Abstract
Obstructive Sleep Apnoea (OSA) is a globally prevalent health concern associated with severe neurocognitive and cardiovascular implications. Despite affecting approximately 1 billion adults globally, the condition remains underdiagnosed, emphasising the need for accessible diagnostic methods and effective treatments. While there are multiple sites of airway obstruction during sleep, the tongue base is recognised as the key role player in the pathogenesis of OSA. The tongue base could be visualised as the gateway for airflow between the upper airway (pharynx) and the tracheo – bronchial airway (of the lungs). Various treatment modalities, such as Continuous Positive Airway Pressure (CPAP), Mandibular Advancement Devices (MADs), Hypoglossal Nerve Stimulation (HNS), and Maxillomandibular Advancement (MMA) surgery, will ultimately have an effect on the anatomical position and function of the tongue and its extrinsic muscular foundation attachments. Thus, medical or surgical modulation of airflow through the upper pharyngeal airspace is entirely dependent upon the response of the tongue base to these interventions. The elimination of apnoea and improvements in its sequelae makes CPAP the gold standard for OSA. While CPAP effectively splints the tongue base in a forward direction, patient adherence remains a significant issue. This paper addresses the limitations and challenges associated with each method, advocating for sustainable and accessible solutions. Furthermore, the paper introduces a polypropylene tongue suspension device as a potential non-invasive alternative to orthognathic surgery, demonstrating promising results in reversing OSA by advancing the tongue base. This concept, currently under investigation, presents an exciting prospect for future OSA treatment options.
INTRODUCTION
Obstructive Sleep Apnoea (OSA) is a pervasive and serious health condition with profound neurocognitive and cardiovascular consequences (Redline and Young, Citation1993; Lévy et al., Citation2015; Malhotra et al., Citation2015). Following American Academy of Sleep Medicine (AASM) diagnostic criteria, it is estimated that 936 million adults aged 30–69 years have mild to severe OSA, whereas 425 million adults have moderate to severe OSA, globally. The prevalence is particularly high, exceeding 50%, in China, the USA, Brazil, and India. In South Africa 23% of adults between the ages of 30 and 69 is estimated, via statistical modelling, to have moderate to severe OSA. If mild OSA is included, the number increases to 41% (Benjafield et al., Citation2019). Despite its prevalence, 70–80% of cases still remain undiagnosed, underscoring the need for accessible diagnostic methods and effective treatment strategies (Kapur et al., Citation2002; Young et al., Citation2002; Foster et al., Citation2009). It is well documented that 40–60% of patients with atrial fibrillation will have significant OSA, which, in turn, significantly reduces the success rate of cardiac ablation by half in patients with OSA (Geovanini and Lorenzi-Filho, Citation2018). Metabolic disorders, e.g., metabolic syndrome, insulin resistance and Type 2 diabetes is seen in 50–60% of OSA patients (Epstein et al., Citation2009). Resistant hypertension is found in 80–100% of patients with OSA. Hypertension in general is seen in 40% of OSA patients (Pedrosa et al., Citation2011). Any other cerebrovascular and cardiovascular disease e.g. Stroke, TIA`s, Pulmonary Hypertension with Cor Pulmonale, Myocardial Infarction, Coronary Artery Disease and Cardiac arrythmias also have a 30–60% affiliation to OSA (Morrison et al., Citation2007; Selim et al., Citation2010).
A history of snoring with witnessed apnoeas is key to alerting clinicians to the presence of OSA. Predictive, validated questionnaires developed to increase the level of suspicion include the Berlin and STOPBANG questionnaires (Nagappa et al., Citation2015). Questions relating to Snoring, Tiredness or fatigue, Observed apnoeas, and treatment for High Blood Pressure (STOP), followed by examination revealing a BMI >30 kg/m2, Average age >50yrs, Neck circumference (>43 cm in males, 41 cm for females) and Gender (males), (BANG) are noted. A score of 5/8 indicates an 80% chance of diagnosing OSA. The Epworth Sleepiness Scale (ESS) looks at sleepiness over a period of time. A score of 10/24 and above raises the concern for OSA (Johns, Citation1991). The severity of OSA is assessed using the Apnoea-Hypopnea Index (AHI). A comprehensive Polysomnogram (PSG) is conducted to rule out various disorders such as Narcolepsy, Idiopathic Hypersomnia, Periodic Limb Movements Disorder and R.E.M. Behaviour Disorder. Specific abnormalities are identified through an electroencephalogram (Sunwoo et al., Citation2020).
The AHI considers apnoeas lasting 10 s, causing > 4% oxygen desaturation and resulting in an arousal. OSA can be classified as Mild: (5 < AHI ≤ 15 / hr), Moderate: (15 < AHI ≤ 30 /hr) and Severe: (AHI > 30 / hr). Minor respiratory events are deemed equally significant (Budhiraja et al., Citation2019). Hypertension raises suspicion of OSA due to sympathetic drive during the apnoeas. Oxygen saturation drops markedly at the onset, leading to rapid deoxygenation culminating in arousal from sleep. Many patients are unaware of these arousals, often describing them as “panic attacks”. Patients with severe OSA may experience frequent wake-ups, accompanied by tachycardia. Blood pressure recordings reveal a surge during apnoeas, contributing to cardiac and metabolic complications (Venkataraman et al., Citation2020; Brown et al., Citation2022). The increased workload on the heart during apnoeas coupled with low oxygen levels can lead to atrial fibrillation and hypertension. Non-dipping blood pressure at night is indicative of sleep apnoea, highlighting the link between OSA and cardiovascular issues (Qin et al., Citation2021). Diagnostic tests for OSA, particularly the Apnoea Specific Test, can be home based and self-administered with the primary focus on breathing parameters such as AHI, oxygen desaturation index (ODI), and minimum/mean oxygen saturation. The development of home-based devices for the specific diagnosis of OSA has advanced and their quality is gradually improving (Varghese et al., Citation2022). The lack of sleep related tests in the public hospital system emphasises the need for lower cost home-based testing. Considering the high prevalence of cardiac and metabolic disease as well as depression in South Africa it is urgent that the diagnosis of sleep disorders is made accessible to as many patients as possible (Chattu et al., Citation2019; Rhoda and Bentley, Citation2022).
Body position during sleep is crucial for treatment considerations. Severe desaturation, especially in the supine position and during REM sleep, is evident in the diagnostic data. Snoring, a common symptom, is monitored using a snore sensor. Trend lines for each parameter provide a visual representation of sleep events. Understanding the intricacies of sleep disorders is crucial, as untreated OSA can lead to a plethora of health complications (Eiseman et al., Citation2012). The diagnostic emphasis on breathing patterns, oxygen levels and related indices helps tailor interventions for improved patient outcomes. Treating OSA is essential not only for alleviating immediate symptoms but also for preventing long-term cardiovascular and metabolic consequences associated with this disorder (Yeghiazarians et al., Citation2021). There appears to be a linear correlation between obesity and OSA. In obese people, fat deposits in the upper respiratory tract can narrow the airway. In addition, there is a decrease in muscle activity in this region, leading to hypoxic and apnoeic episodes, ultimately resulting in OSA. The decreased oxygenation causes tissue hypoxia, which is the main contributing factor to atherosclerosis known as the main risk factor for cardiovascular diseases. A longitudinal study of overweight and obese American adults demonstrates that change in weight is directly proportionate to sleep disordered breathing. Those with the greatest weight gain had a more severe AHI (Jehan et al., Citation2017). A more detailed discussion on the relationship of obesity and OSA is beyond the scope of this paper.
TONGUE BASE INVOLVEMENT IN OSA
The maintenance of the upper airway in humans relies upon muscle activation and soft tissue structures, distinct from the rigid skeletal support found in most mammals (Haponik et al., Citation1983; Suratt et al., Citation1983; Schwab et al., Citation1995). The absence of rigid support to the hyoid bone, potentially related to the evolution of speech, exposes the airway to collapse influenced by external and internal factors (Remmers, Citation1979). This delicate balance involves the interaction between dilating forces, primarily driven by muscle activation and collapsing forces produced by negative inspiratory pressures (Van de Graaff, Citation1988; Kobayashi et al., Citation1996).
Isono et al. (Citation1997) investigated pharyngeal airway diameter in healthy controls and OSA patients, revealing a reduced diameter and increased susceptibility to collapse in OSA patients (Isono et al., Citation1997). The intricate motor control system governing the upper airway involves over twenty muscles, with the hypoglossal motor system regulating the genioglossus muscle, a key dilator of the upper pharyngeal muscular architecture (Onal et al., Citation1981; Malhotra et al., Citation2000; Malhotra et al., Citation2002).
In addition to the cholinergic, adrenergic, serotonergic, and orexinergic systems, pharyngeal dilator muscle control is influenced by a multitude of neurochemical systems (Kubin et al., Citation1994; Horner, Citation1996; Kubin et al., Citation1996). Although it is possible to regulate hypoglossal neural activity precisely, this regulation is significantly impacted upon by the transition from wakefulness to sleep (Van de Graaff, Citation1988; Van de Graaff, Citation1991).
Mechanical effects and neuromuscular reflexes play a significant role in maintaining airway patency, with extraluminal tissue and intraluminal negative pressures contributing to upper airway collapse (Kobayashi et al., Citation2003). The complex and synchronised contractions of the pharyngeal dilator muscles, particularly the genioglossus and tensor palatini muscles (see ), counterbalance the collapsing forces within the upper pharynx (Schwab et al., Citation1995).
Figure 1. The interplay of forces on the upper pharyngeal airway. Inspiratory negative pressure and extraluminal positive pressure tend to promote pharyngeal collapse while upper airway dilator muscles and increased lung volume tend to maintain pharyngeal patency (Image by authors).
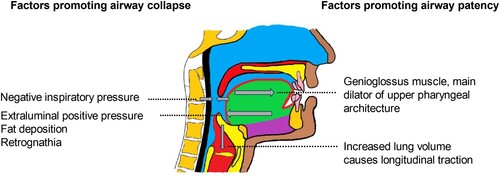
The genioglossus muscle, the main dilator of the upper pharyngeal muscular architecture, is primarily regulated by three neural inputs: afferent activity in mechanoreceptors, neurons that generate respiratory patterns, and serotonergic or noradrenergic neurons that impact upon arousal states (Mathew et al., Citation1982; Fogel et al., Citation2003). Collectively, these inputs regulate muscle activity and maintain airway patency during inspiration.
Pharyngeal muscle activity is subject to the effects of respiration, local airway conditions, and arousal states while sleeping. Although the negative-pressure reflex declines during sleep, the muscles maintain a certain degree of responsiveness, albeit with reduced intensity and speed. This decreased activity increases the susceptibility of the airway to collapse during sleep (Wheatley et al., Citation1993; Wheatley and White, Citation1993; Horner et al., Citation1994; Shea et al., Citation1998; Malhotra et al., Citation2000).
Significant roles are played by lung volume modifications, particularly those that are induced by lung inflation, in maintaining pharyngeal patency. By applying longitudinal tension to the pharyngeal airway, pulmonary inflation reduces collapsibility; conversely, decreased pulmonary volume during wakefulness and sleep increases susceptibility to airway collapse. Hence, airway collapse leading to OSA comprises a lack of compliance of the upper pharyngeal musculature in combination with reduced inspiratory effort (Ballard et al., Citation1990).
THE SIGNIFICANCE OF CPAP
For OSA patients who prefer to avoid surgery, continuous positive airway pressure (CPAP), invented by Sullivan et al. (Citation1981) is the treatment of choice (Sullivan et al., Citation1981; Epstein et al., Citation2009). The elimination of apnoea and improvements in its sequelae makes CPAP the gold standard for OSA (Faccenda et al., Citation2001; Pepperell et al., Citation2002; Rudnick et al., Citation2007; Epstein et al., Citation2009). It acts by delivering air into the airway of the patient through a specifically constructed nasal mask or cushion. During inhalation, the positive pressure created by the input of air keeps the airway open by propelling and splinting the base of the tongue in a forward position. Maintaining airway compliance and patency in this manner is essential. According to Marin et al. (Citation2005), randomised trials of nasal CPAP have revealed significant benefits for patients in terms of drowsiness and neurocognitive performance compared to individuals receiving placebo or subtherapeutic CPAP. Additionally, BP reductions have been documented with CPAP therapy. Significant reduction in cardiovascular risk in untreated OSA is modulated by CPAP when usage is for at least 4 h each night (Marin et al., Citation2005).
The high short term CPAP adherence rate (80%), as seen in Belgium recently, is due to a bundled approach of in-hospital PSGs for OSA diagnoses coupled with the medical and paramedical infrastructure within a multi-disciplinary system geared towards diagnosing and treating OSA patients, including good follow-up practice (Buyse et al., Citation2022).
Patients with an AHI >30 (i.e. severe OSA) have been shown to benefit from nasal CPAP therapy, thereby reducing their risk of cognitive morbidity (Shelton et al., Citation1993; Peppard et al., Citation2000; Redline et al., Citation2007; Punjabi, Citation2008).
ADHERENCE FAILURE TO CPAP THERAPY
Despite CPAP being the universally recognised and recommended initial treatment for OSA, it is known that some patients either do not accept the therapy or stop using it within the first few weeks. The significant abandonment rate (27–46%) can be attributed to non-compliance issues as well as psychological and social variables (Wolkove et al., Citation2008). As a result, many people look to upper airway surgery as a means of alleviating their symptoms (Gay et al., Citation2006). Recent studies have found that the typical CPAP user wears their mask for only 2.6–2.8 h per night (Dissanayake et al., Citation2021).
The use of warm humidification, nasal decongestants and steroids, in addition to comprehensive assistance with regular follow-up, has been shown to enhance CPAP adherence; yet the problem persists (Hoy et al., Citation1999).
Adherence (using the CPAP for at least 4 h per night, in 7 out of 10 nights, for at least 30 days in a row) in the first 90 days was just 72.6% according to recent data from 789 260 individuals initiated on CPAP in the US Centre for Medicare & Medicaid Services database.
To address this issue, many interventions have been implemented, including better mask design, educational programmes, and patient involvement applications (Sunwoo et al., Citation2020). However, the efficacy of these strategies has been modest and this method of treatment still faces issues of non-compliance. Indeed, nonadherence consistently runs between 30% and 40%, especially in healthcare systems where CPAP is not covered by insurance. Given the aforegoing, it is not unexpected that many people with cardiovascular disease, who tend to be less drowsy, were unable to tolerate the CPAP long enough to reap clinically significant advantages from the trials (Rotenberg et al., Citation2016; Tan et al., Citation2018; Keenan and Schwab, Citation2021).
Local issues, such as bloating, nasal drying, dry eyes, and irritation of face skin, are another challenge to using the CPAP machine. In addition, the effects extend to the bedroom. However, it is advised that any treatment-related side effects, such as those caused by the mask or the air pressure, be detected and addressed (Gooneratne et al., Citation2011).
Poor mask fit can lead to leakage, skin pressure and irritation, claustrophobia, dry mouth and nasal congestion, among other uncomfortable symptoms. Although there appears to be no correlation between the type of CPAP mask interface used at the start of treatment and patient adherence, many options have since emerged (Chai-Coetzer et al., Citation2006). Although heated humidification was created to combat dryness, there is currently insufficient evidence to conclude that it improves adherence. Condensation in the tubing or rainout can be prevented by using heated tubing or tube covers to reduce exposure of the tubing air to the cooler surrounding environment (Neill et al., Citation2003; Mador et al., Citation2005).
Rhinitis, congestion, and a dry throat are all symptoms of high blood pressure. Ciliary dysfunction (ciliary beat), nasal mucociliary clearance, nasal inflammation, CSF leak, aerophagy, Gastroesophageal Reflux Disease (GERD), midface hypoplasia, middle ear pressure and pain, and abnormal dental occlusion are further side effects of CPAP usage. Infectious issues, voice alterations, claustrophobia and the noise of the device are among further possible negative outcomes (Ghadiri and Grunstein, Citation2020).
Perhaps due to the poor treatment acceptance, a large disease load remains untreated by CPAP (Lugaresi and Plazzi, Citation1997; Punjabi, Citation2008) prompting sleep specialists to investigate other treatments for OSA sufferers.
MANDIBULAR ADVANCEMENT DEVICES (MAD)
These are removable oral appliances used to advance the lower jaw and chin. Studies utilising cone beam tomography have explored skeletal/dental changes during MAD use, revealing the impact of mandibular protrusion on the vertical relationship between the mandible and maxilla, as well as the antero-superior displacement and rotation of the hyoid bone. These insights help identify suitable candidates for MAD treatment planning (Kim et al., Citation2020). MAD treatment aims to keep the upper airways open during sleep, thereby reducing resistance and the frequency/duration of apnoeas, hypopneas, and snoring events (Mogell et al., Citation2019). Improved night-time oxygenation benefits adult patients with sleep-disordered breathing, positively impacting their quality of life and reducing daytime sleepiness (Ramar et al., Citation2015; Mogell et al., Citation2019).
Since the 1980s, various MAD models have been developed and categorised as prefabricated or customised. Prefabricated MAD, though bulkier, have demonstrated efficacy, especially titratable thermoplastic MAD (Cooke and Battagel, Citation2006; Vanderveken et al., Citation2008). Custom-made MAD (MADc) offer increased comfort, a broader protrusive movement range, and higher therapeutic effectiveness. MADc can either be adjustable (bi-block) or non-adjustable (monoblock) appliances (Ramar et al., Citation2015; Mogell et al., Citation2019).
MAD INDICATIONS, EFFECTIVENESS AND ADHERENCE
MAD are indicated for patients with mild to moderate OSA and primary snoring. However, MAD are also an accepted therapy for patients with severe OSA who do not respond to or do not tolerate positive airway pressure (PAP) therapies (Ramar et al., Citation2015; Mogell et al., Citation2019). Combining MAD with CPAP or other modalities may enhance OSA control (Scherr et al., Citation2014; Ramar et al., Citation2015; Mogell et al., Citation2019). Adherence to MAD is generally higher than CPAP, leading to significant improvements in clinical and polysomnographic outcomes (Vanderveken et al., Citation2013; Sutherland et al., Citation2015).
SURGICAL APPROACHES TO THE TONGUE BASE
Surgical treatments aimed at the tongue base can be approached from 3 perspectives:
Firstly, reducing the volume of the tongue base. Secondly, by protruding the tongue base (tongue base advancement) by using surgical implants to improve compliance of the upper pharyngeal architecture and, thirdly, to increase the pharyngeal volume by simultaneously ostetomising and advancing both the maxilla and mandible, a procedure known as maxillo-mandibular advancement or MMA. This procedure concomitantly advances the tongue base which is attached to the inside of the chin and the body of the hyoid bone. Hence, MMA, has proven to be the most successful surgical intervention and is recognised and accepted globally as the surgical gold standard in OSA management (Zaghi et al., Citation2016).
Radiofrequency (RF) ablation of the tongue base
Coblation, the use of radiofrequency energy and saline is used to shrink and tighten muscle tissue near the back of the tongue. The surgery results in a “permanent reduction” in tongue size and does not affect the surrounding areas. This modality has produced promising early results but was not consistent in the long term (Foster et al., Citation2009). Unfortunately, this procedure has been ineffective in the treatment of OSA (Powell et al., Citation1997; Powell et al., Citation1998). However, in patients reporting snoring, with little or no apnoea found on formal testing, this procedure may be considered. As snoring is a common problem, it is generally not associated with important adverse medical sequelae, in the absence of OSA. Snoring can, however, be a social issue for the bed partners of prominent snorers. In this situation, treatment for snoring with laser-assisted somnoplasty (radiofrequency ablation) may be considered.
(ii) Surgical implants for tongue base advancement (tongue tethering devices)
Tongue suspension devices attempt to advance the base of the tongue and stabilise the genioglossus muscle in an anterior position (Miller et al., Citation2002). Approved methods of tongue suspension in the USA include the AIRvance® (formerly Repose®) system by Medtronic and the EncoreTM system by Siesta Medicals. Both systems rely on a titanium screw fixed to the chin supporting a suture passed through the base of the tongue. Despite significant improvements in AHI and daytime sleepiness, the overall success rate of tongue suspension as a standalone surgical procedure was 35% (20–57%) in a review by Kezirian and Goldberg in 2006 (Kezirian and Goldberg, Citation2006). While most cases of tongue suspension show early successful outcomes, long-term results are poor due to fracture, slippage or migration of devices. The inherent weakness of the intrinsic muscle of the tongue complicates the adherence of implantation devices (Woodson et al., Citation2000; Miller et al., Citation2002; Thomas et al., Citation2003; Fernández-Julián et al., Citation2009; Fibbi et al., Citation2009; Woodson et al., Citation2010; Pavelec et al., Citation2011; Handler et al., Citation2014). The current concepts of tongue suspension have thus not been appraised as successful standalone surgical procedures in the treatment of OSA (HealthCare Citation2016).
| (iii) | Hypoglossal Nerve Stimulation (HNS) | ||||
As a result of inadequate adherence and refractory outcomes from the use of CPAP, alternative therapies have evolved despite the fact that CPAP is presently the first-line treatment for OSA.
Stimulation of the Hypoglossal Nerve, a motor nerve innervating the Protrusor and Retractor muscles of the tongue, via an electrical transducer implanted in the chest wall, can improve OSA by protruding the tongue base (Van de Heyning et al., Citation2012). Electrical pulses are transmitted to the Hypoglossal Nerve between the end of expiration and the beginning of the next expiratory phase. At 6 and 12 months, patients who received HNS have significantly improved AHI and ODI, as well as improvements in symptoms of sleepiness, mood, sleep quality and quality of life (Van de Heyning et al., Citation2012; Strollo et al., Citation2014; Heiser et al., Citation2017).
(iv) Maxillo-Mandibular Advancement (MMA)
MMA is an invasive surgical procedure performed under general anaesthesia, whereupon both the upper and lower jaws are systematically osteotomised and advanced by 8–10 mm. Because the tongue musculature is attached to both the Mandible and Hyoid bones, this MMA procedure successfully opens and increases the volume and 3-dimensional architecture of the upper pharyngeal airspace.
A seminal study by Boyd et al. (Citation2015) evaluated the long-term clinical efficacy and safety of MMA for the treatment of moderate-to-severe OSA. Clinical outcomes included AHI, BP, subjective sleepiness, %REM sleep, and Quality of Life (QOL). The primary outcome measured as AHI, showed that MMA is a clinically effective long-term treatment for patients with moderate-to-severe OSA, with AHI reaching the normal range (AHI < 5 events/h) in 46.7% of patients and 83.4% of patients attaining an AHI of ≤15 events/h without significant subjective sleepiness (ESS ≤ 10) or cardiopulmonary comorbidities. Furthermore, significant improvements in secondary outcomes were observed including decreases in diastolic BP and subjective sleepiness, and significant improvements in QOL. The addition of uvulopalatopharyngoplasty (UPPP) or genioglossal advancement, as adjunctive surgical procedures, did not add significant benefit beyond MMA performed alone. Few adverse events were observed objectively and subjectively following MMA surgery, including long-term sequelae. Surprisingly these long-term results were achieved despite significant weight gain compared to the preoperative state. The average length of follow-up for this study – greater than six years – indicates that the clinical effectiveness of MMA is likely to endure for many years. The mean long-term AHI reduction of 76.9% observed in this study is comparable to the results of three other studies that have reported mean long-term reductions in the AHI ranging from 69% to 84% following MMA performed as the primary procedure (Conradt et al., Citation1997; Jaspers et al., Citation2013). In addition, staged surgical therapy consisting of UPPP was performed in combination with MMA (Riley et al., Citation2000). The results of this study compare well to the results of a recent systematic review and meta-analysis that reported an overall mean reduction in the AHI of 87% when MMA was performed as a primary procedure (Caples et al., Citation2010).
DISCUSSION
While there are multiple sites of obstruction during sleep, the tongue base is recognised as the key role player in the pathogenesis of OSA (Carrera et al., Citation1999; Van de Heyning et al., Citation2012).
The tongue base could be visualised as the gateway for airflow between the upper airway (pharynx) and the tracheo – bronchial airway (of the lungs). All modalities of treatment, albeit medical or conservative e.g. medication/ CPAP or mechanical e.g. MAD, HNS or Surgery, will ultimately have an effect on the anatomical position and function of the tongue and its extrinsic muscular foundation attachments. Thus, medical or surgical modulation of airflow through the upper pharyngeal airspace is entirely dependent upon the response of the tongue base to these interventions.
The successful splinting of the tongue base in an anterior position, as effected by the usage of CPAP, is undoubtedly a reliable and proven method of treating OSA patients and modulating the deleterious sequalae of OSA (Marin et al., Citation2005). By opening the upper airway of OSA sufferers, CPAP not only reverses OSA but at the same time, provide the much-needed oxygenation to the brain and other vital organs, hence reducing the harmful effects of sympathetic drive caused by OSA (Peppard et al., Citation2000; Redline et al., Citation2007; Punjabi, Citation2008).
Undoubtedly, CPAP is currently regarded as the medical gold standard and the first line treatment modality for the successful modulation of OSA (Pepperell et al., Citation2002; Rudnick et al., Citation2007; Epstein et al., Citation2009).
However, the drawback of CPAP therapy, lies not within the treatment itself, but rather in the continuous application and adherence thereto. Hence, failure of CPAP adherence is the main reason why OSA sufferers look elsewhere for alternate comfort in management. If compliance to CPAP usage was simple and easy to cope with, the need for alternate therapies would never have arisen. Patients with moderate to severe OSA have a hard time accepting CPAP. This modality is poorly tolerated by many patients and therefore has poor adherence (Jenkinson et al., Citation1999). A significant proportion (46%) of those so diagnosed either do not initiate or eventually abandon therapy (Wolkove et al., Citation2008). Initiating CPAP therapy or maintaining adherence can be challenging for patients for a number of reasons.
In an effort to enhance the efficacy of long-term therapy, researchers conducted a retrospective study to evaluate compliance over the course of 3.5 years on average. There are two crucial measures to undertake to guarantee CPAP adherence. The first step in preventing a primary failure is convincing patients to start treatment following a successful CPAP titration night. The second is to ensure consistent and prolonged application to forestall secondary failure. According to a long-term adherence investigation conducted by Gabryelska et al. (Citation2021), only 53.7% of patients continued to use their CPAP devices after 42 months (Gabryelska et al., Citation2021). Their findings are consistent with those of other study teams during the past 30 years. Another study found a similar decline in CPAP compliance of 20% over the first 10 months, with compliance dropping to 80% after 3 months. And moreover, Pépin et al., Citation2021, found a 50% CPAP failure after 3 years in a French database analysis (Pepin et al., Citation1999; Pépin et al., Citation2021).
Results from the Randomised Intervention with CPAP in Coronary Artery Disease and Obstructive Sleep Apnoea (RICCADSA) trial showed that 38% of CPAP users quit using their machines during the first year (Peker et al., Citation2016). Patients who utilised CPAP for >4 h per night had a decreased risk of stroke (p = 0.05) and a composite end point of cerebral events (p = 0.02) than those in the usual care group, according to an adjusted on-treatment analysis of cardiovascular risk.
In addition, patients with acute coronary syndrome and OSA, who participated within the Impact of Sleep Apnea syndrome in the evolution of Acute Coronary syndrome (ISAACC) trial, found very low CPAP adherence. Indeed, 1 year after initiating CPAP, the average adherence was only 2.8 ± 2.6 h/night, with only 36% of the patients in the CPAP group reaching ≥4 h/night (Sánchez-de-la-Torre et al., Citation2020).
MAD have evolved as effective tools in treating mild to moderate OSA (Ramar et al., Citation2015). By advancing the mandible in a titrated manner, these devices prevent the collapse of the oropharyngeal tissues and stabilise the base of the tongue. MAD primarily focus on generating mandibular advancement and stabilisation during sleep, thereby promoting anterior traction of the mandible (Haddad and Gregório, Citation2017). This leads to increased tension in the genioglossus and supra-hyoid musculature, resulting in expansion of the air space in the pharyngeal region (Ferguson et al., Citation2006). While MAD have shown efficacy in managing mild OSA, their effectiveness in treating moderate to severe OSA remains uncertain. A retrospective study comparing MMA and MAD devices found that MMA was considerably more effective than MAD at achieving therapeutic success (Jalbert et al., Citation2012; Garreau et al., Citation2014). Short-term side effects of MAD treatment include mild and transient issues like excessive salivation, dry mouth, discomfort in the teeth, irritation in the gingiva, headaches, discomfort in the temporomandibular joint and masticatory muscles (de Almeida et al., Citation2005). Long-term effects may involve dental occlusal changes (de Almeida et al., Citation2006; Hamoda et al., Citation2019). Among these effects are: decreased overbite and overjet, lingual inclination of upper incisors, vestibular inclination of lower incisor teeth, mesialization of lower molars, distalization of upper molars, changes in dental arch crowding, appearance of posterior open bite, and decreased occlusal contacts (Rose et al., Citation2002; de Almeida et al., Citation2006; Alessandri-Bonetti et al., Citation2017; Araie et al., Citation2018). Dental changes develop as a result of the MAD exerting forces on the upper and lower dental arches to maintain protrusion, and jaw resistant counter-forces to maintain the de-novo position. Regular follow-up, including sleep testing is crucial for monitoring adherence, detecting device deterioration, and evaluating oral/craniofacial health. Qualified MAD providers, trained in sleep medicine, play a significant role in achieving optimal therapeutic outcomes (Rose et al., Citation2002).
It has been shown that electrical stimulation of the hypoglossal nerve, thereby protruding the tongue base, can improve OSA (Van de Heyning et al., Citation2012). While this invasive surgical procedure has excellent short-term success, it is also however, costly and there are attendant complications (Gillespie et al., Citation2017). As the HNS device induces the genioglossus muscle to protrude the tongue against the teeth, tongue abrasions were frequent. Using a mouth guard or device reprogramming, these abrasions were remedied (Strollo et al., Citation2014). In addition, complications such as paraesthesias, abnormal sensations, alterations in salivary flow and weakness of the lips have also been documented (Friedman and Wilson, Citation2009; Eastwood et al., Citation2011; Van de Heyning et al., Citation2012; Kezirian et al., Citation2014; Strollo et al., Citation2014; Kent et al., Citation2016; Steffen et al., Citation2018).
The long-term outcome of this invasive and costly form of therapy is currently undergoing further evaluation. Furthermore, MMA has been shown to be more effective than Hypoglossal Nerve Stimulation in reducing AHI (Strollo et al., Citation2014). The incontrovertible evidence for tongue base advancement can be found in documented research with MMA.
In MMA, the tongue base is advanced (on average) by 8–10 mm in both upper and lower jaws. Cephalometric radiographs of the post-operative airway space elucidate the immense success that MMA provides to the airways of OSA sufferers (Abramson et al., Citation2011). MMA is highly effective in treating OSA, with significant improvements in AHI, respiratory disturbance index (RDI), SpO2 nadir and ESS scores (Waite et al., Citation1989; Goh and Lim, Citation2003; Fairburn et al., Citation2007; Serra et al., Citation2012). The overall surgical success and cure rates for MMA were 85.5% and 38.5% for AHI data, and 64.7% and 19.1% for RDI data, respectively. Patients with higher preoperative OSA severity were more likely to achieve surgical success and cure, and experienced greater improvements in AHI and RDI (Sher et al., Citation1996; Friedman and Wilson, Citation2009; Kezirian et al., Citation2011).
A randomised clinical trial conducted by Vicini et al. (Citation2010), compared the efficacy of MMA and auto-titrating positive airway pressure (APAP) in the treatment of OSA. The study found that both treatments significantly improved the mean AHI and ESS scores, with no significant differences between the two groups (Vicini et al., Citation2010). The improvements in subjective sleepiness and quality of life following MMA are also similar to or slightly better than those observed with CPAP therapy (Patel et al., Citation2003; Antic et al., Citation2011; Crawford et al., Citation2012). These improvements have been shown to be sustained in the long term (Dattilo and Drooger, Citation2004; Lye et al., Citation2008; Goodday and Bourque, Citation2012). Additionally, the reductions in blood pressure observed following MMA are comparable to those seen with continuous CPAP therapy (Schein et al., Citation2014). In addition, a meta-analysis by Holty and Guilleminault (Citation2010a) found a significant reduction in the AHI after MMA surgery to treat OSA (Holty and Guilleminault, Citation2010b). Patient factors such as age, preoperative BMI and AHI influence the likelihood and magnitude of surgical success and OSA cure (Boyd et al., Citation2015).
Long-term outcomes of MMA, as documented in this study, showed sustained reductions in subjective sleepiness and improvements in quality of life, as measured by the Functional Outcomes of Sleep Questionnaire (FOSQ) (Dattilo and Drooger, Citation2004; Lye et al., Citation2008; Goodday and Bourque, Citation2012; Doff et al., Citation2013; Strollo et al., Citation2014).
Complications with MMA surgery include pain, swelling, malocclusion, poor cosmetic result, facial numbness, tingling, jaw stiffness, and postsurgical relapse of advancement. Minor haemorrhage, local infection, and extrusion of hardware have also been reported (Hochban et al., Citation1997; Riley et al., Citation2000; Holty and Guilleminault, Citation2010a; Pirklbauer et al., Citation2011). Facial paraesthesia due to stretching or injury to the inferior alveolar nerve is universally common (100% of patients) but has been reported to resolve in 85% to 90% of patients within 6–12 postoperative months. Patient perception of facial aesthetics has generally been positive after MMA. Modified MMA techniques, such as using counterclockwise rotation and presurgical or postsurgical orthodontics, have been developed to prevent maxillary protrusion and to improve facial aesthetics (Li et al., Citation2000a). The mean duration of surgery according to a study was 6.0 (±1.0) hours (Vicini et al., Citation2010). After undergoing MMA, patients require a mean of 3.5 days of hospitalisation. Most patients can return to their regular functional status within 2–10 weeks after surgery (Prinsell, Citation1999). Major complications are rare (approximately 1%) and are associated with being older and having preoperative medical comorbidities (Holty and Guilleminault, Citation2010b). Because many patients with OSA undergoing MMA are obese (mean BMI >30.2) and have compromised airways, careful postoperative care is warranted, including postoperative evaluation by nasopharyngolaryngoscopy (Li et al., Citation2000b). Finally, longer post operative follow-up to MMA is advocated because recurrences of OSA have been noted at 10–15 years after MMA surgery (Zaghi et al., Citation2016). Hence, regular follow-up and repeat sleep studies after all surgical procedures for OSA management are helpful.
Surgical implants to the tongue base have also been used to treat OSA. The Advance® system made by Medtronic uses a titanium screw that is inserted into the lingual plate of the symphysis of the mandible at the floor of the mouth (Miller et al., Citation2002). A loop of suture is passed through the base of the tongue and attached to the screw. This procedure provides a hammock or suspension for the tongue, making it less likely for the base of the tongue to collapse during sleep. However, during wakefulness, the suture material acts as a cutting device and migrates anteriorly losing its efficacy within a few months.
Another tongue suspension device known as the Repose® again has only limited efficacy (Woodson et al., Citation2010). This device also utilises the suture and screw concept. Again, the gains are only short term and below is some evidence relating to its long-term prognosis as a tongue suspension device: The Harvard Pilgrim Healthcare Medical policy on surgical tongue base suspension published in September 2008, stated that “the Repose system does not result in permanent anatomical change in the posterior airway” and this is due to the slippage that occurs in the long term with this particular method. Furthermore, in the ERS Taskforce publication by Randerath et al. (Citation2011) on page 1015 paragraph 2 states “As the aim of the tongue suspension is to stabilize and support the tongue base rather than to advance it is not surprising that Miller and co-workers (Citation2002) and Terris and co-workers (Citation2002) fail to find relevant changes in the posterior airway space”. This confirms the concept that tongue advancement as opposed to only stabilisation is a way forward in the surgical management of OSA. A Proof-of – Concept study was conducted by the first author (R.H.) in 2019 whereupon a polypropylene (PP) tongue suspension device was implanted into 10 moderate to severe OSA in Fujita type III OSA patients (see ) with simultaneous advancement of the tongue base by 10 mm (Fujita et al., Citation1981; Hendricks et al., Citation2019). This device relied on the biological principles of tissue formation aimed at the ingrowth of tissue into the implanted device (Ramrattan et al., Citation2005). The rationale was to facilitate tongue anchorage to increase posterior airway space without restricting tongue movement essential for speech, swallowing etc. However, fibrous encapsulation of the PP mesh lent firmness to the device, but at the same time prevented slippage and failure. The evidence for this fibrous encapsulation was confirmed in an ovine model conducted by the first author (R.H.) in 2016 (Hendricks et al., Citation2021).
Figure 2. Fujita classification: Type 1 obstruction at retropalatal level; Type 2 obstruction at retropalatal and retroglossal level Type 3: obstruction at retroglossal level.
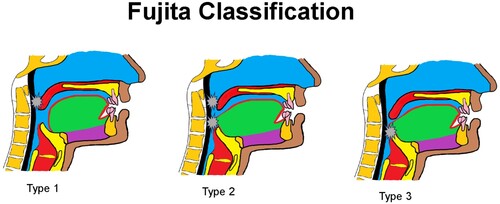
In this study, the severity of OSA was very significant (AHI average of 50.4 events/h), clearly indicating the successful outcome of tongue base advancement. An audit carried out after 7 years, confirmed complete reversal of OSA in all 10 patients who underwent tongue base advancement surgery (Hendricks et al., Citation2019).
CONCLUSION
The role of the tongue base in OSA has been identified and discussed in detail. It has been shown that the various modalities of treatment for OSA albeit medical or surgical have an influence on the tongue base itself. In the case of CPAP which effectively splints the tongue base in an anterior position hence providing a patent upper airway sustained by the constant pressure exerted upon the pharyngeal architecture by the CPAP machine, the modulation of OSA is sustainable. The benefits of optimal CPAP adherence has been discussed.
Nevertheless, a significant drawback pertains to patient adherence, as a considerable percentage of individuals either abandon or fail to tolerate CPAP therapy.
In the case of mild to moderate OSA, MAD offers an alternative treatment option by stabilising the tongue base and preventing the collapse of oropharyngeal tissues. Concerns emerge with respect to the potential long-term side effects of MAD on the dental occlusion and the necessity for consistent monitoring by certified professionals, despite the demonstrated effectiveness of these devices.
Hypoglossal Nerve Stimulation (HNS) which employs electrical stimulation to protrude the base of the tongue, has demonstrated limited long-term efficacy. However, the procedure of installation is invasive with known complications and difficulties such as tongue abrasions, device malfunctions, tongue pain and discomfort, speech changes and tongue weakness and fatigue.
MMA surgery is recognised as a comprehensive and efficacious remedy for OSA. While MMA exhibits comparable outcomes to CPAP therapy in terms of enduring reductions in subjective sleepiness, reducing the risks for cardiovascular events, enhancements in quality of life, and sustained reductions in subjective sleepiness, it is not devoid of complications including pain, swelling and malocclusion etc. In addition, this form of therapy is limited to specialised hospital facilities with high care/ICU post operative management demands not withstanding the high cost of treatment per capita. Thus, despite its unbridled efficacy, MMA is not a sustainable solution globally.
While surgical tongue tethering implants, such as the Advance® system, are intended to advance and maintain the anterior position of the tongue base during sleep, their effectiveness over an extended period has been demonstrated to be insufficient. Tongue suspension devices, such as Repose®, demonstrate restricted efficacy for a permanent anatomical alteration in the upper airway.
A proof-of-concept investigation by the primary author (R.H) presents a tongue suspension device made of polypropylene, which demonstrates encouraging results in the reversal of OSA via advancement of the tongue base. Without the invasive orthognathic surgery advocated by the MMA protagonists, it is also possible to attain a successful surgical result comparable to that of MMA, in which the tongue base is advanced by 10 mm. This exciting concept is currently under investigation by the primary author (R.H).
ACKNOWLEDGEMENTS
The proof of concept and surgical technique of the BTTT is protected by University of Cape Town Patent filed internationally under the number of WO 2017/2012449 A1.
DISCLOSURE STATEMENT
No potential conflict of interest was reported by the author(s).
REFERENCES
- Abramson, Z., Susarla, S.M., Lawler, M., Bouchard, C., Troulis, M. & Kaban, L.B. 2011. Three-dimensional computed tomographic airway analysis of patients with obstructive sleep apnea treated by maxillomandibular advancement. Journal of Oral and Maxillofacial Surgery 69 (3): 677–686.
- Alessandri-Bonetti, G., D’Antò, V., Stipa, C., Rongo, R., Incerti-Parenti, S. & Michelotti, A. 2017. Dentoskeletal effects of oral appliance wear in obstructive sleep apnoea and snoring patients. European Journal of Orthodontics 39 (5): 482–488.
- Antic, N.A., Catcheside, P., Buchan, C., Hensley, M., Naughton, M.T., Rowland, S., Williamson, B., Windler, S. & McEvoy, R.D. 2011. The effect of CPAP in normalizing daytime sleepiness, quality of life, and neurocognitive function in patients with moderate to severe OSA. Sleep 34 (1): 111–119.
- Araie, T., Okuno, K., Minagi, H.O. & Sakai, T. 2018. Dental and skeletal changes associated with long-term oral appliance use for obstructive sleep apnea: a systematic review and meta-analysis. Sleep Medicine Reviews 41: 161–172.
- Ballard, R.D., Irvin, C.G., Martin, R.J., Pak, J., Pandey, R. & White, D.P. 1990. Influence of sleep on lung volume in asthmatic patients and normal subjects. Journal of Applied Physiology 68 (5): 2034–2041.
- Benjafield, A.V., Ayas, N.T., Eastwood, P.R., Heinzer, R., Ip, M.S., Morrell, M.J., Nunez, C.M., Patel, S.R., Penzel, T., Pépin, J.L. & Peppard, P.E. 2019. Estimation of the global prevalence and burden of obstructive sleep apnoea: a literature-based analysis. The Lancet Respiratory Medicine 7 (8): 687–698.
- Boyd, S.B., Walters, A.S., Waite, P., Harding, S.M. & Song, Y. 2015. Long-term effectiveness and safety of maxillomandibular advancement for treatment of obstructive sleep apnea. Journal of Clinical Sleep Medicine 11 (7): 699–708.
- Brown, J., Yazdi, F., Jodari-Karimi, M., Owen, J.G. & Reisin, E. 2022. Obstructive sleep apnea and hypertension: updates to a critical relationship. Current Hypertension Reports 24 (6): 173–184.
- Budhiraja, R., Javaheri, S., Parthasarathy, S., Berry, R.B. & Quan, S.F. 2019. The association between obstructive sleep apnea characterized by a minimum 3 percent oxygen desaturation or arousal hypopnea definition and hypertension. Journal of Clinical Sleep Medicine 15 (9): 1261–1270.
- Buyse, B., Bruyneel, M., Verbraecken, J. & Testelmans, D. 2022. High adherence to continuous positive airway pressure (CPAP) in patients with obstructive sleep apnea (OSA) in Belgium: a narrative review. Acta Clinica Belgica 77 (3): 710–720.
- Caples, S.M., Rowley, J.A., Prinsell, J.R., Pallanch, J.F., Elamin, M.B., Katz, S.G. & Harwick, J.D. 2010. Surgical modifications of the upper airway for obstructive sleep apnea in adults: a systematic review and meta-analysis. Sleep 33 (10): 1396–1407.
- Carrera, M., Barbe, F., Sauleda, J., Tomas, M., Gomez, C. & Agusti, A.G. 1999. Patients with obstructive sleep apnea exhibit genioglossus dysfunction that is normalized after treatment with continuous positive airway pressure. American Journal of Respiratory and Critical Care Medicine 159 (6): 1960–1966.
- Chai-Coetzer, C.L., Pathinathan, A. & Smith, B.J. 2006. Continuous positive airway pressure delivery interfaces for obstructive sleep apnoea. Cochrane Database of Systematic Reviews (2006 (4): 1–25. CD 005308.
- Chattu, V.K., Manzar, M.D., Kumary, S., Burman, D., Spence, D.W. & Pandi-Perumal, S.R. 2019. The global problem of insufficient sleep and its serious public health implications. Healthcare 7 (1): 1. MDPI.
- Conradt, R., Hochban, W., Brandenburg, U., Heitmann, J. & Peter, J.H. 1997. Long-term follow-up after surgical treatment of obstructive sleep apnoea by maxillomandibular advancement. European Respiratory Journal 10 (1): 123–128.
- Cooke, M.E. & Battagel, J.M. 2006. A thermoplastic mandibular advancement device for the management of non-apnoeic snoring: a randomized controlled trial. The European Journal of Orthodontics 28 (4): 327–338.
- Crawford, M.R., Bartlett, D.J., Coughlin, S.R., Phillips, C.L., Neill, A.M., Espie, C.A., Dungan, G.C., Wilding, J.P., Calverley, P.M., Grunstein, R.R. & Marshall, N.S. 2012. The effect of continuous positive airway pressure usage on sleepiness in obstructive sleep apnoea: real effects or expectation of benefit? Thorax 67 (10): 920–924.
- Dattilo, D.J. & Drooger, S.A. 2004. Outcome assessment of patients undergoing maxillofacial procedures for the treatment of sleep apnea: comparison of subjective and objective results. Journal of Oral and Maxillofacial Surgery 62 (2): 164–168.
- de Almeida, F.R., Lowe, A.A., Otsuka, R., Fastlicht, S., Farbood, M. & Tsuiki, S. 2006. Long-term sequellae of oral appliance therapy in obstructive sleep apnea patients: Part 2. Study-model analysis. American Journal of Orthodontics and Dentofacial Orthopedics 129 (2): 205–213.
- de Almeida, F.R., Lowe, A.A., Tsuiki, S., Otsuka, R., Wong, M., Fastlicht, S. & Ryan, F. 2005. Long-term compliance and side effects of oral appliances used for the treatment of snoring and obstructive sleep apnea syndrome. Journal of Clinical Sleep Medicine 1 (02): 143–152.
- Dissanayake, H.U., Colpani, J.T., Sutherland, K., Loke, W., Mohammadieh, A., Ou, Y.H., de Chazal, P., Cistulli, P.A. & Lee, C.H. 2021. Obstructive sleep apnea therapy for cardiovascular risk reduction—time for a rethink? Clinical Cardiology 44 (12): 1729–1738.
- Doff, M.H., Hoekema, A., Wijkstra, P.J., van der Hoeven J.H., Huddleston Slater J.J.R., de Bont L.G.M., & Stegenga B. 2013. Oral appliance versus continuous positive airway pressure in obstructive sleep apnea syndrome: a 2-year follow-up. Sleep 36: 1289–96.
- Eastwood, P.R., Barnes, M., Walsh, J.H., Maddison, K.J., Hee, G., Schwartz, A.R., Smith, P.L., Malhotra, A., McEvoy, R.D., Wheatley, J.R. & O'Donoghue, F.J. 2011. Treating obstructive sleep apnea with hypoglossal nerve stimulation. Sleep 34 (11): 1479–1486.
- Eiseman, N.A., Westover, M.B., Ellenbogen, J.M. & Bianchi, M.T. 2012. The impact of body posture and sleep stages on sleep apnea severity in adults. Journal of Clinical Sleep Medicine 8 (6): 655–666.
- Epstein, L.J., Kristo, D., Strollo, P.J., Jr, Friedman, N., Malhotra, A., Patil, S.P., Ramar, K., Rogers, R., Schwab, R.J., Weaver, E.M. & Weinstein, M.D. 2009. Adult obstructive sleep apnea task force of the American Academy of sleep medicine. Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults. Journal of Clinical Sleep Medicine 5 (3): 263–76.
- Faccenda, J.F., Mackay, T.W., Boon, N.A. & Douglas, N.J. 2001. Randomized placebo-controlled trial of continuous positive airway pressure on blood pressure in the sleep apnea–hypopnea syndrome. American Journal of Respiratory and Critical Care Medicine 163 (2): 344–348.
- Fairburn, S.C., Waite, P.D., Vilos, G., Harding, S.M., Bernreuter, W., Cure, J. & Cherala, S. 2007. Three-dimensional changes in upper airways of patients with obstructive sleep apnea following maxillomandibular advancement. Journal of Oral and Maxillofacial Surgery 65 (1): 6–12.
- Ferguson, K.A., Cartwright, R., Rogers, R. & Schmidt-Nowara, W. 2006. Oral appliances for snoring and obstructive sleep apnea: a review. Sleep 29 (2): 244–262.
- Fernández-Julián, E., Muñoz, N., Achiques, M.T., García-Pérez, M.A., Orts, M. & Marco, J. 2009. Randomized study comparing two tongue base surgeries for moderate to severe obstructive sleep apnea syndrome. Otolaryngology—Head & Neck Surgery 140 (6): 917–923.
- Fibbi, A., Ameli, F., Brocchetti, F., Mignosi, S., Cabano, M.E. & Semino, L. 2009. Tongue base suspension and radiofrequency volume reduction: a comparison between 2 techniques for the treatment of sleep-disordered breathing. American Journal of Otolaryngology 30 (6): 401–406.
- Fogel, R.B., Trinder, J., Malhotra, A., Stanchina, M., Edwards, J.K., Schory, K.E. & White, D.P. 2003. Within-breath control of genioglossal muscle activation in humans: effect of sleep-wake state. The Journal of Physiology 550 (3): 899–910.
- Foster, G.D., Sanders, M.H., Millman, R., Zammit, G., Borradaile, K.E., Newman, A.B., Wadden, T.A., Kelley, D., Wing, R.R., Pi Sunyer, F.X. & Darcey, V. 2009. Obstructive sleep apnea among obese patients with type 2 diabetes. Diabetes Care 32 (6): 1017–1019.
- Friedman, M. & Wilson, M. 2009. Re-redefining success in airway surgery for obstructive sleep apnea. Sleep 32 (1): 17.
- Fujita, S., Conway, W., Zorick, F. & Roth, T. 1981. Surgical correction of anatomic abnormalities in obstructive sleep apnea syndrome: uvulopalatopharyngoplasty. Otolaryngology-Head & Neck Surgery 89 (6): 923–934.
- Gabryelska, A., Mokros, Ł, Kardas, G., Panek, M., Riha, R. & Białasiewicz, P. 2021. The predictive value of BOAH scale for screening obstructive sleep apnea in patients at a sleep clinic in Scotland. Sleep & Breathing 25: 355–359.
- Garreau, E., Wojcik, T., Bouscaillou, J., Ferri, J. & Raoul, G. 2014. Comparative effectiveness of maxillomandibular advancement surgery versus mandibular advancement device for patients with moderate or severe obstructive sleep area. French Orthodontics 85 (2): 163–173.
- Gay, P., Weaver, T., Loube, D. & Iber, C. 2006. Positive airway pressure task force; standards of practice committee; American Academy of sleep medicine. Evaluation of positive airway pressure treatment for sleep related breathing disorders in adults. Sleep 29 (3): 381–401.
- Geovanini, G.R. & Lorenzi-Filho, G. 2018. Cardiac rhythm disorders in obstructive sleep apnea. Journal of Thoracic Disease 10 (Suppl 34): S4221.
- Ghadiri, M. & Grunstein, R.R. 2020. Clinical side effects of continuous positive airway pressure in patients with obstructive sleep apnoea. Respirology 25 (6): 593–602.
- Gillespie, M.B., Soose, R.J., Woodson, B.T., Strohl, K.P., Maurer, J.T., de Vries, N., Steward, D.L., Baskin, J.Z., Badr, M.S., Lin, H.S. & Padhya, T.A. 2017. Upper airway stimulation for obstructive sleep apnea: patient-reported outcomes after 48 months of follow-up. Otolaryngology–Head & Neck Surgery 156 (4): 765–771.
- Goh, Y.H. & Lim, K.A. 2003. Modified maxillomandibular advancement for the treatment of obstructive sleep apnea: a preliminary report. The Laryngoscope 113 (9): 1577–1582.
- Goodday, R. & Bourque, S. 2012. Subjective outcomes of maxillomandibular advancement surgery for treatment of obstructive sleep apnea syndrome. Journal of Oral and Maxillofacial Surgery 70 (2): 417–420.
- Gooneratne, N.S., Richards, K.C., Joffe, M., Lam, R.W., Pack, F., Staley, B., Dinges, D.F. & Pack, A.I. 2011. Sleep disordered breathing with excessive daytime sleepiness is a risk factor for mortality in older adults. Sleep 34 (4): 435–442.
- Haddad, F.L.M. & Gregório, L.C. 2017. Manual do residente: medicina do sono. Tratamento dos distúrbios obstrutivos com aparelhos de pressão positiva: visão prática. 1st ed., Barueri, Manole.
- Hamoda, M.M., Almeida, F.R. & Pliska, B.T. 2019. Long-term side effects of sleep apnea treatment with oral appliances: nature, magnitude and predictors of long-term changes. Sleep Medicine 56: 184–191.
- Handler, E., Hamans, E., Goldberg, A.N. & Mickelson, S. 2014. Tongue suspension: an evidence-based review and comparison to hypopharyngeal surgery for OSA. The Laryngoscope 124 (1): 329–336.
- Haponik, E.F., Smith, P.L., Bohlman, M.E., Allen, R.P., Goldman, S.M. & Bleecker, E.R. 1983. Computerized tomography in obstructive sleep apnea: correlation of airway size with physiology during sleep and wakefulness. American Review of Respiratory Disease 127 (2): 221–226.
- HealthCare HP. 2016. Medical policy surgical tongue base suspension 2016. https://www.harvardpilgrim.org/pls/portal/docs/page/providers/medmgmt/statements/tongue_suspension_updates_clean.pdf (accessed August 2016)
- Heiser, C., Maurer, J.T., Hofauer, B., Sommer, J.U., Seitz, A. & Steffen, A. 2017. Outcomes of upper airway stimulation for obstructive sleep apnea in a multicenter German postmarket study. Otolaryngology–Head and Neck Surgery 156 (2): 378–384.
- Hendricks, R., Davids, M., Khalfey, H., Landman, H.J., Theron, A.E., Engela, E. & Dheda, K. 2019. Sleepiness score-specific outcomes of a novel tongue repositioning procedure for the treatment of continuous positive airway pressure-resistant obstructive sleep apnea. Annals of Maxillofacial Surgery 9 (1): 28.
- Hendricks, R., Hofmann, E., Peres, J., Prince, S., Hille, J., Davies, N.H. & Bezuidenhout, D. 2021. Tendon-like tether formation for tongue-base advancement in an ovine model using a novel implant device intended for the surgical management of obstructive sleep apnoea. Journal of Biomedical Materials Research Part B: Applied Biomaterials 109 (7): 1005–1016.
- Hochban, W., Conradt, R., Brandenburg, U., Heitmann, J. & Peter, J.H. 1997. Surgical maxillofacial treatment of obstructive sleep apnea. Plastic & Reconstructive Surgery 99 (3): 619–626.
- Holty, J.E.C. & Guilleminault, C. 2010a. Maxillomandibular advancementfor the treatment of obstructive sleep apnoea: a systemic review and meta- analysis. Sleep Medicine Reviews 14 (5): 287–297.
- Holty, J.E.C & Guilleminault, C. 2010b. Surgical options for the management of obstructive sleep apnoea. Medical Clinics 94 (3): 479–515.
- Horner, R.L. 1996. Motor control of the pharyngeal musculature and implications for the pathogenesis of obstructive sleep apnea. Sleep 19 (10): 827–853.
- Horner, R.L., Innes, J.A., Morrell, M.J., Shea, S.A. & Guz, A. 1994. The effect of sleep on reflex genioglossus muscle activation by stimuli of negative airway pressure in humans. The Journal of Physiology 476 (1): 141–151.
- Hoy, C.J., Vennelle, M., Kingshott, R.N., Engleman, H.M. & Douglas, N.J. 1999. Can intensive support improve continuous positive airway pressure use in patients with the sleep apnea/hypopnea syndrome? American Journal of Respiratory &Critical Care Medicine 159 (4): 1096–1100.
- Isono, S., Remmers, J.E., Tanaka, A., Sho, Y., Sato, J. & Nishino, T. 1997. Anatomy of pharynx in patients with obstructive sleep apnea and in normal subjects. Journal of Applied Physiology 82 (4): 1319–1326.
- Jalbert, F., Lacassagne, L., Bessard, J., Dekeister, C., Paoli, J.R. & Tiberge, M. 2012. Orthèse d’avancée mandibulaire ou ostéotomie maxillo-mandibulaire pour le traitement des syndromes d’apnées obstructives du sommeil sévères refusant la PPC. Revue de Stomatologie et de Chirurgie Maxillo-faciale 113 (1): 19–26.
- Jaspers, G.W., Booij, A., De Graaf, J. & De Lange, J. 2013. Long-term results of maxillomandibular advancement surgery in patients with obstructive sleep apnoea syndrome. British Journal of Oral and Maxillofacial Surgery 51 (3): e37–e39.
- Jehan, S., Zizi, F., Pandi-Perumal, S.R., Wall, S., Auguste, E., Myers, A.K., Jean-Louis, G. & McFarlane, S.I. 2017. Obstructive sleep apnea and obesity: implications for public health. Sleep Medicine & Disorders: International Journal 1 (4): 1–15.
- Jenkinson, C., Davies, R.J., Mullins, R. & Stradling, J.R. 1999. Comparison of therapeutic and subtherapeutic nasal continuous positive airway pressure for obstructive sleep apnoea: a randomised prospective parallel trial. The Lancet 353 (9170): 2100–2105.
- Johns, M.W. 1991. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep 14 (6): 540–545.
- Kapur, V., Strohl, K.P., Redline, S., Iber, C., O'connor, G. & Nieto, J. 2002. Underdiagnosis of sleep apnea syndrome in US communities. Sleep and Breathing 6 (02): 049–054.
- Keenan, B.T. & Schwab, R.J. 2021. Using the remote monitoring framework to promote adherence to continuous positive airway pressure. Sleep Medicine Clinics 16 (1): 85–99.
- Kent, D.T., Lee, J.J., Strollo, P.J., Jr & Soose, R.J. 2016. Upper airway stimulation for OSA: early adherence and outcome results of one centre. Otolaryngology–Head and Neck Surgery 155 (1): 188–193.
- Kezirian, E.J., Goding, G.S., Jr, Malhotra, A., O'donoghue, F.J., Zammit, G., Wheatley, J.R., Catcheside, P.G., Smith, P.L., Schwartz, A.R., Walsh, J.H. & Maddison, K.J. 2014. Hypoglossal nerve stimulation improves obstructive sleep apnea: 12-month outcomes. Journal of Sleep Research 23 (1): 77–83.
- Kezirian, E.J. & Goldberg, A.N. 2006. Hypopharyngeal surgery in obstructive sleep apnea: an evidence-based medicine review. Archives of Otolaryngology–Head & Neck Surgery 132 (2): 206–213.
- Kezirian, E.J., Weaver, E.M., Criswell, M.A., De Vries, N., Woodson, B.T. & Piccirillo, J.F. 2011. Reporting results of obstructive sleep apnea syndrome surgery trials. Otolaryngology–Head and Neck Surgery 144 (4): 496–499.
- Kim, D.I., Lagravère Vich, M., Mayoral, P. & Miguez, M. 2020. Three-dimensional changes in skeletal/dental landmarks with use of mandibular advancement devices. Journal of Dental Sleep Medicine 7 (2): 1–18.
- Kobayashi, I., Perry, A., Rhymer, J., Wuyam, B., Hughes, P., Murphy, K., Innes, J.A., McIvor, J., Cheesman, A.D. & Guz, A. 1996. Inspiratory coactivation of the genioglossus enlarges retroglossal space in laryngectomized humans. Journal of Applied Physiology 80 (5): 1595–1604.
- Kobayashi, M., Sakaida, H., Yuta, A., Takeuchi, K., Shimizu, T. & Majima, Y. 2003. Therapeutic results of respiratory disturbance during sleep in children. Nihon Jibiinkoka Gakkai Kaiho 106 (8): 815–22.
- Kubin, L., Reignier, C., Tojima, H., Taguchi, O., Pack, A.I. & Davies, R.O. 1994. Changes in serotonin level in the hypoglossal nucleus region during carbachol-induced atonia. Brain Research 645 (1-2): 291–302.
- Kubin, L., Tojima, H., Reignier, C., Pack, A.I. & Davies, R.O. 1996. Interaction of serotonergic excitatory drive to hypoglossal motoneurons with carbachol-induced, REM sleep-like atonia. Sleep 19 (3): 189–195.
- Lévy, P., Kohler, M., McNicholas, W.T., Barbé, F., McEvoy, R.D., Somers, V.K., Lavie, L. & Pépin, J.L. 2015. Obstructive sleep apnoea syndrome. Nature Reviews Disease Primers 1 (1): 1–21.
- Li, K.K., Riley, R.W., Powell, N.B. & Guilleminault, C. 2000a. Maxillomandibular advancement for persistent obstructive sleep apnea after phase I surgery in patients without maxillomandibular deficiency. The Laryngoscope 110 (10): 1684–1688.
- Li, K.K., Riley, R.W., Powell, N.B. & Zonato, A. 2000b. Fiberoptic nasopharyngolaryngoscopy for airway monitoring after obstructive sleep apnea surgery. Journal of Oral and Maxillofacial Surgery 58 (12): 1342–1345.
- Lugaresi, E. & Plazzi, G. 1997. Heavy snorer disease: from snoring to the sleep apnea syndrome-an overview. Respiration 64 (1): 11–14.
- Lye, K.W., Waite, P.D., Meara, D. & Wang, D. 2008. Quality of life evaluation of maxillomandibular advancement surgery for treatment of obstructive sleep apnea. Journal of Oral and Maxillofacial Surgery 66 (5): 968–972.
- Mador, M.J., Krauza, M., Pervez, A., Pierce, D. & Braun, M. 2005. Effect of heated humidification on compliance and quality of life in patients with sleep apnea using nasal continuous positive airway pressure. Chest 128 (4): 2151–2158.
- Malhotra, A., Orr, J.E. & Owens, R.L. 2015. On the cutting edge of obstructive sleep apnoea: where next? The Lancet Respiratory Medicine 3 (5): 397–403.
- Malhotra, A., Pillar, G., Fogel, R.B., Beauregard, J., Edwards, J.K., Slamowitz, D.I., Shea, S.A. & White, D.P. 2000. Genioglossal but not palatal muscle activity relates closely to pharyngeal pressure. American Journal of Respiratory & Critical Care Medicine 162 (3): 1058–1062.
- Malhotra, A., Pillar, G., Fogel, R.B., Edwards, J.K., Ayas, N., Akahoshi, T., Hess, D. & White, D.P. 2002. Pharyngeal pressure and flow effects on genioglossus activation in normal subjects. American Journal of Respiratory & Critical Care Medicine 165 (1): 71–77.
- Malhotra, A. & White, D.P. 2002. Obstructive sleep apnoea. The Lancet 360 (9328): 237–245.
- Marin, J.M., Carrizo, S.J., Vicente, E. & Agusti, A.G. 2005. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. The Lancet 365 (9464): 1046–1053.
- Mathew, O.P., Abu-Osba, Y.K. & Thach, B.T. 1982. Genioglossus muscle responses to upper airway pressure changes: afferent pathways. Journal of Applied Physiology 52 (2): 445–450.
- Miller, F.R., Watson, D. & Malis, D. 2002. Role of the tongue base suspension suture with The Repose System bone screw in the multilevel surgical management of obstructive sleep apnea. Otolaryngology—Head and Neck Surgery 126 (4): 392–398.
- Mogell, K., Blumenstock, N., Mason, E., Rohatgi, R., Shah, S. & Schwartz, D. 2019. Definition of an effective oral appliance for the treatment of obstructive sleep apnea and snoring: an update for 2019. Journal of Dental Sleep Medicine 6 (3): 1–4.
- Morrison, J.A., Friedman, L.A. & Gray-McGuire, C. 2007. Metabolic syndrome in childhood predicts adult cardiovascular disease 25 years later: the Princeton Lipid Research clinics follow-up study. Pediatrics 120 (2): 340–345.
- Nagappa, M., Liao, P., Wong, J., Auckley, D., Ramachandran, S.K., Memtsoudis, S., Mokhlesi, B. & Chung, F. 2015. Validation of the STOP-Bang questionnaire as a screening tool for obstructive sleep apnea among different populations: a systematic review and meta-analysis. PLoS One 10 (12): e0143697.
- Neill, A.M., Wai, H.S., Bannan, S.P.T., Beasley, C.R., Weatherall, M. & Campbell, A.J. 2003. Humidified nasal continuous positive airway pressure in obstructive sleep apnoea. European Respiratory Journal 22 (2): 258–262.
- Onal, E., Lopata, M. & O'connor, T.D. 1981. Diaphragmatic and genioglossal electromyogram responses to CO2 rebreathing in humans. Journal of Applied Physiology 50 (5): 1052–1055.
- Patel, S.R., White, D.P., Malhotra, A., Stanchina, M.L. & Ayas, N.T. 2003. Continuous positive airway pressure therapy for treating gess in a diverse population with obstructive sleep apnea: results of a meta-analysis. Archives of Internal Medicine 163 (5): 565–571.
- Pavelec, V., Hamans, E. & Stuck, B.A. 2011. A study of the new generation of the advance system tongue implants: three-and six-month effects of tongue to mandible tethering for obstructive sleep apnea. The Laryngoscope 121 (11): 2487–2493.
- Pedrosa, R.P., Drager, L.F., Gonzaga, C.C., Sousa, M.G., de Paula, L.K., Amaro, A.C., Amodeo, C., Bortolotto, L.A., Krieger, E.M., Bradley, T.D. & Lorenzi-Filho, G. 2011. Obstructive sleep apnea: the most common secondary cause of hypertension associated with resistant hypertension. Hypertension 58 (5): 811–817.
- Peker, Y., Glantz, H., Eulenburg, C., Wegscheider, K., Herlitz, J. & Thunström, E. 2016. Effect of positive airway pressure on cardiovascular outcomes in coronary artery disease patients with nonsleepy obstructive sleep apnea. The RICCADSA randomized controlled trial. American Journal of Respiratory & Critical Care Medicine 194 (5): 613–620.
- Pépin, J.L., Bailly, S., Rinder, P., Adler, D., Szeftel, D., Malhotra, A., Cistulli, P.A., Benjafield, A., Lavergne, F., Josseran, A. & Tamisier, R. 2021. CPAP therapy termination rates by OSA phenotype: a French nationwide database analysis. Journal of Clinical Medicine 10 (5): 936.
- Pepin, J.L., Krieger, J., Rodenstein, D., Cornette, A., Sforza, E., Delguste, P., Deschaux, C., Grillier, V. & Levy, P. 1999. Effective compliance during the first 3 months of continuous positive airway pressure: a European prospective study of 121 patients. American Journal of Respiratory & Critical Care Medicine 160 (4): 1124–1129.
- Peppard, P.E., Young, T., Palta, M. & Skatrud, J. 2000. Prospective study of the association between sleep-disordered breathing and hypertension. New England Journal of Medicine 342 (19): 1378–1384.
- Pepperell, J.C., Ramdassingh-Dow, S., Crosthwaite, N., Mullins, R., Jenkinson, C., Stradling, J.R. & Davies, R.J. 2002. Ambulatory blood pressure after therapeutic and subtherapeutic nasal continuous positive airway pressure for obstructive sleep apnoea: a randomised parallel trial. The Lancet 359 (9302): 204–210.
- Pirklbauer, K., Russmueller, G., Stiebellehner, L., Nell, C., Sinko, K., Millesi, G. & Klug, C. 2011. Maxillomandibular advancement for treatment of obstructive sleep apnea syndrome: a systematic review. Journal of Oral and Maxillofacial Surgery 69 (6): e165–e176.
- Powell, N.B., Riley, R.W., Troell, R.J., Blumen, M.B. & Guilleminault, C. 1997. Radiofrequency volumetric reduction of the tongue: a porcine pilot study for the treatment of obstructive sleep apnea syndrome. Chest 111 (5): 1348–1355.
- Powell, N.B., Riley, R.W., Troell, R.J., Li, K., Blumen, M.B. & Guilleminault, C. 1998. Radiofrequency volumetric tissue reduction of the palate in subjects with sleep-disordered breathing. Chest 113 (5): 1163–1174.
- Prinsell, J.R. 1999. Maxillomandibular advancement surgery in a site-specific treatment approach for obstructive sleep apnea in 50 consecutive patients. Chest 116 (6): 1519–1529.
- Punjabi, N.M. 2008. The epidemiology of adult obstructive sleep apnea. Proceedings of the American Thoracic Society 5 (2): 136–143.
- Qin, H., Steenbergen, N., Glos, M., Wessel, N., Kraemer, J.F., Vaquerizo-Villar, F. & Penzel, T. 2021. The different facets of heart rate variability in obstructive sleep apnea. Frontiers in Psychiatry 12: 642333.
- Ramar, K., Dort, L.C., Katz, S.G., Lettieri, C.J., Harrod, C.G., Thomas, S.M. & Chervin, R.D. 2015. Clinical practice guideline for the treatment of obstructive sleep apnea and snoring with oral appliance therapy: an update for 2015: an American Academy of Sleep Medicine and American Academy of Dental Sleep Medicine clinical practice guideline. Journal of Clinical Sleep Medicine 11 (7): 773–827.
- Ramrattan, N.N., Heijkants, R.G., Tienen, T.G.V., Schouten, A.J., Veth, R.P. & Buma, P. 2005. Assessment of tissue in-growth rates in polyurethane scaffolds for tissue engineering. Tissue Engineering 11 (7-8): 1212–1223.
- Randerath, W.J., Verbraecken, J., Andreas, S., Bettega, G., Boudewyns, A., Hamans, E., Jalbert, F., Paoli, J.R., Sanner, B., Smith, I. & Stuck, B.A. 2011. Non-CPAP therapies in obstructive sleep apnoea: 1000–1028.
- Redline, S., Storfer-Isser, A., Rosen, C.L., Johnson, N.L., Kirchner, H.L., Emancipator, J. & Kibler, A.M. 2007. Association between metabolic syndrome and sleep-disordered breathing in adolescents. American Journal of Respiratory & Critical Care Medicine 176 (4): 401–408.
- Redline, S. & Young, T. 1993. Epidemiology and natural history of obstructive sleep apnea. Ear, Nose & Throat Journal 72 (1): 20–26.
- Remmers, J.E. 1979. Pathogenesis of upper airway occlusion during sleep. Journal of Applied Physiology 46: 772–779.
- Rhoda, M. & Bentley, A. 2022. Home – based diagnostic studies and CPAP titrations for obstructive sleep apnea in Johannesburg. Book of abstracts Pg 14. South African Society for Sleep and Health. https://sassh.org.za/documents/.
- Riley, R.W., Powell, N.B., Li, K.K., Troell, R.J. & Guilleminault, C. 2000. Surgery and obstructive sleep apnea: long-term clinical outcomes. Otolaryngology—Head and Neck Surgery 122 (3): 415–420.
- Rose, E.C., Staats, R., Virchow, C., Jr & Jonas, I.E. 2002. Occlusal and skeletal effects of an oral appliance in the treatment of obstructive sleep apnea. Chest 122 (3): 871–877.
- Rotenberg, B.W., Murariu, D. & Pang, K.P. 2016. Trends in CPAP adherence over twenty years of data collection: a flattened curve. Journal of Otolaryngology-Head & Neck Surgery 45: 1–9.
- Rudnick, E.F., Walsh, J.S., Hampton, M.C. & Mitchell, R.B. 2007. Prevalence and ethnicity of sleep-disordered breathing and obesity in children. Otolaryngology–Head and Neck Surgery 137 (6): 878–882.
- Sánchez-de-la-Torre, M., Sánchez-de-la-Torre, A., Bertran, S., Abad, J., Duran-Cantolla, J., Cabriada, V., Mediano, O., Masdeu, M.J., Alonso, M.L., Masa, J.F., Barceló, A., de la Peña, M., Mayos, M., Coloma, R., Montserrat, J.M., Chiner, E., Perelló, S., Rubinós, G., Mínguez, O., Pascual, L., Cortijo, A., Martínez, D., Aldomà, A., Dalmases, M., McEvoy, R.D., Barbé, F., Abad, L., Muñoz, A., Zamora, E., Vicente, I., Inglés, S., Egea, C., Marcos, J., Fernández, A., Ullate, J., Durán Carro, J., Rodríguez, J.L., Mendoza, M.J., Labeaga, R., Diez, D., Muria, B., Amibilia, C., Urrutia, A., Castro, S., Serrano, L., Salinas, I., Diez, R., Martínez, A., Florés, M., Galera, E., Mas, A., Martínez, M., Arbonés, M., Ortega, S., Martín, A., Román-Sánchez, J.M., Valiente-Diaz, M.I., Viejo-Ayuso, M.E., Rodríguez-García, C., Sánchez-Rodríguez, N., Mayoral, N., Rubio, F.J., Anta-Mejias, Y., Romera-Peralta, S., Resano, P., Arroyo-Espilguero, R., Bienvenido-Villalba, M., Vigil, L., Ramírez, E., Piñar, M., Martínez, E., Múñoz, C., Ordax, E., Surname, N., Corral, J., Gómez de Terreros Caro, F.J., García-Ledesma, E., Gallego, R., Cabrero, J.L., Pereira, R., Giménez, P., Carrera, M., Pierola, J., Villena, C., Campaner, M., Fortuna, A.M., Peñacoba, P., Martínez García, A.J., García Castillo, S., Navas, L., Garmendia, O., Suárez, M., Sancho, J., Farre, N., Bonet, G., Bardaji, A., Villares, A. & Vázquez, M.J. 2020. Effect of obstructive sleep apnoea and its treatment with continuous positive airway pressure on the prevalence of cardiovascular events in patients with acute coronary syndrome (ISAACC study): a randomised controlled trial. The Lancet Respiratory Medicine 8 (4): 359–367.
- Schein, A.S., Kerkhoff, A.C., Coronel, C.C., Plentz, R.D. & Sbruzzi, G. 2014. Continuous positive airway pressure reduces blood pressure in patients with obstructive sleep apnea; a systematic review and meta-analysis with 1000 patients. Journal of Hypertension 32 (9): 1762–1773.
- Scherr, S.C., Dort, L.C., Almeida, F.R., Bennett, K.M., Blumenstock, N.T., Demko, B.G., Essick, G.K., Katz, S.G., McLornan, P.M., Phillips, K.S. & Prehn, R.S. 2014. Definition of an effective oral appliance for the treatment of obstructive sleep apnea and snoring: a report of the American Academy of Dental Sleep Medicine. Journal of Dental Sleep Medicine 1 (1): 39–50.
- Schwab, R.J., Gupta, K.B., Gefter, W.B., Metzger, L.J., Hoffman, E.A. & Pack, A.I. 1995. Upper airway and soft tissue anatomy in normal subjects and patients with sleep-disordered breathing. Significance of the lateral pharyngeal walls. American Journal of Respiratory & Critical Care Medicine 152 (5): 1673–1689.
- Selim, B., Won, C. & Yaggi, H.K. 2010. Cardiovascular consequences of sleep apnea. Clinics in Chest Medicine 31 (2): 203–220.
- Serra, M.M., Greenburg, D., Barnwell, M., Fallah, D., Keith, K. & Mysliwiec, V. 2012. Maxillomandibular advancement as surgical treatment for obstructive sleep apnea in active duty military personnel: a retrospective cohort. Military Medicine 177 (11): 1387–1392.
- Shea, S.A., Edwards, J.K. & White, D.P. 1998. Effects of sleep-wake transitions and REM sleep on genioglossal response to upper airway negative pressure. American Journal of Respiratory & Critical Care Medicine 157: A653.
- Shelton, K.E., Woodson, H., Gay, S. & Suratt, P.M. 1993. Pharyngeal fat in obstructive sleep apnoea. American Review of Respiratory Disease 148 (2): 462–466.
- Sher, A.E., Schechtman, K.B. & Piccirillo, J.F. 1996. The efficacy of surgical modifications of the upper airway in adults with obstructive sleep apnea syndrome. Sleep 19 (2): 156–177.
- Steffen, A., Sommer, J.U., Hofauer, B., Maurer, J.T., Hasselbacher, K. & Heiser, C. 2018. Outcome after one year of upper airway stimulation for obstructive sleep apnea in a multicenter German post-market study. The Laryngoscope 128 (2): 509–515.
- Strollo, P.J., Jr, Soose, R.J., Maurer, J.T., De Vries, N., Cornelius, J., Froymovich, O., Hanson, R.D., Padhya, T.A., Steward, D.L., Gillespie, M.B. & Woodson, B.T. 2014. Upper-airway stimulation for obstructive sleep apnea. New England Journal of Medicine 370 (2): 139–149.
- Sullivan, C.E., Berthon-Jones, M., Issa, F. & Eves, L. 1981. Reversal of obstructive sleep apnoea by continuous positive airway pressure applied through the nares. The Lancet 317: 862–865.
- Sunwoo, B.Y., Light, M. & Malhotra, A. 2020. Strategies to augment adherence in the management of sleep-disordered breathing. Respirology 25 (4): 363–371.
- Suratt, P.M., Dee, P., Atkinson, R.L., Armstrong, P. & Wilhoit, S.C. 1983. Fluoroscopic and computed tomographic features of the pharyngeal airway in obstructive sleep apnea. American Review of Respiratory Disease 127 (4): 487–492.
- Sutherland, K., Phillips, C.L. & Cistulli, P.A. 2015. Efficacy versus effectiveness in the treatment of obstructive sleep apnea: CPAP and oral appliances. Journal of Dental Sleep Medicine 2 (4): 175–181.
- Tan, B., Tan, A., Chan, Y.H., Mok, Y., Wong, H.S. & Hsu, P.P. 2018. Adherence to continuous positive airway pressure therapy in Singaporean patients with obstructive sleep apnea. American Journal of Otolaryngology 39 (5): 501–506.
- Terris, D.J., Kunda, L.D. & Gonella, M.C. 2002. Minimally invasive tongue base surgery for obstructive sleep apnoea. The Journal of Laryngology & Otology 116 (9): 716–721.
- Thomas, A.J., Chavoya, M. & Terris, D.J. 2003. Preliminary findings from a prospective, randomized trial of two tongue-base surgeries for sleep-disordered breathing. Otolaryngology—Head and Neck Surgery 129 (5): 539–546.
- Van de Graaff, W.B. 1988. Thoracic influence on upper airway patency. Journal of Applied Physiology 65 (5): 2124–2131.
- Van de Graaff, W.B. 1991. Thoracic traction on the trachea: mechanisms and magnitude. Journal of Applied Physiology 70 (3): 1328–1336.
- Van de Heyning, P.H., Badr, M.S., Baskin, J.Z., Cramer Bornemann, M.A., De Backer, W.A., Dotan, Y., Hohenhorst, W., Knaack, L., Lin, H.S., Maurer, J.T. & Netzer, A. 2012. Implanted upper airway stimulation device for obstructive sleep apnea. The Laryngoscope 122 (7): 1626–1633.
- Vanderveken, O.M., Devolder, A., Marklund, M., Boudewyns, A.N., Braem, M.J., Okkerse, W., Verbraecken, J.A., Franklin, K.A., De Backer, W.A. & Van de Heyning, P.H. 2008. Comparison of a custom-made and a thermoplastic oral appliance for the treatment of mild sleep apnea. American Journal of Respiratory & Critical Care Medicine 178 (2): 197–202.
- Vanderveken, O.M., Dieltjens, M., Wouters, K., De Backer, W.A., Van de Heyning, P.H. & Braem, M.J. 2013. Objective measurement of compliance during oral appliance therapy for sleep-disordered breathing. Thorax 68 (1): 91–96.
- Varghese, L., Rebekah, G., Oliver, A. & Kurien, R. 2022. Oxygen desaturation index as alternative parameter in screening patients with severe obstructive sleep apnea. Sleep Science 15 (S 01): 224–228.
- Venkataraman, S., Vungarala, S., Covassin, N. & Somers, V.K. 2020. Sleep apnea, hypertension and the sympathetic nervous system in the adult population. Journal of Clinical Medicine 9 (2): 591.
- Vicini, C., Dallan, I., Campanini, A., De Vito, A., Barbanti, F., Giorgiomarrano, G., Bosi, M., Plazzi, G., Provini, F. & Lugaresi, E. 2010. Surgery vs ventilation in adult severe obstructive sleep apnea syndrome. American Journal of Otolaryngology 31 (1): 14–20.
- Waite, P.D., Wooten, V., Lachner, J. & Guyette, R.F. 1989. Maxillomandibular advancement surgery in 23 patients with obstructive sleep apnea syndrome. Journal of Oral and Maxillofacial Surgery 47 (12): 1256–1261.
- Wheatley, J.R., Tangel, D.J., Mezzanotte, W.S. & White, D.P. 1993. Influence of sleep on response to negative airway pressure of tensor palatini muscle and retropalatal airway. Journal of Applied Physiology 75 (5): 2117–2124.
- Wheatley, J.R. & White, D.P. 1993. The influence of sleep on pharyngeal reflexes. Sleep 16 (suppl_8): S87–S89.
- Wolkove, N., Baltzan, M., Kamel, H., Dabrusin, R. & Palayew, M. 2008. Long-term compliance with continuous positive airway pressure in patients with obstructive sleep apnea. Canadian Respiratory Journal 15: 365–369.
- Woodson, B.T., Derowe, A., Hawke, M., Wenig, B., Ross, E.B., Jr, Katsantonis, G.P., Mickelson, S.A., Bonham, R.E. & Benbadis, S. 2000. Pharyngeal suspension suture with Repose bone screw for obstructive sleep apnea. Otolaryngology—Head and Neck Surgery 122 (3): 395–401.
- Woodson, B.T., Steward, D.L., Mickelson, S., Huntley, T. & Goldberg, A. 2010. Multicenter study of a novel adjustable tongue-advancement device for obstructive sleep apnea. Otolaryngology—Head and Neck Surgery 143 (4): 585–590.
- Yeghiazarians, Y., Jneid, H., Tietjens, J.R., Redline, S., Brown, D.L., El-Sherif, N., Mehra, R., Bozkurt, B., Ndumele, C.E. & Somers, V.K. 2021. Obstructive sleep apnea and cardiovascular disease: a scientific statement from the American Heart Association. Circulation 144 (3): e56–e67.
- Young, T., Peppard, P.E. & Gottlieb, D.J. 2002. Epidemiology of obstructive sleep apnea: a population health perspective. American Journal of Respiratory & Critical Care Medicine 165 (9): 1217–1239.
- Zaghi, S., Holty, J.E.C., Certal, V., Abdullatif, J., Guilleminault, C., Powell, N.B., Riley, R.W. & Camacho, M. 2016. Maxillomandibular advancement for treatment of obstructive sleep apnea: a meta-analysis. JAMA Otolaryngology–Head & Neck Surgery 142 (1): 58–66.
