vineyards and deciduous fruit, as well as forestry plantations, cover 25.9% of the area, mostly on the nutrient-rich soils of the renosterveld vegetation region (Rouget et al., Citation2003). The CFK, characterised by its Mediterranean climate, has an average annual rainfall that varies dramatically, from 2000 mm on the high mountains facing the coastline to under 200 mm on the leeward slopes, with most of the precipitation occurring during winter (Goldblatt, Citation1997).
Figure 1. Extent of the Cape Floristic Region in southwestern South Africa, with the main protected areas (excluding the West Coast) indicated.
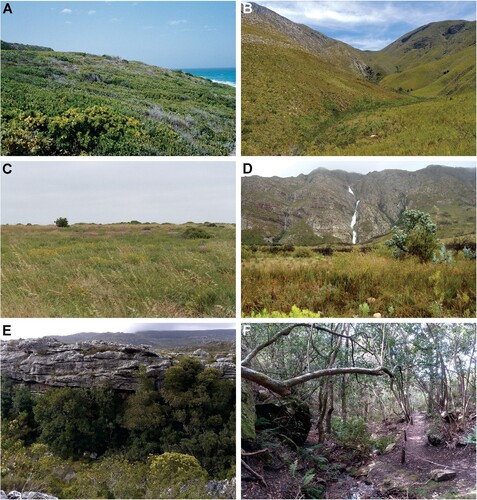
Figure 2. Representative biotopes of the Cape Floristic Kingdom. A, Coastal thicket, De Hoop Nature Reserve; B, Montane fynbos approximately one year after burning, Garcia Nature Reserve; C, Strandveld vegetation at Jacobsbaai; D, Montane fynbos, Dutoitskloof Pass; E, Montane fynbos and Afromontane Forest patch near Wynberg Caves, Table Mountain National Park; F, Interior of Afromontane Forest, Fernkloof Nature Reserve. Photographs: Charles Haddad.
Information from databases
Data on spider species richness for the CFK were obtained from three forms of datasets. This includes primary data from preserved specimens housed in natural history collections; data from published taxonomic and faunistic surveys; as well as a digital photographic database containing images of species recorded by the public and housed in Virtual Museums that could be accurately identified to species level. Also included are records from unpublished MSc and PhD theses and longer-term surveys that have been undertaken since the 1970s. One of the biggest constraints for SANSA is the lack of good taxonomic revisions for many of the larger spider families in Africa, such as Agelenidae, Dictynidae, Linyphiidae, Lycosidae and Theridiidae.
Fieldwork
Field studies were undertaken between 1997 and 2022 at several CFK sites as part of SANSA surveys (Dippenaar-Schoeman et al., Citation2015) and various student and research projects conducted by the Universities of Cape Town, Rhodes, Stellenbosch, Free State and Venda. A large proportion of the specimens were also collected by reserve and national park managers and the public. Ground-dwelling spiders were mainly collected using pitfall traps, leaf litter sifting, and active searching at the base of grass tussocks and under rocks. Plant-dwelling species were collected by vacuum sampling, beating, sweeping, actively searching on vegetation and flowers, and tree fogging. Voucher specimens sampled by the students are housed in the NCA and the National Museum, Bloemfontein.
Conservation assessments
Endemicity value
An endemicity value was provided for each species (Appendix 1) based on its currently known distribution. Seven endemicity categories are recognised: 0 = species known from Africa and wider (C); 1 = AE, endemic to the Afrotropical Region (African Endemic); 2 = STHE, endemic to southern Africa (south of Zambezi and Kunene Rivers); 3 = SAE, endemic to South Africa, and known from more than two provinces or two provinces not adjoining; 4–5 = species recorded from the CFK in both the Western Cape and Eastern Cape provinces (4), or in only one of the provinces (5); 6 = CFK endemic, known only from type locality in the CFK.
Conservation status
As part of the Red Listing Spider project, the preliminary conservation status of all South African spider species was determined using the IUCN Red List criteria (Foord et al., Citation2020). Immatures or those species represented by new or undetermined taxa were not evaluated (NE), while species known from only one sex, or where only very old material was available, or where a species could not be accurately identified, were listed as DD (data deficient for taxonomic reasons or a lack of distribution data). Species with a broad distribution (categories 0–4) were of Least Concern (LC), while species of special concern were listed as Rare, Critically Rare, Vulnerable, or Near Threatened, as they belong to categories 5 or 6 (Appendix 1).
RESULTS AND DISCUSSION
Faunistic composition
In compiling the spider species records from the three primary data sources, approximately 11 500 records from 130 localities were recorded in the CFK until the end of 2023, representing 62 families, 334 genera and 960 species (; Appendix 1). A further two families, Synotaxidae and Theridiosomatidae, have been collected in the CFK but none of the species have been described, so they are not dealt with further here. The most species-rich site sampled to date is the Table Mountain National Park (260 spp.), followed closely by the De Hoop Nature Reserve (252 spp.).
Table 1. Spider diversity of the Cape Floristic Kingdom (CFK), indiating the total number of genera (GEN) and species (SPP) sampled, their relative proportion, and the number of species endemic to the CFK.
The most species-rich families include Salticidae (128 spp.), which represents 13.33% of the fauna sampled, followed by Gnaphosidae (107 spp., 11.15%), Thomisidae (86 spp., 8.96%) and Lycosidae (49 spp., 5.10%). Six families are only represented by a single species (). The most species-rich spider genera recorded from the CFK are Anyphops Benoit, 1968 (Selenopidae, 24 spp.), followed by Heliophanus C. L. Koch, 1833 (Salticidae, 22 spp.), Zelotes Gistel, 1848 (Gnaphosidae, 20 spp.), Xerophaeus Purcell, 1907 (Gnaphosidae, 16 spp.) and Moggridgea O. Pickard-Cambridge, 1875 (Migidae, 14 spp.) (Appendix 1).
Representative taxa
The spider fauna of the CFK, as elsewhere, can be broadly divided into hunting spiders, which actively forage and capture prey, and web-builders, which construct silk webs with a range of shapes and structures in which prey is trapped, immobilised and consumed. Within each group, further distinction can be made between species living on the ground surface and closely associated structures, such as rocks and logs, and those primarily associated with plants. Of the 960 species recorded from the CFK, 728 spp. (75.83%) are hunters while 232 spp. (24.17%) are web-builders. The two broad guilds will be treated separately.
Hunting spiders
The ground-dwelling spider fauna is largely shaped by the biotopes sampled and the methods used. In open fynbos and thicket habitats, Chummidae (A), Corinnidae (B), some Gallieniellidae (E), Lycosidae (F, G), Scytodidae (M, N) and some Thomisidae (R) are commonly collected. Species of Bemmeridae, Gnaphosidae, Scytodidae, Sicariidae (O) and Theraphosidae are often collected under rocks and logs. In Afromontane forests, the litter fauna is dominated by Ctenidae (C), some Gallieniellidae (D), jumping spiders (Salticidae), particularly members of the tribeEuophryini (I, L), and Zoropsidae (W, X).
Figure 3. Representative hunting spiders of the Cape Floristic Kingdom. A, Chumma gastroperforata (Amaurobiidae); B, Pronophaea natalica (Corinnidae); C, Undescribed Anahita sp. (Ctenidae); D, Drassodella aurostriata (Gallieniellidae); E, D. quinquelabecula; F, Undetermined Hogna sp. (Lycosidae); G, Proevippa dregei (Lycosidae); H, Chiasmopes lineatus (Pisauridae); I, Euophrys cochlea (Salticidae); J, Undescribed Hispo sp. (Salticidae); K, Myrmarachne lesserti (Salticidae); L, Thyenula leighi (Salticidae); M, N, Undescribed Scytodes spp. (Scytodidae); O, Loxosceles spinulosa (Sicariidae); P, Harpactira cafreriana (Theraphosidae); Q, Undescribed Harpactira sp.; R, Heriaeus crassispinus (Thomisidae); S, Synema riflense (Thomisidae); T, Pherecydes tuberculatus (Thomisidae); U, Jocquestus capensis (Trachelidae); V, Griswoldia acaenata (Zoropsidae); W, Phanotea peringueyi (Zoropsidae); X, P. digitata. Photographs: A, C, E–J, M, N, Q, R, U–X Ruan Booysen; B, D, K, L, O, S, T, Rudolph Steenkamp; P, Charles Haddad. Photographers retain copyright of their images.

Common foliage-dwelling hunters in fynbos and thickets include species of Clubionidae, Philodromidae, Pisauridae (H), Salticidae (J, K) and Thomisidae (S, T), while the fauna in forests is dominated by Clubionidae, Thomisidae and Trachelidae (U).
Web-building spiders
Rare, tiny Anapidae (B) and common Amaurobiidae and Linyphiidae (N) are typically found in fynbos, thicket and Afromontane forest leaf litter. Common foliage-dwelling web-builders in forests include Amaurobiidae, especially Chresiona Simon, 1903 (A), several species of Araneidae, particularly Caerostris Thorell, 1868 and Neoscona Simon, 1864, Synotaxidae (Q) and Theridiidae. The dominant groups of web-builders associated with vegetation in open fynbos and thicket habitats include various species of Araneidae (C–H), Dictynidae (J) and Theridiidae (R–X).
Figure 4. Representative web-building spiders of the Cape Floristic Kingdom. A, Undetermined Chresiona sp. (Amaurobiidae); B, Undetermined Crozotelus sp. (Anapidae); C, Argiope aurocincta (Araneidae); D, A. australis; E, Bijoaraneus legonensis (Araneidae); F, Gea infuscata (Araneidae); G, Isoxya cicatricosa (Araneidae); H, Larinia chloris (Araneidae); I, Trichonephila fenestrata (Araneidae); J, Undetermined Dictynidae sp.; K, Gandanameno fumosa (Eresidae); L, G. spenceri; M, Stegodyphus tentoriicola (Eresidae); N, Limoneta sirimoni (Linyphiidae); O, Vidole capensis (Phyxelididae); P, Spermophora pembai (Pholcidae); Q, Undescribed Synotaxidae sp.; R, Undetermined Argyrodes sp. (Theridiidae); S, Latrodectus renivulvatus (Theridiidae); T, Platnickina mneon (Theridiidae); U, Undescribed Ruborrhidion sp. (Theridiidae); V, Steatoda capensis (Theridiidae); W, Undetermined Theridion sp. (Theridiidae); X, Undetermined Tidarren sp. (Theridiidae). Photographs: A, D–H, J, P–W, Rudolph Steenkamp; B, N, X, Ruan Booysen; C, I, K–M, O, Charles Haddad. Photographers retain copyright of their images.

Species of Eresidae (K, L) and Phyxelididae (O) are found under rocks in fynbos and thickets, while Drymusidae and Phyxelididae are common under logs in forests. Non-social (M) and social species of Stegodyphus Simon, 1873 (Eresidae) are occasionally found associated with woody vegetation and fences in fynbos and thickets, while the introduced Australian brown house spider, Badumna longinqua (L. Koch, 1867) (Desidae), is common in synanthropic habitats in coastal and subcoastal areas from Cape Town to Gqeberha (Port Elizabeth), building webs around houses and on fencing (Haddad and Foord, Citation2021).
Spiders in conserved areas
In 2004, the “Cape Floral Region Protected Areas” was inscribed as a World Heritage Site, i.e., areas that receive legal protection by the United Nations Educational, Scientific and Cultural Organization (UNESCO). In the CFK, nine representative protected areas have been identified, namely the Cederberg Wilderness Area, West Coast National Park, Table Mountain National Park, Groot Winterhoek Wilderness Area, Boland Mountain Complex, De Hoop Nature Reserve, Boosmansbos Wilderness Area, Swartberg complex, and Baviaanskloof Mega Reserve (). As spider surveys have been undertaken in all nine sites, survey results are accordingly listed below.
Cederberg Wilderness Area
The Cederberg Wilderness Area (CWA) lies about 250 km north of Cape Town. This vast region encompasses approximately 182 000 hectares of rugged mountainous terrain, stretching from the Pakhuis Pass in the east near the Springbok Flats, and from the Middelberg Pass in the north to the Koue Bokkeveld Mountains and the Skurweberge in the south.
From 2004 to 2009, a transect consisting of 17 sites, starting at sea level in the west (Lambert’s Bay), peaking at 1920m (Sneeukop) and ending at Wupperthal (500 m a.s.l.) in the east, was sampled using pitfall traps. A total of 10 094 spiders, representing 178 species in 44 families and 100 genera were caught over the 6-year period and the voucher specimens are housed in the NCA (Foord and Dippenaar-Schoeman, Citation2016). Levels of spider endemicity mirrored that of the plants in the region. Sixty-five percent (116 spp.) of the spider species sampled in the study were found to be endemic to South Africa, 57 spp. are undescribed, but 20 are in the process of being described.
| 2. | West Coast | ||||
No long-term spider surveys have been undertaken on the West Coast. Presently, 350 records are listed for the region in the NCA. Four regions are recognised in the West Coast and the localities where spiders have been sampled are listed as follows: the Bergriver region (Piketberg, Veldrift); Swartland region (Darling, Malmesbury, Moorreesburg, Yzerfontein); Peninsula region (Jacobsbaai, Langebaan, Paternoster, Saldanha Bay, St Helena Bay) and the Namaqua West Coastal region (Lutzville, Koekenaap, Vanrhynsdorp).
| 3. | Groot Winterhoek Wilderness Area | ||||
The Groot Winterhoek Mountains, located about 120 km north of Cape Town, near Porterville, are particularly important for protecting mountain fynbos and wildlife. They form part of the Cape Fold Belt comprising a watershed area of 552 606 hectares. Up to 83% of the range is still classified as being in a natural state and up to 72% of the range is protected. The towns of Saron and Porterville are located at the foot the mountain range's western side. Unfortunately, the Groot Winterhoek Wilderness Area is not well sampled for spiders and only 20 spp. have been recorded so far, although it is the type locality for numerous species of Gnaphosidae and Zodariidae (Tucker, Citation1920b, Citation1923).
| 4. | Table Mountain National Park (TMNP) | ||||
TMNP is a national park in Cape Town, South Africa, proclaimed on 29 May 1998, for the purpose of protecting the natural environment of the Table Mountain Chain, and in particular the rare fynbos vegetation. The area is also known as the Cape Peninsula and contains Table Mountain, for which the park is named, the Cape of Good Hope,and Kirstenbosch National Botanical Gardens. Several faunistic surveys were undertaken by the Universities of Stellenbosch and Cape Town to address ecological questions around the conservation of biodiversity in this area (Picker and Samways, Citation1996; Pryke, Citation2008; Pryke and Samways, Citation2008, Citation2009a, Citation2009b, Citation2010, Citation2012; Rebelo et al., Citation2011; Tucker, Citation1920a; Uys, Citation2012). Surveys from temperate sandstone caves falling within the TMNP (Dippenaar-Schoeman and Myburgh, Citation2009; Ferreira et al., Citation2020; Sharratt and Samways, Citation2000) were also undertaken. The first records were sampled from the Kirstenbosch National Botanical Garden by Tucker (Citation1920a), and more recently, by Pryke and Samways (Citation2009a). A checklist for the TMNP was recently completed (Haddad and Dippenaar-Schoeman, Citationin press) and it contains records of all the species sampled in the area, listing a total of 260 species from 50 families and 167 genera.
| 5. | Boland Mountain Complex | ||||
The Boland is a region situated to the northeast of Cape Town in the middle and upper courses of the Berg and Breede Rivers, around the Boland Mountains of the central Cape Fold Belt. It is sometimes also referred to as the Cape Winelands, because it is the primary region for the production of Western Cape wine and its core lies around the Boland Mountains and the towns of Stellenbosch, Paarl and Worcester. In the CFK, agriculture has transformed a quarter of the landscape, with growth of the industry predicted to continue (Rouget et al., Citation2003). The vineyard industry has experienced a slight decline in extent in South Africa in recent years, with 92 067 ha under vines in 2019, most of which occurs in the CFK (South African Wine Industry Information and Systems, Citation2019).
Throughout the years several surveys have been undertaken in the vineyards, with >2000 specimens housed in the NCA. Different aspects of wine grape production and its impacts on biodiversity conservation were addressed by several universities and the Agricultural Research Council (Gaigher and Samways, Citation2010, Citation2014; Gaigher et al., Citation2016; Halleen and Dippenaar-Schoeman, Citation2013; Magoba et al., Citation2015; Theron, Citation2017; Theron et al., Citation2020a, Citation2020b). From surveys conducted in the Boland vineyards, a total of 45 families represented by 210 species were sampled. Surveys were also undertaken in the Hermanus area, including the Fernkloof Nature Reserve (Hamilton-Attwell and Dippenaar-Schoeman, Citation2023), the Bontebok National Park (Dippenaar-Schoeman et al., Citation2021) and the Gondwana Private Game Reserve (Dippenaar-Schoeman and Buck, Citation2023). Further surveys have also been conducted in the Kogelberg Biosphere Reserve, focusing on the effects of fire on arthropods, including spiders (Yekwayo et al., Citation2018, Citation2019), and the buffer zones between conserved areas and surrounding transformed land (Van Schalkwyk et al., Citation2019a, Citation2019b, Citation2020). Spiders were also included in an assessment of vegetation-dwelling arthropods in the Jonkershoek Valley (Swart et al., Citation2017) and evaluations of the effects of invasive trees on arthropod diversity in the Boland Mountains (Maoela et al., Citation2016).
| 6. | De Hoop Nature Reserve (DHNR) | ||||
DHNR is situated on the south coast of the Western Cape Province and covers an area of 32 279 hectares terrestrially. In addition, the coastline and adjacent marine areas are also included in the reserve for the protection of the marine environment and its biodiversity. Surveys by the University of the Free State were carried out between 1999 and 2007 and consisted of five intensive surveys between two and 12 days in duration. Arachnids were sampled in five broad habitat types, namely fynbos, wetlands (De Hoop Vlei), Eucalyptus plantations at Potberg and Cupido’s Kraal, coastal dunes near Koppie Alleen and the intertidal zone at Koppie Alleen. A total of 274 species representing five arachnid orders were recorded, of which spiders were the dominant taxon (252 spp., 174 genera, 53 families) (Haddad and Dippenaar-Schoeman, Citation2009).
| 7. | Boosmansbos Wilderness Area and Garden Route | ||||
Boosmansbos Wilderness Area, approximately 270 km east of Cape Town, is a large, rugged forest reserve in the Langeberg range. The area includes several towns, such as Barrydale, Suurbraak, Swellendam and Heidelberg. The Garden Route includes fynbos and extensive Afromontane forests along the southern coastline. Spiders were collected by several reserve managers but the most comprehensive survey of this extended area formed part of a Ph.D study at Stellenbosch University (Swart, Citation2020), where several forest sites were sampled by canopy fogging and other methods at five sites: Oubos at Riviersonderend; Grootvadersbosch near Heidelberg; Kleinbos near Friemersheim; Woodville near Wilderness and Witelsbos near Stormsrivier, both in the Garden Route National Park. This resulted in several papers that investigated land-use and anthropogenic impacts on spiders and other arthropods (Swart et al., Citation2018, Citation2019), and effects of tree surroundings and physiological traits on arthropods (Swart et al., Citation2020a, Citation2020b).
| 8. | Swartberg complex | ||||
The Swartberg Nature Reserve is situated in the Large Swartberg mountain range in the Oudtshoorn district of the Western Cape Province. Spiders were collected by the reserve manager over a 10-year period. A total of 45 families comprising 136 genera and 186 species were collected, all of which were new records for the area. This represents about 8.2% of the total known South African spider fauna (Dippenaar-Schoeman et al., Citation2005). Additional samples were also taken from Touwsrivier, Gouritz, Witteberg Nature Reserve, Prince Albert, Gamkapoort Nature Reserve and Towerkop Nature Reserve.
| 9. | Baviaanskloof Mega Reserve | ||||
Baviaanskloof lies between the Baviaanskloof and Kouga mountain ranges. The easternmost point of the valley is about 95 km NW of the coastal city of Gqeberha (Port Elizabeth). The Baviaanskloof area includes a cluster of formal protected areas managed by the Eastern Cape Parks Board, totalling around 500 000 hectares. The best-known of these is the 184 385 ha Baviaanskloof Nature Reserve – the third largest protected area in South Africa – whichwas established in 1920. The Baviaanskloof Mega Reserve includes the Groendal Nature Reserve and Formosa Nature Reserve, and also encompasses private land. The Baviaanskloof area is one of outstanding natural beauty, owing to its spectacular landscapes and a diverse array of plants and animals. The area has been included as part of the Cape Floristic Region World Heritage Site since 2004. Surveys are currently ongoing in the area, which has historically been undersampled for arachnids. Recently, the first checklist of the spiders of the Baviaanskloof was published (Dippenaar-Schoeman et al., Citation2023a, Citation2023b), in which 190 species representing 48 families were recorded. Additional surveys near the border of the reserve include checklists of the Addo Elephant National Park (Dippenaar-Schoeman et al., Citation2020), Thyspunt (Dippenaar-Schoeman and Wiese, Citation2022) and Jeffreys Bay (Wiese and Dippenaar-Schoeman, Citation2023).
Spiders in agroecosystems
Predacious mites and insects have received much attention in biological control programmes of pests in Africa, while spiders appear to have been neglected. Research showed that spiders are one of the most common predator groups found in agroecosystems in South Africa (Dippenaar-Schoeman et al., Citation2013). In some crops, they represent 70% of the predator complex (Van den Berg and Dippenaar-Schoeman, Citation1991). Reviews on the role of spiders in agroecosystems indicate an increasing interest in, and recognition of, spiders as natural control agents of insects and mites in crop systems. Spiders form part of a complex predatory community in crops and are important in regulating pest species in both commercial and small-scale farms (Dippenaar-Schoeman et al., Citation2013).
In the CFK of South Africa, agriculture is dominated by wine and table grapes, citrus and deciduous fruit production, as well being as a major region for the production of berry crops, hops and wheat. Wine grape production and diversity conservation are of major importance in the CFK, and innovative management of the landscape is necessary (Gaigher and Samways, Citation2010, Citation2014; Gaigher et al., Citation2016). In one survey conducted in the CFK, a total of 940 spiders were sampled with paper traps or by hand to investigate their possible role in the transfer of vinestem virus (Halleen and Dippenaar-Schoeman, Citation2013). A total of 13 families and 36 species were recorded, with Cheiracanthium furculatum Karsch, 1879 (Cheiracanthiidae), Euryopis episinoides (Walckenaer, 1847) (Theridiidae) and Pelecopsis janus Jocqué, 1984 (Linyphiidae) the most abundant species.
In a separate study in the CFK, Magoba and Samways (Citation2012) investigated the effects of vineyards and invasive alien trees on arthropods, finding that the most common predatory arthropods were spiders (50 spp.). In a comparison of fynbos with organic and conventional vineyards, Gaigher and Samways (Citation2014) found that the vineyards had comparable ground-dwelling spider diversity to fynbos, although fynbos supported several unique and rare families and species that were not collected in the vineyards. Gaigher et al. (Citation2016) compared vineyards, old fields and fynbos, collecting 50 spider species, of which 11 were considered to be of conservation importance. In a subsequent comparison of vinyeards, old fields, alien tree plantations and fynbos, Theron et al. (Citation2020a, Citation2020b) found variable impacts of biotope structure and vegetation composition on plant- and ground-dwelling spiders. Vineyards had the lowest diversity, and the quantity and quality of natural vegetation in the landscape had an effect on spider assemblages in the transformed biotopes.
Using various sampling methods across different vineyards, Geldenhuys et al. (Citation2021) sampled 112 spider morphospecies, which represented the third most species-rich arthropod order. In a separate study comparing vineyards and fynbos, they found that fynbos supported a significantly higher species richness of spiders, particularly plant-dwellers, although both biotopes had relatively high alpha-diversity (Geldenhuys et al., Citation2022). They attributed the distinct differences in the spider assemblages between the two biotopes to species associations with a particular biotope.
Another survey conducted in the CFK used pitfall traps to look at the impacts of alternative farming methods, such as organic and biodynamic farming, on biodiversity (Gaigher, Citation2008). A total of 17 spider families represented by 42 species were recorded during this study. In vineyards, Pelecopsis janus Jocqué, 1984 was the most abundant species, followed by Agyneta habra (Locket, 1968) and Limoneta sirimoni (Bosmans, 1979), all belonging to Linyphiidae. More recently, Birkhofer et al. (Citation2019) investigated the effects of ground cover management in organic deciduous fruit orchards on various taxonomic groups, spiders included, while Arvidsson et al. (Citation2020) investigated the impacts of mulching in these orchards on spider assemblages and their prey composition.
Conservation status and endemicity
Endemicity
A large percentage of the 960 species recorded in the CFK (21.35%) have a wide distribution throughout Africa, with a further 58 spp. (6.04%) also found in countries beyond Africa and 156 spp. (16.25%) occurring more widely in southern Africa. A total of 269 species (28.02%) are endemic to the CFK, including species from the Western Cape and parts of the Eastern Cape ().
Table 2. Conservation status and endemicity of the spider species sampled in the Cape Floristic Kingdom.
Among the 53 families with more than two species recorded from the CFK, half or more of the species of 17 families are endemic to the CFK (; Appendix 1). All but one of these families is represented by fewer than 20 species, the exception being Zodariidae (36 spp.). Families with very high levels of endemism (75% or more CFK endemics) were dominated by ground-dwelling Mygalomorphae families (trapdoor and baboon spiders), which are sedentary and have limited dispersal capabilities (Dippenaar-Schoeman, Citation2002): Bemmeridae (90.9%), Cyrtaucheniidae (83.3%), Theraphosidae (81.3%) and Stasimopidae (75%). Only two families belong to Araneomorphae, i.e. Gallieniellidae (87.5%) and Drymusidae (75%).
For comparison, using the same qualifying criteria, only five of 53 families recorded from the Savanna Biome had 50% of more of their species endemic to the biome (Archaeidae, Barychelidae, Idiopidae, Migidae and Zodariidae), and none had an endemism level of 75% (Foord et al., Citation2011). From the 44 qualifying families from the Grassland Biome, none had an endemism level exceeding 50% of their species, with the highest being Stasimopidae (then Ctenizidae, 46.7%) (Haddad et al., Citation2013). This data therefore emphasises the significance of the CFK as a biodiversity hotspot with high spider endemism, already indicated in Foord et al. (Citation2020), and highlights the need to prioritise the conservation of its unique fauna.
Conservation status
Of the 960 species sampled, 229 spp. (23.85%) are data deficient (DD), i.e., lacking taxonomic or distribution data (). Most of the species (682 spp., 71.04%) sampled are listed as being of Least Concern (LC). Twenty-three species are listed as Rare and 26 are of special concern, ranging from Critically Rare to Near Threatened ().
Table 3. Spider species of special conservation concern in the Cape Floristic Kingdom, indicating their endemism score (END). B, F and M indicate that species are known from both sexes, females or males only, respectively.
Taxonomic challenges
One of the largest tasks facing researchers in the biological sciences globally is addressing the “taxonomic deficit” – considered as the ratio between described and expected species in a taxon (Blaxter et al., Citation2005). This is a shared concern for researchers studying the diversity of protists (Mitchell et al., Citation2011), fungi (Heilmann-Clausen et al., Citation2019), insects (Dijkstra et al., Citation2014), spiders (Jocqué et al., Citation2013) and other organismal groups. Across the tree of life, taxonomists responsible for describing species are facing shared challenges of funding and infrastructure limitations and a lack of development of new researchers in the field to replace aging and retiring colleagues (Anderson and Majka, Citation2009; Bik, Citation2017).
Resolving the true diversity of spiders in South Africa is no different. Although the country has the richest described fauna on the continent that has been researched most thoroughly in the past (Dippenaar-Schoeman et al., Citation2023a, Citation2023b; Jocqué et al., Citation2013), many families have never been subjected to revision and continue to present a considerable identification challenge to taxonomists and ecologists alike. This includes families with a large proportion of undescribed species (e.g. Agelenidae, Amaurobiidae, Dictynidae, Linyphiidae, Miturgidae, Theridiidae and Trachelidae), as well as poorly studied groups with currently misplaced species or with a number of undescribed genera (e.g. Amaurobiidae, Lycosidae and Trachelidae).
As such, the list of 960 described species recorded from the CFK in this paper (Appendix 1) is a considerable under-representation of the true diversity of the order. Several recent taxonomic revisions of South African taxa have elucidated a largely undescribed fauna of particular genera, including species endemic to the CFK (Appendix 1). For Chumma Jocqué, 2002 (Amaurobiidae), a revision quadrupled the species richness of the genus, which now includes four species from the CFK, two of which are endemic (Jocqué and Alderweireldt, Citation2018). Among the Entypesidae trapdoor spiders, the genus Lepthercus Purcell, 1902 increased from two to 11 species, with one CFK endemic (Ríos-Tamayo and Lyle, Citation2020); Hermacha Simon, 1899 increased from eight to 11 species, with six of the seven species from the CFK being endemics; a CFK endemic Hermachola Hewitt, 1915 was also described in the same paper (Ríos-Tamayo et al., Citation2021). As part of a concerted revision of Afrotropical Trachelidae, 20 of the 26 species recorded from the CFK have been described in the last two decades, including several new genera, and 12 of these species are endemics (Appendix 1).
These are only a few examples of how poorly studied many spider families are and the considerable proportion of newly described species that are CFK endemics. If projections can be made for the other understudied groups, then it is likely that the spider species richness in the CFK could potentially double once the fauna has been comprehensively studied. This can only be achieved through a concerted collecting effort in undersampled areas, as well as developing taxonomic expertise dedicated to revise and describe the fauna. This will require investment in capacity building through postgraduate student development, sourcing targeted funding from government and the private sector, attracting international researchers to work on the South African spider fauna, and promoting the importance of natural history collections to society and improving financial support to ensure their long-term use and development (Hamer, Citation2012; Jacobs, Citation2020).
CONCLUSION
The CFK represents a global biodiversity hotspot of considerable international significance, as it represents a unique floristic kingdom and is under severe threat from anthropogenic factors, particularly agriculture and urban development. Fortunately, its significance has been widely recognised and enabled the establishment of a broad network of protected areas to conserve the fauna and flora. To date, 960 spider species have been recorded from the CFK, representing 42.4% of the South African fauna, with a significant proportion of endemic species and species of conservation concern that need to be monitored in the future. This conservation effort has benefitted greatly from a broad range of ecological studies in the CFK that have included spiders as an indicator taxon, which not only elucidates the impacts of various anthropogenic factors on their faunistic composition, but has provided invaluable material to better understand the distribution patterns of the fauna. However, there is still a considerable taxonomic deficit for many groups, and resolving this shortfall is essential to better conserve the unique taxa occurring in the CFK.
NOTES ON CONTRIBUTORS
A.S.D. identified specimens, processed species records and data, and wrote part of the manuscript; C.R.H. identified specimens, prepared figure plates, analysed data and wrote part of the manuscript; S.H.F. analysed data and prepared the map; L.N.L. identified specimens and helped with data analysis. All authors assisted with the conservation assessments and editing the final manuscript.
ACKNOWLEDGEMENTS
This paper is dedicated to the memory of our late colleague and co-author, Stefan Foord, whose incredible contribution to the success of SANSA as an ecologist, data analyst and taxonomist will be sorely missed in the years to come. The authors would like to thank the Agricultural Research Council (ARC) and the South African National Biodiversity Institute (SANBI), Threatened Species Programme for funding the South African National Survey of Arachnida (SANSA) phase 2. CapeNature and SANParks are thanked for permits and facilitating projects to collect in the reserves and national parks. We acknowledge support from the DST-NRF Centre for Invasion Biology and the National Research Foundation of South Africa. The staff of the Arachnology section of the ARC –National Collection of Arachnida and the National Museum, Bloemfontein are thanked for their assistance with processing and databasing the material collected during SANSA. Members of the Spider Club and the SANSA volunteers (Linda Wiese, Helen Lieben, Victor Hamilton-Attwell, Elton le Roux, Walter Jubber, Renata Kruyswijk and the late Zanie van der Walt), reserve managers, and all the students from the universities of Stellenbosch, Rhodes, Cape Town, the Free State and Venda are thanked for their assistance with surveys and providing material. The contribution of photographers submitting images to the virtual museums is also acknowledged.
DISCLOSURE STATEMENT
No potential conflict of interest was reported by the author(s).
REFERENCES
- Anderson, R. & Majka, C. 2009. Biodiversity and biosystematic research in a brave new 21st century information-technology world. ZooKeys 22: 1–4. doi:10.3897/zookeys.22.222.
- Arvidsson, F., Addison, P., Addison, M., Haddad, C.R. & Birkhofer, K. 2020. Weed species, not mulching, affect web-building spiders and their prey in organic fruit orchards in South Africa. Ecosphere 11: e03059. doi:10.1002/ecs2.3059.
- Balmford, A. & Gaston, K.J. 1999. Why biodiversity surveys are good value. Nature 398: 204–205. doi:10.1038/18339.
- Bengtsson, J., Jones, H. & Setala, H. 1997. The value of biodiversity. Trends in Ecology & Evolution 12: 334–336. doi:10.1016/S0169-5347(97)01135-X.
- Bik, H.M. 2017. Let's rise up to unite taxonomy and technology. PLoS Biology 15: e2002231. doi:10.1371/journal.pbio.2002231.
- Birkhofer, K., Addison, P., Addison, M.F., Arvidsson, F., Bazelet, C., Bengtsson, J., Haddad, C., Booysen, R., Conlong, D., Janion-Scheepers, C., Kapp, C., Lindborg, R., Louw, S., Malan, A.P., Storey, S.G. & Swart, W.J. 2019. Effects of ground cover management on biotic communities, ecosystem services and disservices in organic deciduous fruit orchards in South Africa. Frontiers in Sustainable Food Systems 3: 107. doi:10.3389/fsufs.2019.00107.
- Blaxter, M., Mann, J., Chapman, T., Thomas, F., Whitton, C., Floyd, R. & Abebe, E. 2005. Defining operational taxonomic units using DNA barcode data. Philosophical Transactions of the Royal Society B: Biological Sciences 360: 1935–1943. doi:10.1098/rstb.2005.1725.
- Coetzee, J.H., Dippenaar-Schoeman, A.S. & Van Den Berg, A. 1990. Spider assemblages on five species of Proteaceous plants in the fynbos biome of South Africa. Phytophylactica 22: 443–447.
- Cowling, R.M., Rundel, P.W., Desmet, P.G. & Esler, K.J. 1998. Extraordinary high regional-scale plant diversity in southern African arid lands: subcontinental and global comparisons. Diversity and Distributions 4: 27–36.
- Dijkstra, K.D., Monaghan, M.T. & Pauls, S.U. 2014. Freshwater biodiversity and aquatic insect diversification. Annual Review of Entomology 59: 143–163.
- Dippenaar-Schoeman, A.S. 2002. Baboon and Trapdoor Spiders of Southern Africa: An identification manual. Plant Protection Research Institute Handbook no. 13. Pretoria, Agricultural Research Council, 128 pp.
- Dippenaar-Schoeman, A.S. & Buck, J. 2023. A checklist of the spiders (Arachnida, Araneae) of Gondwana Private Game Reserve, in the Western Cape, South Africa. SANSA Newsletter 46: 17–24. doi:10.5281/zenodo.8137378.
- Dippenaar-Schoeman, A.S., Foord, S.H., Haddad, C.R. & Le Roux, E. 2021. A checklist of the spiders (Arachnida, Araneae) of the Bontebok National Park in the Western Cape Province, South Africa. SANSA Newsletter 38: 13–21. doi:10.5281/zenodo.5947479.
- Dippenaar-Schoeman, A.S., Haddad, C.R., Foord, S.H., Lyle, R., Lotz, L.N., & Jocqué, R. 2010. First Atlas of the Spiders of South Africa (Arachnida: Araneae). South African National Survey of Arachnida Technical Report 2010, version 1, 1160 pp. doi:10.5281/zenodo.7628808.
- Dippenaar-Schoeman, A.S., Haddad, C.R., Foord, S.H., Lyle, R., Lotz, L.N. & Marais, P. 2015. South African National Survey of Arachnida (SANSA): review of current knowledge, constraints and future needs for documenting spider diversity (Arachnida: Araneae). Transactions of the Royal Society of South Africa 70: 245–275. doi:10.1080/0035919X.2015.1088486.
- Dippenaar-Schoeman, A.S., Haddad, C.R., Lotz, L.N., Booysen, R., Steenkamp, R.C. & Foord, S.H. 2023a. Checklist of the spiders (Araneae) of South Africa. African Invertebrates 64: 221–289. doi:10.3897/AfrInvertebr.64.111047.
- Dippenaar-Schoeman, A.S. & Myburgh, J.G. 2009. A review of the cave spiders (Arachnida: Araneae) from South Africa. Transactions of the Royal Society of South Africa 64: 53–61. doi:10.1080/00359190909519237.
- Dippenaar-Schoeman, A.S., Van Den Berg, A.M., Haddad, C.R. & Lyle, R. 2013. Current knowledge of spiders in South African agroecosystems (Arachnida, Araneae). Transactions of the Royal Society of South Africa 68: 57–74. doi:10.1080/0035919X.2012.755136.
- Dippenaar-Schoeman, A.S., Van Der Walt, A.E., De Jager, M., Le Roux, E. & Van Den Berg, A. 2005. The spiders of the Swartberg Nature Reserve in South Africa (Arachnida: Araneae). Koedoe 48: 77–86. doi:10.4102/koedoe.v48i1.167.
- Dippenaar-Schoeman, A.S. & Wiese, L. 2022. SANSA Eastern Cape surveys: A checklist of the spiders (Arachnida, Araneae) of Thyspunt, South Africa. SANSA Newsletter 40: 23–29. doi:10.5281/zenodo.5916721.
- Dippenaar-Schoeman, A.S., Wiese, L., Foord, S.H. & Haddad, C.R. 2020. A list of spider species found in the Addo Elephant National Park (Eastern Cape Province, South Africa). KOEDOE - African Protected Area Conservation and Science 62: a1578. doi:10.4102/koedoe.v62i1.1578.
- Dippenaar-Schoeman, A.S., Wiese, L. & Lotz, L.N. 2023b. Eastern Cape surveys: A checklist of the spiders (Arachnida, Araneae) of the Baviaanskloof Mega Reserve, South Africa. SANSA Newsletter 47: 33–42. doi:10.4102/koedoe.v62i1.1578.
- Drinkrow, D.R., Cherry, M.I. & Siegfried, W.R. 1994. The role of natural history museums in preserving biodiversity in South Africa. South African Journal of Science 90: 470–479. https://hdl.handle.net/10520/AJA00382353_7811.
- Ferreira, R.L., Giribet, G., Du Preez, G., Ventouras, O., Janion, C. & Silva, M.S. 2020. The Wynberg Cave System, the most important site for cave fauna in South Africa at risk. Subterranean Biology 36: 73–81. doi:10.3897/subtbiol.36.60162.
- Foord, S.H. & Dippenaar-Schoeman, A.S. 2016. The effect of elevation and time on mountain spider diversity: a view of two aspects in the Cederberg mountains of South Africa. Journal of Biogeography 43: 2354–2365. doi:10.1111/jbi.12817.
- Foord, S.H., Dippenaar-Schoeman, A.S., Haddad, C.R., Lotz, L.N. & Lyle, R. 2011. The faunistic diversity of spiders (Arachnida: Araneae) of the Savanna Biome in South Africa. Transactions of the Royal Society of South Africa 66: 170–201. doi:10.1080/0035919X.2011.639406.
- Foord, S.H., Dippenaar-Schoeman, A.S., Haddad, C.R., Lyle, R., Lotz, L.N., Sethusa, T. & Raimondo, D. 2020. The South African National Red List of spiders: patterns, threats, and conservation. Journal of Arachnology 48: 110–118. doi:10.1636/0161-8202-48.2.110.
- Gaigher, R. 2008. The effect of different vineyard management systems on the epigaeic arthropod assemblages in the Cape Floristic Region, South Africa. Unpublished M.Sc thesis, Stellenbosch University, Stellenbosch, 118 pp.
- Gaigher, R., Pryke, J.S. & Samways, M.J. 2016. Old fields increase habitat heterogeneity for arthropod natural enemies in an agricultural mosaic. Agriculture, Ecosystems and Environment 230: 242–250. doi:10.1016/j.agee.2016.06.014.
- Gaigher, R. & Samways, M.J. 2010. Surface-active arthropods in organic vineyards, integrated vineyards and natural habitat in the Cape Floristic Region. Journal of Insect Conservation 14: 595–605. doi:10.1007/s10841-010-9286-2.
- Gaigher, R. & Samways, M.J. 2014. Landscape mosaic attributes for maintaining ground-living spider diversity in a biodiversity hotspot. Insect Conservation and Diversity 7: 470–479. doi:10.1111/icad.12070.
- Geldenhuys, M., Gaigher, R., Pryke, J.S. & Samways, M.J. 2021. Diverse herbaceous cover crops promote vineyard arthropod diversity across different management regimes. Agriculture, Ecosystems & Environment 307: 107222. doi:10.1016/j.agee.2020.107222.
- Geldenhuys, M., Gaigher, R., Pryke, J.S. & Samways, M.J. 2022. Vineyards compared to natural vegetation maintain high arthropod species turnover but alter trait diversity and composition of assemblages. Agriculture, Ecosystems & Environment 336: 108043. doi:10.1016/j.agee.2022.108043.
- Goldblatt, P. 1997. Floristic diversity in the Cape Flora of South Africa. Biodiversity and Conservation 6: 359–377. doi:10.1023/A:1018360607299.
- Haddad, C.R. & Dippenaar-Schoeman, A.S. 2009. A checklist of the non-acarine arachnids (Chelicerata: Arachnida) of the De Hoop Nature Reserve, Western Cape Province, South Africa. Koedoe 51: 149. doi:10.4102/koedoe.v51i1.149.
- Haddad, C.R. & Dippenaar-Schoeman, A.S. in press. A checklist of the spiders (Arachnida, Araneae) of the Table Mountain National Park in the Western Cape Province, South Africa, Koedoe.
- Haddad, C.R., Dippenaar-Schoeman, A.S., Foord, S.H., Lotz, L.N. & Lyle, R. 2013. The faunistic diversity of spiders (Arachnida, Araneae) of the Grassland Biome in South Africa. Transactions of the Royal Society of South Africa 68: 97–122. doi:10.1080/0035919X.2013.773267.
- Haddad, C.R. & Foord, S.H. 2021. Future climate may limit the spread of the Australian house spider Badumna longinqua (Araneae: Desidae) in South Africa. Journal of Arachnology 49: 332–339. doi:10.1636/JoA-S-20-069.
- Halleen, F. & Dippenaar-Schoeman, A.S. 2013. Spiders in vineyards. SANSA Newsletter 19: 10.
- Hamer, M. 2012. An assessment of zoological research collections in South Africa. South African Journal of Science 108: 1090. doi:10.4102/sajs.v108i11/12.1090.
- Hamilton-Attwell, V. & Dippenaar-Schoeman, A.S. 2023. Spiders of Fernkloof Nature Reserve. Hermanus, Hermanus Botanical Society, 121 spp.
- Heilmann-Clausen, J., Bruun, H.H., Ejrnæs, R., Frøslev, T.G., Læssøe, T. & Petersen, J. 2019. How citizen science boosted primary knowledge on fungal biodiversity in Denmark. Biological Conservation 237: 366–372. doi:10.1016/j.biocon.2019.07.008.
- Jacobs, A. 2020. The importance of natural science collections in South Africa. South African Journal of Science 116: 8145. doi:10.17159/sajs.2020/8145.
- Jocqué, R. & Alderweireldt, M. 2018. New Chummidae (Araneae): quadrupling the size of the clade. European Journal of Taxonomy 412: 1–25. doi:10.5852/ejt.2018.412.
- Jocqué, R., Alderweireldt, M. & Dippenaar-Schoeman, A.S. 2013. Biodiversity: An African perspective. In Penney, D. (Ed.) Spider Research in the 21st Century. Manchester, Siri Scientific Press. pp. 18–57.
- Magoba, R.N. & Samways, M.J. 2012. Comparative footprint of alien, agricultural and restored vegetation on surface-active arthropods. Biological Invasions 14: 165–177. doi:10.1007/s10530-011-9994-x.
- Magoba, R.N., Simaika, J.P. & Samways, M.J. 2015. Soil compaction and surface-active arthropods in historic, agricultural, alien, and recovering vegetation. Journal of Insect Conservation 19: 501–508. doi:10.1007/s10841-015-9771-8.
- Maoela, M.A., Roets, F., Jacobs, S.M. & Esler, K.J. 2016. Restoration of invaded Cape Floristic Region riparian systems leads to a recovery in foliage-active arthropod alpha- and beta-diversity. Journal of Insect Conservation 20: 85–97. doi:10.1007/s10841-015-9842-x.
- Mitchell, E.A.D., Belbahri, L., Job, D., Pawlowski, D. & Lara, E. 2011. Exploring the Terra incognita of unknown eukaryotic diversity in Soils – A major challenge we now have the tools to tackle!. Bulletin de la Société Suisse de Pédologie 32: 57–62. doi:10.1111/jeu.12095.
- Picker, M.D. & Samways, M.J. 1996. Faunal diversity and endemicity of the Cape Peninsula, South Africa – a first assessment. Biodiversity and Conservation 5: 591–606. doi:10.1007/BF00137611.
- Pryke, J.S. 2008. Conservation of Invertebrate Fauna of the Cape Peninsula. Unpublished Ph.D thesis, Stellenbosch University, Stellenbosch, 262 pp.
- Pryke, J.S. & Samways, M.J. 2008. Conservation of invertebrate biodiversity on a mountain in a global biodiversity hotspot, Cape Floral Region. Biodiversity and Conservation 17: 3027–3043. doi:10.1007/s10531-008-9414-4.
- Pryke, J.S. & Samways, M.J. 2009a. Recovery of invertebrate diversity in a rehabilitated city land-scape mosaic in the heart of a biodiversity hotspot. Landscape Urban Planning 93: 54–62. doi:10.1016/j.landurbplan.2009.06.003.
- Pryke, J.S. & Samways, M.J. 2009b. Conservation of the insect assemblages of the Cape Peninsula biodiversity hotspot. Journal of Insect Conservation 13: 627–641. doi:10.1007/s10841-009-9213-6.
- Pryke, J.S. & Samways, M.J. 2010. Significant variables for the conservation of mountain invertebrates. Journal of Insect Conservation 14: 247–256. doi:10.1007/s10841-009-9253-y.
- Pryke, J.S. & Samways, M.J. 2012. Importance of using many taxa and having adequate controls for monitoring impacts of fire for arthropod conservation. Journal of Insect Conservation 16: 177–185. doi:10.1007/s10841-011-9404-9.
- Rebelo, T.G., Freitag, S., Cheney, C. & Mcgeoch, M.A. 2011. Prioritising species of special concern for monitoring in Table Mountain National Park: The challenge of a species-rich, threatened ecosystem. Koedoe 53: 1019. doi:10.4102/koedoe.v53i2.1019.
- Ríos-Tamayo, D., Engelbrecht, I. & Goloboff, P.A. 2021. A revision of the genus Hermacha Simon, 1889 (Mygalomorphae: Entypesidae), in southern Africa with revalidation of Hermachola Hewitt, 1915, and Brachytheliscus Pocock, 1902. American Museum Novitates 3977: 1–80. doi:10.1206/3977.1.
- Ríos-Tamayo, D. & Lyle, R. 2020. The South African genus Lepthercus Purcell, 1902 (Araneae: Mygalomorphae): phylogeny and taxonomy. Zootaxa 4766: 261–305. doi:10.11646/zootaxa.4766.2.2.
- Robertson, M.P., Cumming, G.S. & Erasmus, B.F.N. 2010. Getting the most out of atlas data. Diversity and Distributions 16: 363–375. doi:10.1111/j.1472-4642.2010.00639.x.
- Rouget, M., Richardson, D.M., Cowling, R.M., Lloyd, J.W. & Lombard, A.T. 2003. Current patterns of habitat transformation and future threats to biodiversity in terrestrial ecosystems of the Cape floristic region, South Africa. Biological Conservation 112: 63–85. doi:10.1016/S0006-3207(02)00395-6.
- Schnitzler, J., Barraclough, T.G., Boatwright, J.S., Goldblatt, P., Manning, J.C., Powell, M.P., Rebelo, T. & Savolainen, V. 2011. Causes of plant diversification in the Cape biodiversity hotspot of South Africa. Systematic Biology 60: 343–357. doi:10.1093/sysbio/syr006.
- Scoble, M. 2010. Rationale and value of natural history collections digitisation. Biodiversity Informatics 7: 77–80. doi:10.17161/bi.v7i2.3994.
- Sharratt, N.J., Picker, M.D. & Samways, M.J. 2000. The invertebrate fauna of the sandstone caves of the Cape Peninsula (South Africa): patterns of endemism and conservation priorities. Biodiversity and Conservation 9: 107–143. doi:10.1023/A:1008968518058.
- Smith, G.F., Steenkamp, Y., Klopper, R.R., Siebert, S.J. & Arnold, T.H. 2003. The price of collecting life. Nature 422: 375–376. doi:10.1038/422375a.
- South African Wine Industry Information and Systems (SAWIS). 2019. Macro-Economic Impact of the Wine Industry on the South African Economy. Unpublished Report, FTI Consulting, 90 pp.
- Swart, R.C. 2020. Interactions between indigenous southern Afrotemperate forest trees and arthropod diversity. Unpublished Ph.D thesis, Stellenbosch University, Stellenbosch, 277 pp.
- Swart, R., Pryke, J.S. & Roets, F. 2017. Optimising the sampling of foliage arthropods from scrubland vegetation for biodiversity studies. African Entomology 25: 164–174. doi:10.4001/003.025.0164.
- Swart, R.C., Pryke, J.S. & Roets, F. 2018. Arthropod assemblages deep in natural forests show different responses to surrounding land use. Biodiversity and Conservation 27: 583–606. doi:10.1007/s10531-017-1451-4.
- Swart, R.C., Pryke, J.S. & Roets, F. 2019. The intermediate disturbance hypothesis explains arthropod beta-diversity responses to roads that cut through natural forests. Biological Conservation 236: 243–251. doi:10.1016/j.biocon.2019.03.045.
- Swart, R.C., Samways, M.J., Pryke, J.S. & Roets, F. 2020b. Individual tree context and contrast dictate tree physiological features and arthropod biodiversity patterns across multiple trophic levels. Ecological Entomology 45: 333–344. doi:10.1111/een.12804.
- Swart, R.C., Samways, M.J. & Roets, F. 2020a. Tree canopy arthropods have idiosyncratic responses to plant ecophysiological traits in a warm temperate forest complex. Scientific Reports 10: 19905. doi:10.1038/s41598-020-76868-8.
- Theron, K.J. 2017. Conservation of spider diversity within an agricultural mosaic: insights from the Greater Cape Floristic Region, biodiversity hotspot. Unpublished M.Sc thesis, Stellenbosch University, Stellenbosch, 88 pp.
- Theron, K.J., Gaigher, R., Pryke, J.S. & Samways, M.J. 2020a. High quality remnant patches in a complex agricultural landscape sustain high spider diversity. Biological Conservation 243: 108480. doi:10.1016/j.biocon.2020.108480.
- Theron, K.J., Gaigher, R., Pryke, J.S. & Samways, M.J. 2020b. Abandoned fields and high plant diversity support high spider diversity within an agricultural mosaic in a biodiversity hotspot. Biodiversity and Conservation 29: 3757–3782. doi:10.1007/s10531-020-02048-9.
- Tucker, R.W.E. 1920a. Spiders of Kirstenbosch. Journal of the Botanical Society of South Africa 6: 21–24.
- Tucker, R.W.E. 1920b. Contributions to the South African Arachnid Fauna. II. On some new South African spiders of the families Barychelidae, Dipluridae, Eresidae, Zodariidae, Heracliidae, Urocteidae, Clubionidae. Annals of the South African Museum 17: 439–488. pl. 28–29.
- Tucker, R.W.E. 1923. The Drassidae of South Africa. Annals of the South African Museum 19: 251–437. pl. 8–11.
- Uys, C.J. 2012. The impact of pine plantations and alien invertebrates on native forest and fynbos invertebrate communities in Table Mountain National Park. Unpublished Ph.D thesis, University of Cape Town, 194 pp.
- Van Den Berg, A.M. & Dippenaar-Schoeman, A.S. 1991. Spiders, predacious insects and mites on South African cotton. Phytophylactica 23: 85–86.
- Van Der Colff, D., Dreyer, L.L., Valentine, A. & Roets, F. 2015. Invasive plant species may serve as biological corridor for the invertebrate fauna of naturally isolated hosts. Journal of Insect Conservation 19: 863–875. doi:10.1007/s10841-015-9804-3.
- Van Schalkwyk, J., Pryke, J.S., Samways, M.J. & Gaigher, R. 2019a. Congruence between arthropod and plant diversity in a biodiversity hotspot largely driven by underlying abiotic factors. Ecological Applications 29: e01883. doi:10.1002/eap.1883.
- Van Schalkwyk, J., Pryke, J.S., Samways, M.J. & Gaigher, R. 2019b. Complementary and protection value of a Biosphere Reserve buffer zone for increasing local representativeness of ground-living arthropods. Biological Conservation 239: 108292. doi:10.1016/j.biocon.2019.108292.
- Van Schalkwyk, J., Pryke, J.S., Samways, M.J. & Gaigher, R. 2020. Environmental filtering and spillover explain multi-species edge responses across agricultural boundaries in a biosphere reserve. Scientific Reports 10: 14800. doi:10.1038/s41598-020-71724-1.
- Visser, D., Wright, M.G., Van Den Berg, A. & Giliomee, J. 1999. Species richness of arachnids associated with Protea (Proteaceae) in the Cape fynbos. African Journal of Ecology 37: 334–343. doi:10.1046/j.1365-2028.1999.00182.x
- Wiese L. & Dippenaar-Schoeman A.S. 2023. SANSA Eastern Cape surveys: A checklist of the spiders (Arachnida, Araneae) of Jeffreys Bay, South Africa. SANSA Newsletter 45: 19–28. doi:10.5281/zenodo.7827670
- Yekwayo, I., Pryke, J.S., Gaigher, R. & Samways, M.J. 2018. Only multi-taxon studies show the full range of arthropod responses to fire. PLoS One 13: e0195414. doi:10.1371/journal.pone.0195414.
- Yekwayo, I., Pryke, J.S., Gaigher, R. & Samways, M.J. 2019. Wandering spiders recover more slowly than web building spiders after fire. Oecologia 191: 231–240. doi:10.1007/s00442-019-04471-4.
APPENDIX 1.
CHECKLIST OF THE DESCRIBED SPIDER SPECIES OF THE CAPE FLORISTIC KINGDOM, SOUTH AFRICA. NEW SPECIES AND GENERA REPRESENTED BY UNDETERMINED SPECIES ARE NOT INCLUDED HERE.
Endemism scores (ES) and categories (END): 0 = species known from Africa and wider (C); 1 = AE, endemic to the Afrotropical Region (African Endemic); 2 = STHE, endemic to southern Africa (south of Zambezi and Kunene Rivers); 3 = SAE, endemic to South Africa, and known from more than two provinces or two provinces not adjoining; 4–5 = species recorded from the CFK in both the Western Cape and Eastern Cape provinces (4), or in only one of the provinces (5); 6 = CFK endemic, known only from type locality in the CFK.
Conservation status (CON): CR = Critically Endangered; EN = Endangered; VU = Vulnerable; NT = Near Threatened; R = Rare; LC = Least Concern; DD = Data Deficient; NE = Not Evaluated.
