Abstract
Hyperparathyroidism (HPT), including normocalcaemic, vitamin D sufficient (Serum (S)-25(OH)D ≥ 50 nmol/L) hyperparathyroidism (nHPT), has increasingly been diagnosed in the last few decades due to the more common use of the serum parathyroid hormone (S-PTH) assay. We investigated if men with HPT had higher morbidity and mortality than men without HPT during 21 years’ follow-up.
A random population sample of 750 men, all 50 years of age, was examined in 1993. Endpoints were retrieved 21 years later at 71 years of age.
Albumin-corrected serum (S) calcium, S-25-hydroxyvitamin D and S-PTH were assessed along with data on cardiovascular risk factors and medication. Outcome data on fractures, stroke, myocardial infarction, cancer and death were retrieved in 2014; 21 years after primary assessment.
The prevalence of HPT at 50 years of age was 9.3%; nHPT 2.8%, primary HPT 0.4%, secondary HPT 0.4%, and HPT with vitamin D insufficiency 6%. Fracture rate, myocardial infarction, stroke, cancer and death occurred similarly in men with or without HPT, as well as in men with nHPT as compared with men without calcium/PTH aberrations during 21 years’ follow-up. S-PTH was evenly distributed in the univariable analyses for each outcome. Cox regression analyses showed no increase in serious morbidity or in mortality in men with HPT, irrespective of cause, compared with men with normal S-PTH over a 21-year period. None had HPT at a S-25(OH)D level of 100 nmol/L.
Introduction
Primary hyperparathyroidism (pHPT) is the third most common endocrine disorder, after diabetes mellitus and thyroid diseases, with a prevalence of approximately 1% depending on age and sex [Citation1,Citation2]. Patients with pHPT suffer from an increased fracture rate and higher cardiovascular morbidity and mortality compared with the general population [Citation3,Citation4].
The introduction of the analysis of serum intact parathyroid hormone (S-PTH) in the early 1990s started a new era in the diagnosis and treatment of pHPT [Citation5]. Until then the diagnosis was based on the clinical symptoms of kidney stones and bone loss, which appear at an advanced stage of the illness. With the wide use of the S-PTH assay and the early diagnosis of pHPT, physicians started using this assay also for the evaluation of undiagnosed bone diseases or unclear fatigue, even in the presence of normal serum calcium (S-Ca). In this context, many individuals with normal S-Ca were found to have elevated S-PTH without any obvious underlying etiology. This new diagnostic entity was first officially described at the Third International Workshop on the Management of Asymptomatic Primary Hyperparathyroidism in 2008 [Citation6].
Normocalcaemic, vitamin D sufficient hyperparathyroidism (nHPT) in men and women has been shown to have a benign course in an earlier small population study [Citation7]. Endocrine diseases are in general more common in women, while cardiovascular morbidity rises at a younger age in men.
The present study was based on clinical and laboratory data sampled in 1993 for the “Study of Men Born in 1943”, which had as main aim to follow and evaluate long-term cardiovascular risk [Citation8,Citation9].
Our hypothesis was that men with all types of HPT, including nHPT, would develop more fractures, cardiovascular disease and cancer than men without HPT during 21 years’ follow-up.
Material and methods
Study population
The aim of the study is to screen a random sample of the general population for calcium and PTH, and to follow this cohort for over 20 years regarding morbidity and mortality.
A random population sample of 50 year-old men (n = 1463), all born in 1943 and living in the city of Gothenburg, Sweden (latitude 57°N), was drawn from the local population register. All men were invited to a health examination in 1993 for the “Study of Men Born in 1943” project [Citation8,Citation9]. Out of the invited cohort, 798 men (55%) participated. Men of outside European origin consisted of 3.5%. Complete biochemical analysis of S-Ca, S-Albumin, S-Creatinine, S-PTH, S-25-hydroxyvitamin D (S-25(OH)D), cholesterol, high-density lipoprotein (HDL) cholesterol, triglycerides, and fasting plasma (P) glucose were available in 750 men. Data on morbidity and mortality for all men in the study was collected through the Swedish Hospital Discharge Registry and the National Board of Health and Welfare, Stockholm, Sweden until 31.12.2014 (21 years of follow-up).
Anthropometric data at baseline
The body weight was measured to the nearest 0.1 kg and the height was recorded barefoot to the nearest 1 centimeter (cm). Body mass index (BMI) (kg/m2) was calculated as body weight divided by height squared. Waist circumference (cm) was measured with a soft tape around the waist at the umbilicus level.
Biochemical analyses and definitions
The examinations including blood sampling, performed between February 1993 and June 1994, excluding July and August 1993. Fasting blood samples were obtained from an antecubital vein. S-Ca and albumin were analyzed according to routine methods at the accredited laboratory for clinical chemistry at the Sahlgrenska University Hospital. Albumin-corrected S-Ca (denoted S-Ca) was calculated as 0.019 × (46 - S-Albumin) + S-Ca [Citation10]. The in-hospital reference range was 2.15 – 2.49 mmol/L and defined as normocalcaemia. Hypocalcaemia was defined as S-Ca ≤ 2.14 mmol/L and hypercalcaemia ≥ 2.50 mmol/L. S-creatinine, glucose, lipids, and albumin were measured with standard methods. The reference level for S-creatinine was 60 – 105 μmol/L. Kidney insufficiency was defined as an elevated S-creatinine level. Low-density lipoprotein (LDL cholesterol) was calculated with Friedewalds’ estimation [Citation11].
Blood samples were stored at −80 ○C for later analysis of hormones in 2015. S-25(OH)D was determined by radioimmunoassay 125I RIA kit (Liaison, Diasorin, Stillwater, MN, USA). The total coefficient of variation (CV) was 17% for the mean level of 26 nmol/L and 12.0% for mean level 66 nmol/L. S-25(OH)D ≥ 50 nmol/L was considered as sufficient level of vitamin D [Citation12]. S-PTH was measured by immunoradiometric assay (Roche Cobas, Rotkreuz, Switzerland), reference range 1.6 – 6.9 pmol/L. CV was 7% for 3 pmol/L and 3% for 10 pmol/L. HPT was defined as a S-PTH > 6.9 pmol/L. Primary HPT (pHPT) was defined as hypercalcaemia and elevated S-PTH. Secondary HPT (sHPT) was defined as hypocalcaemia and elevated S-PTH. Normocalcaemic, vitamin D sufficient HPT (nHPT) was defined as normocalcaemia (2.15 – 2.49 mmol/L), a sufficient S-25(OH)D (≥ 50 nmol/L) and an elevated S-PTH level (> 6.9 pmol/L).
Medical history and medications
Diagnoses were retrieved and coded according to the International Classification of Diseases (ICD) 8, 9, and 10. ICD 8 and 9 were converted to ICD 10. Information about kidney stones (N20, N21), neck operation (BAA40, BAA50, BAA60, BBA40, and BBA50), X-ray verified fractures (S12, S22, S27, S32, S39, S42, S52, S62, S72, S82, S92, and T02-T10), myocardial infarction (I21-I23), hemorrhagic and non-hemorrhagic stroke (I60-I61, I63-I64), hypertension (I10) and cancer (C00-C97) were retrieved from the Swedish Hospital Discharge Registry until 31.12.2014. Mortality data were obtained from the National Cause of Death Registry and included date and cause of death. Questionnaires regarding medical history, lifestyle factors, medications, and origin of birth were used in 1993. Medication was coded according to the Anatomical Therapeutic Chemical Classification System (ATC). Thiazides were used by three men (0.4%). Hypertension was diagnosed based on current antihypertensive therapy. Smoking was defined as a current smoker of cigarettes, pipe or cigars daily. Men who had quit smoking more than 3 months earlier were defined as ex-smokers.
Statistical analysis
Means, medians, standard deviations (SD), and confidence intervals (CI) were calculated using conventional methods. For comparison between groups, t-test and Fisher’s exact test were used for dichotomous variables. The Chi-square test and hazard ratios were expressed with 95% CI. Univariable analysis was used for the event rate in relation to S-PTH levels. Cox proportional hazard regression analyses were performed with maximum-likelihood estimates for a high S-PTH with normal S-Ca and S-25(OH)D and risk for fracture, myocardial infarction, stroke, cancer, and death, respectively. Survival estimates are presented as Hazard Ratio (HR) with 95% CI. Adjustment was also performed for BMI, as this could possibly influence on S-PTH [Citation13]. No other confounding factors were adjusted for as all were men of the same age, 50 years. A p value of < .05 was considered statistically significant.
Ethics
The study complies with the Declaration of Helsinki, and was approved by the Ethics Committee of the University of Gothenburg (Dnr. 157-93 date 14.04.1993 and T529-15 date 18.06.2015) and the National Data Inspection Board. The study is registered in Clinical Trials.gov Identifier: NCT 03138122. All participants gave their written informed consent.
Results
Baseline characteristics, HPT, and biochemistry
Calcium and PTH-aberrations were occurring in 92 of 750 men (12.3%), of whom 70 men (9.3%) had elevated PTH levels, . None hade kidney insufficiency defined as an elevated serum creatinine. pHPT was found in three men (0.4%), sHPT was found in three men (0.4%) and nHPT in 21 men (2.8%). Elevated S-PTH without the mentioned states was found in 43 men (6%) with vitamin D insufficiency, . Vitamin D insufficiency (S-25(OH)D < 50 nmol/L) was common overall, and present in 409 men (55%). Obesity, defined as BMI > 30 kg/m2, was found in 12.5% of the participating men. The correlation between S-Ca and S-PTH and the distribution of the different HPT groups are shown in . Anthropometric and metabolic data for men with HPT (n = 70) in comparison with men with normal S-PTH irrespective of S-Ca and S-25(OH)D (n = 680), are given in . Men with HPT had significantly lower S-25(OH)D levels, higher waist circumference, BMI, fasting glucose, more diabetes mellitus and were more commonly ex-smokers than men with normal S-PTH. Exclusion of the three men with thiazides did not alter the results. Men with HPT smoked less and fewer had antihypertensive treatment compared with men without HPT, . At the time of the study, in 1993, the most commonly used antihypertensive agent was the betablocker.
Figure 1. Flowchart of 798 men included in the study on men born in 1943. S-PTH: serum parathyroid hormone (reference range 1.6 – 6.9 pmol/L); S-Ca: albumin-adjusted serum calcium (reference range 2.15 – 2.49 mmol/L); S-25(OH)D: serum 25-hydroxyvitamin D (sufficiency limit ≥ 50 nmol/L); nHPT: normocalcaemic hyperparathyroidism; pHPT: primary hyperparathyroidism; sHPT: secondary hyperparathyroidism (to hypocalcaemia).
Figure 2. Simple linear regression between albumin-adjusted serum calcium (S-Ca) and serum parathyroid hormone (S-PTH) in a random population sample of 750 men aged 50 years. The circles with cross represent individuals with vitamin D sufficiency (S-25(OH)D ≥ 50 nmol/L).
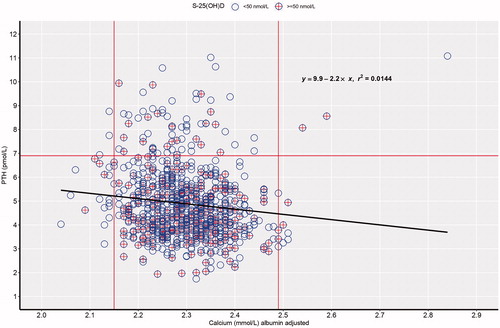
Table 1. Baseline characteristics in men aged 50 years examined in 1993 with hyperparathyroidism (HPT) (n = 70) in comparison with men with normal S-PTH independent of S-Ca or S-25(OH)D level (n = 680).
In , the subgroup of men with nHPT (n = 21) were similarly compared to individuals with normal S-Ca, sufficient S-25(OH)D and normal S-PTH (n = 312). Except for the definitions, individuals with nHPT smoked less than individuals with normal PTH; there was no other difference in cardiovascular risk factors or outcome variables.
Table 2. Baseline characteristics in men aged 50 years examined in 1993 with normocalcaemic, vitamin D sufficient hyperparathyroidism (nHPT) (n = 21) and men with normal S-Ca, sufficient S-25(OH)D levels and normal S-PTH (n = 312).
Calculations were also performed using the cut off for S-25(OH)D of 75 nmol/L instead of 50 nmol/L. This resulted in only five men left having an elevated S-PTH. Furthermore, none had elevated S-PTH at a S-25(OH)D cut off of 100 nmol/L.
Univariable analysis for the event rate in relation to S-PTH levels
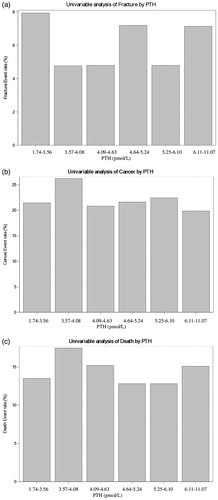
There were no associations between increasing number of fractures, cancer or deaths with levels of S-PTH in all 750 men, .
Morbidity and mortality at 21-year follow-up
Cox proportional regression analyses were performed for estimation of the risk for HPT and endpoint during 21 years. No differences in fractures HR 0.92 (95% CI 0.33–2.57) cancer HR 0.83 (95% CI 0.47–1.46), myocardial infarction HR 1.40 (95% CI 0.92–2.15), stroke HR 1.26 (95%CI 0.31–5.17), or death HR 0.83 (95% CI 0.47–1.46) were seen in men with HPT compared with men without HPT during a 21-year follow-up period.
Diagnoses of HPT and calcium aberrations
Three men (0.4%) fulfilled the criteria for pHPT in 1993, . All of them developed hypertension, but none developed any fracture, cardiovascular disease, cancer, or passed away during 21 years’ follow-up.
Hypercalcaemia with normal S-PTH was found in five individuals (0.7%). Neither of them had cancer and they may have had pHPT, but the PTH levels were in the lower reference range and S-Ca only 2.50 and 2.51 mmol/L. Hypocalcaemia without elevated S-PTH was found in 17 men (2.3%). None of the men with isolated hyper- or hypocalcaemia had increased morbidity or mortality compared with men with normal S-Ca and S-PTH levels during 21 years’ follow-up.
Three individuals had sHPT (hypocalcaemia and elevated S-PTH) in 1993, . One of them had myocardial infarction, stroke and hypertension. No fractures, cancer or death were registered in men with sHPT during follow-up.
Discussion
This study showed that men with HPT, irrespective of S-Ca and S-25(OH)D levels, had no increase in morbidity or mortality compared with men with normal S-PTH during 21 years’ follow-up. Hence, our hypothesis regarding HPT being associated with more fractures, cardiovascular disease or more cancer could not be verified. This is, to the best of our knowledge, the longest follow-up of a non-referral, random population sample of similar sex and age with hard end points as outcome. The present results corroborate those from our previous smaller population study of men and women with two laboratory measurements 13 years apart [Citation7]. The introduction of the PTH analysis in the early 1990 increased the awareness, diagnostics, and treatment of calcium aberrations. However, PTH has often been analyzed since then, even without the presence of an abnormal S-Ca. Hence, it is of utmost importance to know the cause, or long-term consequence, of an isolated elevated PTH level. We could not confirm an association between an elevated PTH level and adverse outcomes during 21 years’ follow-up. Some earlier studies have shown a higher morbidity and mortality in patients with pHPT compared with the general population during follow-up [Citation3,Citation4,Citation14]. On the other hand, reports from the Scandinavian SIPH study did not show any increased morbidity in pHPT, either operated or observed, during follow-up [Citation15,Citation16]. Furthermore, at a recent consensus meeting [Citation17], it was concluded that the natural course of pHPT is hitherto unknown. The authors suggested “observation without intervention” in milder forms of pHPT. It was discussed that the cardiovascular morbidity, fractures and quality of life were not convincingly deteriorated in previous studies on pHPT. No increase in mortality was observed in the few men with pHPT in the present study, but due to the small number of affected individuals no conclusions can be drawn.
Previous studies have indicated that nHPT could be an early stage of pHPT and that many of these individuals develop pHPT [Citation18,Citation19]. These studies followed highly selected patients, who experienced symptoms of hypercalcaemia and therefore had been referred to specialized bone, metabolic and endocrine units [Citation18,Citation19]. A previous study of an unselected, non-referral population showed that none of the 2% with nHPT aged 25–64 years developed clinically overt pHPT during 13 years’ follow-up [Citation7]. Two participants with nHPT might have fulfilled the laboratory criteria for pHPT, but no secondary phenomenon (kidney stones or fractures) could be seen in the subjects during follow-up in that study [Citation7]. At that time, when men and women had reached the age range of 38–78 years, 11% had nHPT [Citation7].
We do not know how many men with HPT (9.3%) or nHPT (2.8%) developed pHPT by time in the present study, but we do know that none of the subjects with HPT or nHPT had been hospitalized due to kidney stones or neck operation during the following 21 years with the exception of one man with pHPT in the primary screening.
Another study on non-referral subjects with nHPT has been performed with a follow-up time of 8 years [Citation20]. The prevalence for nHPT in elderly men was 0.4%, but no analysis of morbidity or mortality was performed, and the authors concluded that larger studies with longer follow-up were needed to understand the natural history of nHPT [Citation20].
HPT, and especially nHPT, is an increasing clinical problem since it is highly uncertain if, or how, we should treat nHPT, and whether a scheduled and systematic follow-up of nHPT is required [Citation20,Citation21]. It remains unclear whether this laboratory finding is pathological, and therefore a target for treatment, or just a part of the aging process [Citation22]. S-PTH levels may rise due to low levels of circulating S-25(OH)D levels and/or low calcium intake. The former was also seen in the present study where quite a few (6% of the study population) had HPT and vitamin D insufficiency but with maintained normocalcaemia. The large proportion of individuals with hypocalcaemia may also be explained by the poor vitamin D status in inhabitants at our latitude, as individuals are at a high risk of vitamin D insufficiency. This, combined with low calcium intake, may provoke hypocalcaemia. We unfortunately have no data on dietary intake of our participants, nor were they investigated regarding malabsorption. One can speculate if the cut off for S-25(OH)D for vitamin D sufficiency, as stipulated by the Institute of Medicine [Citation12], should be higher than 50 nmol/L. The number of men with elevated S-PTH decreased with increasing vitamin D levels in the present study. None with S-25(OH)D at 100 nmol/L had elevated S-PTH. Holick MF et al. proposed a cut of at 75 nmol/L for vitamin D sufficiency [Citation23]. It might well be that S-PTH has its place in the evaluation of titration of the vitamin D dose for supplementation in all subjects with sHPT as well as in HPT in general, and especially in the latter group in order to enhance the diagnostic accuracy of a true pHPT. Hence, the laboratory investigation of both S-25(OH)D and S-PTH could be recommended in the investigation of calcium disorders, malabsorption, treatment, and supplementation and HPT in general, respectively.
The prevalence of nHPT in the present study was 2.8%, which is higher than in previous studies showing prevalence around 0.4% [Citation20,Citation24]. Our data indicated that there was no increase in fractures, cardiovascular diseases, cancer, or death during 21 years in nHPT in comparison with individuals of the same age and gender, but with S-PTH within the normal reference range. Similar prevalence and results were seen in a smaller cohort from the same latitude [Citation7]. Furthermore, there were no correlations between the event rate for fractures, cancer or deaths with increasing S-PTH levels, ), in the present study.
On the other hand, it is important to diagnose pHPT as this disease can lead to kidney stones and increased risk for fractures. The importance of surgery for patients with a true pHPT per se with these related complications has recently been reviewed [Citation25].
Strengths
The strength of the present study was that the population consisted of a random sample of men at the same age and from the same town and latitude, having the same possibility to sun exposure, which influences vitamin D concentrations, under similar circumstances [Citation26]. All citizens in Sweden have a unique identification number. This makes it possible to follow persons included in a study during their entire lifetime and through the death register, provided they are still living in Sweden. Furthermore, the hospital medical system is nearly 100% public which gives the opportunity to follow a study population through hospital registration codes and medical treatments.
Limitations
This study has some limitations. The participation rate was 55%. A low and declining participation rate in epidemiological studies is observed throughout the world [Citation27]. Previous studies have shown that participants in population studies tend to have higher socioeconomic status and to be healthier than non-participants [Citation28]. This may have contributed to a low number of outcome events and reduced the power of the study. It was a retrospective cohort study on an already examined sample in 1993. Although most of the estimates for the endpoints were close to one, the confidence intervals were wide.
Urinary samples were not collected, and analysis of urinary calcium excretion would have helped to identify possible subjects with familial hypocalciuric hypercalcaemia (FHH), which may lead to misclassification [Citation29]. The prevalence of FHH is low and the risk to include someone with this genetic form of hypercalcaemia was hypothetically low and would not have influenced the main outcome of this study. We did not exclude users of thiazide diuretics, since there were only three and they were equally distributed in the groups.
The present results rely on a single blood sample and did not include ionized calcium, but albumin-corrected S-Ca, which, at that time, was the most commonly used method for estimation of the free calcium fraction. S-albumin level is the only factor which can influence S-Ca leading to a false interpretation of total S-Ca. The present results were based on blood samples which were analyzed directly after they were collected (S-Ca, S-creatinine, S-cholesterol, S-HDL, S-triglycerides, and fasting P-glucose). Hormone levels, (S-25(OH)D and S-PTH) together with S-Ca were analyzed from frozen serum samples in 2015. Earlier studies have shown that levels of S-PTH [Citation30,Citation31] and S-25(OH)D [Citation32] remain stable after freezing and thawing. S-Ca analyzed in 1993 and on frozen samples in 2015 were well correlated, r = 0.8, in this study.
In conclusion, HPT, and especially normocalcaemic, vitamin D sufficient HPT (nHPT), were common, 9.3% and 2.8%, respectively, in randomly selected 50 year-old men. No increase in serious morbidity or total mortality was seen in men with elevated S-PTH levels, irrespective of cause, compared with men without, calcium/PTH aberrations over a 21-year period. However, the study had limited statistical power with wide confidence intervals around the estimates. All were followed during the entire 21-year period and, hence, no healthy survival effect could be attributable to. Further follow-up studies from sizeable non-referral populations, which include analyses of S-ionized calcium, S-PTH, S-25(OH)D, and urinary calcium excretion, are warranted to further assess the natural progression and morbidity of elevated S-PTH levels in the population. Reference levels of S-PTH and recommended levels of S-25(OH)D could be reconsidered and vitamin D supplementation tested.
Acknowledgments
The excellent help from the staff at the Section for Preventive Cardiology for coordinating the subjects and the statistical help from Mattias Molin and Georgios Lappas is gratefully acknowledged.
Disclosure statement
Georgios Kontogeorgos, Lennart Welin, Michael Fu, Per-Olof Hansson, Kerstin Landin-Wilhelmsen, and Christine M. Laine declare that they have no conflict of interest.
Additional information
Funding
References
- Palmer M, Jakobsson S, Akerstrom G, et al. Prevalence of hypercalcaemia in a health survey: a 14-year follow-up study of serum calcium values. Eur J Clin Invest. 1988;18:39–46.
- Lundgren E, Rastad J, Thurfjell E, et al. Population-based screening for primary hyperparathyroidism with serum calcium and parathyroid hormone values in menopausal women. Surgery. 1997;121(3):287–294.
- Buizert PJ, van Schoor NM, Simsek S, et al. PTH: a new target in arteriosclerosis? J Clin Endocrinol Metab. 2013;98(10):E1583–90.
- Clifton-Bligh PB, Nery ML, Supramaniam R, et al. Mortality associated with primary hyperparathyroidism. Bone. 2015;74:121–124.
- Nussbaum SR, Zahradnik RJ, Lavigne JR, et al. Highly sensitive two-site immunoradiometric assay of parathyrin, and its clinical utility in evaluating patients with hypercalcemia. Clin Chem. 1987;33(8):1364–1367.
- Bilezikian JP, Khan AA, Potts JT. Jr. Guidelines for the management of asymptomatic primary hyperparathyroidism: summary statement from the third international workshop. J Clin Endocrinol Metab. 2009;94(2):335–339.
- Kontogeorgos G, Trimpou P, Laine CM, et al. Normocalcaemic, vitamin D-sufficient hyperparathyroidism – high prevalence and low morbidity in the general population: a long-term follow-up study, the WHO MONICA project, Gothenburg, Sweden. Clin Endocrinol. 2015;83(2):277–284.
- Rosengren A, Eriksson H, Hansson PO, et al. Obesity and trends in cardiovascular risk factors over 40 years in Swedish men aged 50. J Intern Med. 2009;266(3):268–276.
- Zhong Y, Rosengren A, Fu M, et al. Secular changes in cardiovascular risk factors in Swedish 50-year-old men over a 50-year period: the study of men born in 1913, 1923, 1933, 1943, 1953 and 1963. Eur J Prev Cardiolog. 2017;24(6):612–620.
- Hagstrom E, Ahlstrom T, Arnlov J, et al. Parathyroid hormone and calcium are independently associated with subclinical vascular disease in a community-based cohort. Atherosclerosis. 2015;238:420–426.
- Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18(6):499–502.
- Ross AC, Manson JE, Abrams SA, et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Endocrinol Metab. 2011;96(1):53–58.
- Landin-Wilhelmsen K, Wilhelmsen L, Lappas G, et al. Serum intact parathyroid hormone in a random population sample of men and women: relationship to anthropometry, life-style factors, blood pressure, and vitamin D. Calcif Tissue Int. 1995;56(2):104–108.
- Hagstrom E, Ingelsson E, Sundstrom J, et al. Plasma parathyroid hormone and risk of congestive heart failure in the community. Eur J Heart Fail. 2010;12:1186–1192.
- Bollerslev J, Rosen T, Mollerup CL, et al. Effect of surgery on cardiovascular risk factors in mild primary hyperparathyroidism. J Clin Endocrinol Metab. 2009;94(7):2255–2261.
- Lundstam K, Heck A, Mollerup C, et al. Effects of parathyroidectomy versus observation on the development of vertebral fractures in mild primary hyperparathyroidism. J Clin Endocrinol Metab. 2015;100(4):1359–1367.
- Bollerslev J, Schalin-Jantti C, Rejnmark L, et al. Management of endocrine disease: unmet therapeutic, educational and scientific needs in parathyroid disorders. Eur J Endocrinol. 2019;181:1–19.
- Lowe H, McMahon DJ, Rubin MR, et al. Normocalcemic primary hyperparathyroidism: further characterization of a new clinical phenotype. J Clin Endocrinol Metab. 2007;92(8):3001–3005.
- Bilezikian JP, Silverberg SJ. Normocalcemic primary hyperparathyroidism. Arq Bras Endocrinol Metab.. 2010;54(2):106–109.
- Cusano NE, Maalouf NM, Wang PY, et al. Normocalcemic hyperparathyroidism and hypoparathyroidism in two community-based nonreferral populations. J Clin Endocrinol Metab. 2013;98(7):2734–2741.
- Khan AA, Hanley DA, Rizzoli R, et al. Primary hyperparathyroidism: review and recommendations on evaluation, diagnosis, and management. A Canadian and international consensus. Osteoporos Int. 2017;28(1):1–19.
- Farrell CL, Nguyen L, Carter AC. Parathyroid hormone: data mining for age-related reference intervals in adults. Clin Endocrinol. 2018;88(2):311–317.
- Holick MF, Binkley NC, Bischoff-Ferrari HA, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2011;96(7):1911–1930.
- Vignali E, Cetani F, Chiavistelli S, et al. Normocalcemic primary hyperparathyroidism: a survey in a small village of Southern Italy. Endocr Connect. 2015;4(3):172–178.
- Nilsson IL. Primary hyperparathyroidism: should surgery be performed on all patients? Current evidence and residual uncertainties. J Intern Med. 2019;285(2):149–164.
- Landin-Wilhelmsen K, Wilhelmsen L, Wilske J, et al. Sunlight increases serum 25(OH) vitamin D concentration whereas 1,25(OH)2D3 is unaffected. Results from a general population study in Goteborg, Sweden (The WHO MONICA Project). Eur J Clin Nutr. 1995;49(6):400–407.
- Morton LM, Cahill J, Hartge P. Reporting participation in epidemiologic studies: a survey of practice. Am J Epidemiol. 2006;163(3):197–203.
- Harald K, Salomaa V, Jousilahti P, et al. Non-participation and mortality in different socioeconomic groups: the FINRISK population surveys in 1972–92. J Epidemiol Commun Health. 2007;61(5):449–454.
- Bandeira L, Bilezikian J. Primary hyperparathyroidism.2016; 5:pii:F1000 Faculty Rev-1.
- Inaba M, Nakatsuka K, Imanishi Y, et al. Technical and clinical characterization of the Bio-PTH (1-84) immunochemiluminometric assay and comparison with a second-generation assay for parathyroid hormone. Clin Chem. 2004;50(2):385–390.
- Cavalier E, Carlisi A, Bekaert AC, et al. New insights on the stability of the parathyroid hormone as assayed by an automated 3rd generation PTH assay. Clin Chim Acta. 2012;413(1-2):353–354.
- Antoniucci DM, Black DM, Sellmeyer DE. Serum 25-hydroxyvitamin D is unaffected by multiple freeze-thaw cycles. Clin Chem. 2005;51(1):258–261.