 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Fractional exhaled nitric oxide (FENO) is used to assess eosinophilic inflammation of the airways. FENO values are influenced by the expiratory flow rate and orally produced NO. We measured FENO at four different expiratory flow levels after two different mouthwashes: tap water and carbonated water. Further, we compared the alveolar NO concentration (CANO), maximum airway NO flux (J′awNO) and airway NO diffusion (DawNO) after these two mouthwashes. FENO was measured in 30 volunteers (healthy or asthmatic) with a chemiluminescence NO-analyser at flow rates of 30, 50, 100 and 300 mL/s. A mouthwash was performed before the measurement at every flow rate. The carbonated water mouthwash significantly reduced FENO compared to the tap water mouthwash at all expiratory flows: 50 mL/s (p < .001), 30 mL/s (p = .001), 100 mL/s (p < .001) and 300 mL/s (p = .004). J′awNO was also significantly reduced (p = .017), however, there were no significant differences in CANO and DawNO. In conclusion, a carbonated water mouthwash can significantly reduce oropharyngeal NO compared to a tap water mouthwash at expiratory flows of 30–300 mL/s without affecting the CANO and DawNO. Therefore, mouthwashes need to be taken into account when comparing FENO results.
Introduction
Eosinophilic inflammation of the bronchial epithelium is a chronic process often leading to bronchial hyperreactivity and airway obstruction. During inflammation, fractional exhaled nitric oxide (FENO) is mainly produced in the airway epithelium, catalysed by inducible NO synthase (NOS2) [Citation1]. In clinical practice, assessing FENO facilitates asthma diagnosis [Citation2].
FENO is a combination of nitric oxide (NO) originating in the lung periphery, the bronchioli, the bronchi and the central large airways, and FENO depends highly on the expiratory flow rates. NO dynamics in the lung periphery determine to a large extent FENO measured at higher flow rates, while mainly bronchial NO dynamics determine FENO measured at slower flow rates [Citation3,Citation4]. FENO measurement has been previously recommended at the expiratory flow rate of 50 mL/s [Citation5,Citation6]. There is evidence that FENO values over 50 ppb (>35 ppb in children) measured at this flow indicate eosinophilic airway inflammation and the cut-off can be applied to detect asthma in symptomatic individuals [Citation7,Citation8].
FENO measurement at multiple flows are used to estimate the anatomical origin of NO in exhaled air [Citation9–12]. A simple two compartment model of the airways has been adopted [Citation6] to define the alveolar NO concentration (CANO), maximum airway NO flux (J′awNO) and airway NO diffusion (DawNO) [Citation13]. These estimates have provided extended information in subjects with asthma [Citation12–15].
In healthy subjects, a fraction of FENO seems to originate in the upper airways and oropharynx, partly due to bacterial production of NO and dietary intake of nitrate [Citation16–21].
Rinsing the oral cavity with carbonated water lowers FENO levels significantly in healthy and asthmatic subjects [Citation22,Citation23]. Although the ATS/ERS guidelines suggest that a mouthwash before FENO measurements may reduce oral FENO, it is not part of the standardized clinical procedure [Citation5], but only recommended in physiological research [Citation6]. However, rinsing of the mouth with carbonated water has been routinely adopted in our laboratory at the Helsinki University Hospital prior to FENO measurements since 1995.
The aim of this study was to compare the effects of a carbonated water mouthwash and a tap water mouthwash on FENO at different flow rates, as well to analyse the effects of the mouthwashes on CANO, J′awNO and DawNO. Furthermore, we aimed to investigate the repeatability of the FENO measurements.
Methods
We recruited 30 volunteers aged 16–68 years, either healthcare workers (n = 21) or patients (n = 9). The patients enrolled have had asthmatic symptoms and were previously referred for FENO testing, either to the Laboratory of Clinical Physiology at the University Hospital in Helsinki or the Skin and Allergy Hospital in Helsinki. Healthcare workers who volunteered were included without further selection. None of the subjects were excluded. All participants were asked to fill a questionnaire including questions regarding smoking habits, asthma, allergic rhinitis and COPD.
Nine participants reported having allergic rhinitis, six asthma and one COPD. Two participants smoked regularly, one less than five cigarettes a day, the other from 5 to 15 a day. From available medical records, we searched for respiratory or systemic diseases that could influence FENO values, reason for referral to FENO testing and current medication. Nine participants had a chronic respiratory disease or respiratory symptoms. Among these were four patients with asthma, three with respiratory symptoms due to building dampness but low or negative bronchial hyperreactivity, one with eosinophilic bronchitis and another with Sjögren’s syndrome. The patient with Sjögren’s syndrome had no interstitial lung disease, previously ruled out through a high resolution computed tomography. Six participants had prescriptions for short acting beta2-agonists, four used inhaled corticosteroids regularly, two used antihistamines and one used leukotriene receptor antagonists. Spirometric data were available in 25 subjects. None of these subjects had current bronchodilator reversibility (defined as increase in FEV1 or FVC over 12% and 200 mL) [Citation24]. Demographic, anthropometric and spirometric data are summarised in .
Table 1. Demographic, anthropometric and spirometric data of the study participants.
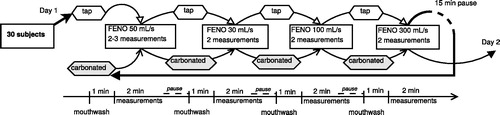
The FENO measurements were made either at the Skin and Allergy Hospital or at the Finnish Institute of Occupational Health, both in Helsinki. The NO analysers used were chemiluminescence CLD 88 sp and the devices were EXHALIZER®’s D with SPIROWARE® software from Eco Medics AG (Dürnten, Switzerland) and calibrated according to the manufacturer’s instructions by using certified span gas (AGA Gas BV, Amsterdam, Netherlands) and NO free air by using a zero-air filter (DENOX 88 unit). The inspired gas was NO free (<5 ppb, maximum recorded fractional inspired Fino was 3.1 ppb). Before the measurements, the ultrasonic flow sensor was calibrated with a calibration syringe (Hans Rudolph Inc., Shawnee, KS). FENO measurements for each subject were performed during two consecutive days. The flowchart in visualizes the order of the procedures. The measurements were made at four different expiratory flow rates V′ (30, 50, 100 and 300 mL/s). The sequence of the flow rates was kept the same, starting with a 50 mL/s flow rate, followed by 30, 100 and 300 mL/s. The mouthwash procedure was defined as rinsing the oral cavity for 30–60 seconds with 100 mL of tap water or carbonated water. On the first day, the subjects performed a mouthwash with tap water before the measurements at the first flow level, and then repeated the mouthwash with tap water before measuring at every flow level. After reaching the highest flow level, i.e. 300 mL/s, there was a 15 min pause before starting a new array. After this time interval, all measurements were repeated, but 100 mL of carbonated water was used to perform the mouthwash. During the second consecutive day, all tests were repeated in the same fashion (i.e. two multiple-flow measurements of FENO with different mouthwashes, totalling two arrays of testing on each day). The mouthwashes were not randomized, since the carbonated water mouthwash has a significantly longer effect on FENO than the tap water mouthwash [Citation22].
Figure 1. Flowchart illustrating the procedures, mouthwashes and repetitions performed during one day. Tap: tap water mouthwash, carbonated: carbonated water mouthwash.
A minimum of two measurements of FENO at each flow were performed to obtain an acceptable value. The measurements at each stage were accepted if its variation was no more than 2 ppb. If more than two attempts were needed for getting a valid measurement, the oral rinsing was repeated. To further analyse the data, a mean value was obtained from four single determinations (two obtained each day) for every subject at each flow and mouthwash setting.
The bottled carbonated water used for the mouthwash was commercially available (HARTWALL VICHY ORIGINAL®, Oy Hartwall Ab, Helsinki, Finland) and had a declared pH of 5.7–5.9; the tap water used had a pH of 8.3. More detailed information regarding the solutions can be found under Lassmann-Klee et al. [Citation22].
All recommendations according to ATS/ERS were followed, except from the selection of a wide range of expiratory flows and using a mouthwash [Citation5]. All subjects refrained from smoking four hours, from drinking coffee two hours and from eating and drinking one hour before the study. Also strenuous exercising prior measuring was discouraged.
Ethical committee approval was received (99/13/03/00/15) and we followed the ethical principles stated in the Declaration of Helsinki [Citation26].
Statistics
Analyses were performed using IBM® SPSS® statistics software version 22 (Armonk, NY), RKWARD® version 0.6.5 frontend and RSTUDIO® version 1.1.383 frontend to the R statistics language (THE R FOUNDATION®, Vienna, Austria), and we partially used GRAPHPAD® PRISM® version 5.04 to obtain the graphs (La Jolla, CA). The comparison of FENO was made using a Wilcoxon test for paired probes. We accepted a significance level of α = 0.05 as significant. The mean (SD) was obtained for each subject from four FENO measurements after each mouthwash at flows 30, 50, 100 and 300 mL/s, respectively. The median and the median absolute deviation (MAD) was calculated too [Citation27]. To prove the accuracy of the measurements, we used the coefficient of variation (cv), defined as the quotient of standard deviation (SD) and mean. We defined a total mean value of cv< 10% as acceptable. CANO, J′awNO and DawNO were calculated using a nonlinear logarithmic transformation (we used starting estimated values of the quadratic T transformation, a second order approximation) according to Eckel et al. [Citation11] using following equation:
(1)
(1)
CANO, J′awNO or DawNO were also calculated using the Högman and Meriläinen algorithm (HMA), with mean FENO and mean V′ values for the flow rates: 30 mL/s, 100 mL/s and 300 mL/s [Citation12]. Cases with negative values for CANO and with failure of consistency check were not ruled out.
The comparison of CANO, J′awNO or DawNO between mouthwashes was made using a Wilcoxon test for paired probes. All target variables were not normally distributed (Shapiro–Wilk’s test). The differences between cv were analysed with a Wilcoxon test when comparing mouthwashes. A comparison of all calculated cv results was made with Friedman’s two-way ANOVA.
Results
The results of FENO measurements at multiple flow levels are listed in and visualised in . Individual FENO values are visualised in . FENO ranged between 4.4 and 221.6 ppb and the median was 14.94 (MAD: 6.76) ppb at a flow rate of 50 mL/s after rinsing with tap water. The mouthwash with carbonated water reduced FENO significantly compared to the tap water mouthwash at every flow rate.
Figure 2. FENO measured at multiple expiratory flow levels after different mouthwashes. Pairs compared with the Wilcoxon signed-rank test.
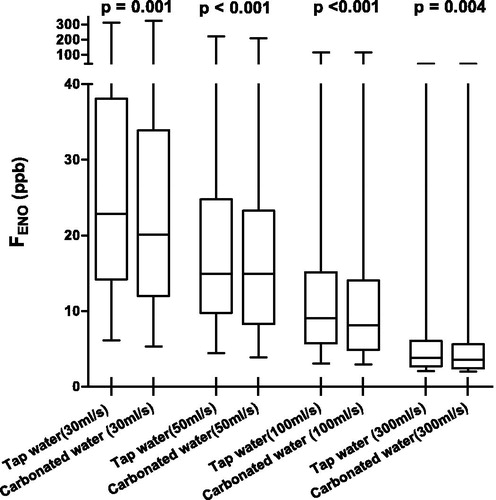
Figure 3. Individual FENO values measured at multiple expiratory flow levels after different mouthwashes. Ordinate represents the individuals (n = 30), abscissa represents FENO (ppb) at multiple expiratory flow levels after: carbonated water mouthwash (black dots), and tap water mouthwash (grey rhombi). Abscissa truncated at 75 ppb. Subjects 1 and 2 had higher FENO (ppb) values (not shown). The missing higher values (tap water/carbonated) for subject 1 are: 128/128, 85/79; and for subject 2 are: 311/322, 222/208, 117/117.
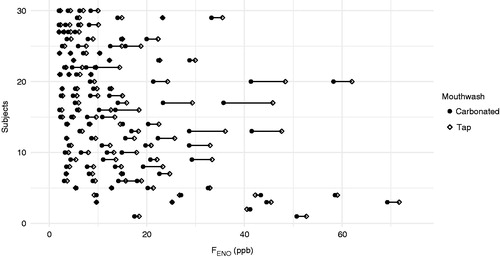
Table 2. Summarized results of FENO at every flow rate and mouthwash either with tap water or carbonated water.
The mean cv of FENO stayed under 10% for all flow levels. Comparing the cv between tap water rinsing and carbonated water rinsing, no significant difference was found at any flow level (at 30 mL/s p = .094; at 50 mL/s p = .125; at 100 mL/s p = .245; at 300 mL/s p= .688). Analysing cv for all flow levels and mouthwashes resulted in no difference (p= .202).
When comparing J′awNO between mouthwashes, the median J′awNO for carbonated water was significantly lower using both models. CANO and DawNO did not differ significantly between both mouthwashes. These results are summarized in .
Table 3. Results of J′awNO, CANO and DawNO, after carbonated water or tap water mouthwash.
The NO fraction of inspired gas was at all times below 20 ppb as recommended, having a mean value of 0.35 ppb (SD 0.34) [Citation5].
Discussion
Mouthwashes
We found a statistically significant difference in FENO between mouthwashes at all expiratory flow levels. FENO was statistically significantly lower after rinsing with carbonated water compared to tap water at all expiratory flow levels. This confirms previous studies in which carbonated water was used to perform a mouthwash [Citation22,Citation23], and suggests endorsement of a carbonated water mouthwash prior multiple-flow testing.
In the present study, the difference between mouthwashes was ca. –5% at all flow levels. This decrease of FENO between mouthwashes equals our previously observed reduction. We demonstrated recently that both carbonated water and tap water mouthwashes lower FENO significantly compared with the baseline and observed an immediate decrease of FENO after a carbonated water mouthwash of –18% and of –13% after a tap water mouthwash [Citation22]. Additionally, Piirilä et al. [Citation23] found at 50 mL/s a decrease from baseline (without mouthwash) of ca. –10% after a carbonated water mouthwash in a healthy population. In comparison, previous multiple flow studies have found a relative decrease in FENO of ca. –10% after a chlorhexidine mouthwash in children and adolescent (both asthmatic and healthy) [Citation28] and of ca. –15% in healthy adults [Citation29].
Furthermore, the tap water mouthwash’s effect is short-lasting, only two minutes. On the other hand, the carbonated water mouthwash’s effect is longer, lasting 12 minutes, and more effective if compared with tap water [Citation22]. For this reason, we did not randomize the subjects in the present study and performed the measurements with tap water first.
Our data were accurately collected, since the intra-individual cv of FENO stayed low and the mean cv below 10%. We did not find a difference in cv between mouthwashes. The cv at 100 mL/s is similar to previously reported by Ekroos et al. [Citation30] for a healthy male population taking into account a time period of maximal 24 hours.
Multiple flow FENO and mouthwashes
As we expected, J′awNO was significantly lower after the carbonated water mouthwash compared to the tap water mouthwash. There was no statistically significant difference between mouthwashes when analysing CANO and DawNO. We verified this results with estimated values for J′awNO, CANO and DawNO and two different models. This further strengthens our result, that the carbonated water mouthwash affects the airway fraction (due to oral NO reduction) and not the peripheral alveolar NO, neither the NO diffusion. We could state the hypothesis that the carbonated water rinsing provides more exact values (without oral contamination) in general. Heijkenskjöld-Rentzhog et al. published similar findings when analysing J′awNO and CANO after a chlorhexidine mouthwash [Citation28]. This is in contrast to results by Malinovschi et al. [Citation29] who found also a decrease in CANO after a chlorhexidine mouthwash. Both used a trumpet-shaped model with corrections for axial diffusion (TMAD). Kerckx et al. [Citation31] demonstrated that, when using a model correcting for axial diffusion to estimate CANO in asthmatic patients (unobstructed and well-managed), CANO is normal, even when J′awNO is elevated. This has been confirmed also for patients with severe asthma and during exacerbation [Citation32].
For our purposes, we employed a two compartment model of the lung [Citation4], without considering axial diffusion [Citation9], and applied a robust mathematical model [Citation11]. This model was tested previously exactly at the same flow levels by Eckel et al. and did not impose flow limitations unlike other models [Citation11]. We obtained also values using the HMA (without axial diffusion) and the results between mouthwashes were similar. Although results from models with and without axial diffusion are not directly equivalent, an analogy can be made between the main findings of different studies.
The strength of our study was using different parameters to examine the effect of the carbonated water mouthwash and for that using two different mathematical models. Our calculations with EquationEquation (1)(1)
(1) provided negative values for CANO in five cases for tap water mouthwash and four cases for carbonated water mouthwash, which were not excluded. A similar amount of negative values were obtained with the HMA. This has been a regular problem with other models providing a greater proportion of negative concentration values. We disregarded the importance of these data, since the comparison of CANO between mouthwashes showed no difference (even when replacing these values for zero). Our extended analysis of J′awNO, CANO and DawNO found these values comparable to previous results in a healthy population [Citation33]. All results for CANO were under 2.3 ppb and none of the subjects had a medical condition associated with interstitial lung disease, therefore we assume that the subjects with measured FENO over 25 ppb (n = 7) had an elevated airway production of NO.
Limitations and implications
We acknowledge the limitation of not randomizing the order of the expiratory flows levels. However, the limitation was imposed by the nature of the flow levels used, i.e. the high flow representing an expiratory burden to the participants. Previous studies reported a decrease in FENO after forced expirations [Citation34–36]. We selected on purpose an incremental flow order to avoid the high flow expiration influencing the slower expiration manoeuvres.
The tap water used to compare the effect of carbonated water had a pH value over 8. The tap water in European countries has a pH of 6.5–9.5 units (Council Directive 98/83/EC of 3 November 1998 on the quality of water intended for human consumption) and this range also complies with WHO guidelines. Many countries may have lower tap water’s pH values than 8. This may enhance the tap water’s effect on oral FENO and lower the differences between tap water and carbonated water. Nevertheless, further studies are needed to elucidate if the pH is the crucial factor of the mouthwash solutions.
Carbonated water poses an ideal candidate for performing a mouthwash before multiple flow measuring. It eliminates oral NO interference more effectively than tap water and for a prolonged period of time. Our recent publication [Citation22] argues for the clinical application of a mouthwash before FENO measurement. Here, we demonstrate it influences only the airway fraction of FENO, and using carbonated water as a mouthwash, in a more pronounced way. Probably one mouthwash procedure with carbonated water may suffice when performing a routine multiple-flow investigation, but repetitions might be useful if exact values are needed, e.g. in physiological research.
Conclusions
We conclude that a carbonated water mouthwash can significantly reduce oropharyngeal NO compared to a tap water mouthwash at expiratory flows of 30–300 mL/s without affecting the CANO nor the DawNO. We imply that a carbonated water mouthwash is suitable for routine multiple-flow FENO-analysis and evidently useful in clinical research. This study strengthens the view that mouthwash procedures shall be taken into account when comparing FENO values.
Päivi Piirilä https://orcid.org/0000-0002-2535-4409
Acknowledgements
We thank the staff members: Sari Fischer, Helena Punkari and Elina Voutilainen for performing the FENO measuring and also Tommi Pallasaho for data collection.
Disclosure statement
No conflicts of interest are declared by the author(s).
Additional information
Funding
References
- Lane C, Knight D, Burgess S, et al. Epithelial inducible nitric oxide synthase activity is the major determinant of nitric oxide concentration in exhaled breath. Thorax. 2004;59:757–760.
- Asthma: diagnosis, monitoring and chronic asthma management. Guidance and guidelines. NICE [Internet]. London [cited 2018 Mar 6]. Available from: https://www.nice.org.uk/guidance/ng80/chapter/Recommendations
- Högman M, Drca N, Ehrstedt C, et al. Exhaled nitric oxide partitioned into alveolar, lower airways and nasal contributions. Respir Med. 2000;94:985–991.
- Tsoukias NM, George SC. A two-compartment model of pulmonary nitric oxide exchange dynamics. J Appl Physiol. 1998;85:653–666.
- ATS/ERS recommendations for standardized procedures for the online and offline measurement of exhaled lower respiratory nitric oxide and nasal nitric oxide, 2005. Am J Respir Crit Care Med. 2005;171:912–930.
- Horváth I, Barnes PJ, Loukides S, et al. A European Respiratory Society technical standard: exhaled biomarkers in lung disease. Eur Respir J. 2017;49:1600965.
- Karrasch S, Linde K, Rücker G, et al. Accuracy of FENO for diagnosing asthma: a systematic review. Thorax. 2017;72:109–116.
- Dweik RA, Boggs PB, Erzurum SC, et al. An official ATS clinical practice guideline: interpretation of exhaled nitric oxide levels (FeNO) for clinical applications. Am J Respir Crit Care Med. 2011;184:602–615.
- Condorelli P, Shin H-W, Aledia AS, et al. A simple technique to characterize proximal and peripheral nitric oxide exchange using constant flow exhalations and an axial diffusion model. J Appl Physiol. 2007;102:417–425.
- George SC, Högman M, Permutt S, et al. Modeling pulmonary nitric oxide exchange. J Appl Physiol. 2004;96:831–839.
- Eckel SP, Linn WS, Berhane K, et al. Estimation of parameters in the two-compartment model for exhaled nitric oxide. PLoS One. 2014;9:e85471.
- Högman M, Meriläinen P. Extended NO analysis in asthma. J Breath Res. 2007;1:024001.
- Heijkenskjöld-Rentzhog C, Nordvall L, Janson C, et al. Alveolar and exhaled NO in relation to asthma characteristics—effects of correction for axial diffusion. Allergy. 2014;69:1102–1111.
- Silkoff PE, Sylvester JT, Zamel N, et al. Airway nitric oxide diffusion in asthma: role in pulmonary function and bronchial responsiveness. Am J Respir Crit Care Med. 2000;161:1218–1228.
- Lehtimäki L, Kankaanranta H, Saarelainen S, et al. Extended exhaled NO measurement differentiates between alveolar and bronchial inflammation. Am J Respir Crit Care Med. 2001;163:1557–1561.
- Alving K, Weitzberg E, Lundberg J. Increased Amount of nitric oxide in exhaled air of asthmatics. Eur Respir J. 1993;6:1368–1370.
- Duncan C, Dougall H, Johnston P, et al. Chemical generation of nitric oxide in the mouth from the enterosalivary circulation of dietary nitrate. Nat Med. 1995;1:546–551.
- Marteus H, Törnberg DC, Weitzberg E, et al. Origin of nitrite and nitrate in nasal and exhaled breath condensate and relation to nitric oxide formation. Thorax. 2005;60:219–225.
- Olin A-C, Aldenbratt A, Ekman A, et al. Increased nitric oxide in exhaled air after intake of a nitrate-rich meal. Respir Med. 2001;95:153–158.
- Törnberg DCF, Marteus H, Schedin U, et al. Nasal and oral contribution to inhaled and exhaled nitric oxide: a study in tracheotomized patients. Eur Respir J. 2002;19:859–864.
- Zetterquist W, Pedroletti C, Lundberg JON, et al. Salivary contribution to exhaled nitric oxide. Eur Respir J. 1999;13:327–333.
- Lassmann-Klee PG, Lindholm T, Metsälä M, et al. Reduction of FENO by tap water and carbonated water mouthwashes: magnitude and time course. Scand J Clin Lab Invest. 2018;78:153–156.
- Piirilä P, Rouhos A, Kainu A, et al. Reduction of fractional exhaled nitric oxide (FENO) and its variation by mouth wash. Scand J Clin Lab Invest. 2012;72:253–257.
- Pellegrino R, Viegi G, Brusasco V, et al. Interpretative strategies for lung function tests. Eur Respir J. 2005;26:948–968.
- Viljanen AA, Halttunen PK, Kreus KE, et al. Spirometric studies in non-smoking, healthy adults. Scand J Clin Lab Investig. 1982;42:5–20.
- World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310:2191.
- Leys C, Ley C, Klein O, et al. Detecting outliers: do not use standard deviation around the mean, use absolute deviation around the median. J Exp Soc Psychol. 2013;49:764–766.
- Heijkenskjöld-Rentzhog C, Alving K, Kalm-Stephens P, et al. The fraction of NO in exhaled air and estimates of alveolar NO in adolescents with asthma: methodological aspects. Pediatr Pulmonol. 2012;47:941–949.
- Malinovschi A, Janson C, Holm L, et al. Basal and induced NO formation in the pharyngo-oral tract influences estimates of alveolar NO levels. J Appl Physiol. 2009;106:513–519.
- Ekroos H, Karjalainen J, Sarna S, et al. Short-term variability of exhaled nitric oxide in young male patients with mild asthma and in healthy subjects. Respir Med. 2002;96:895–900.
- Kerckx Y, Michils A, Muylem AV. Airway contribution to alveolar nitric oxide in healthy subjects and stable asthma patients. J Appl Physiol. 2008;104:918–924.
- Gelb AF, George SC, Silkoff PE, et al. Central and peripheral airway/alveolar sites of exhaled nitric oxide in acute asthma. Thorax. 2010;65:619–625.
- Högman M, Lafih J, Meriläinen P, et al. Extended NO analysis in a healthy subgroup of a random sample from a Swedish population. Clin Physiol Funct Imaging. 2009;29:18–23.
- Deykin A, Halpern O, Massaro AF, et al. Expired nitric oxide after bronchoprovocation and repeated spirometry in patients with asthma. Am J Respir Crit Care Med. 1998;157:769–775.
- Silkoff PE, Wakita S, Chatkin J, et al. Exhaled nitric oxide after beta2-agonist inhalation and spirometry in asthma. Am J Respir Crit Care Med. 1999;159:940–944.
- Kharitonov SA, Barnes PJ. Exhaled markers of pulmonary disease. Am J Respir Crit Care Med. 2001;163:1693–1722.
