Abstract
Branched chain amino acids (BCAA) are implicated in the pathogenesis of cardiometabolic diseases conceivably by affecting insulin resistance and mitochondrial dysfunction. Circulating BCAA levels may predict (subclinical) atherosclerosis, diabetes and hypertension development but the factors involved in BCAA regulation are incompletely understood. Given the key role of thyroid hormones on many metabolic processes including protein metabolism, we aimed to determine effects of thyroid dysfunction on circulating BCAA. Effects of short-term profound hypothyroidism on plasma BCAA were determined in 17 patients who had undergone total thyroidectomy for differentiated thyroid carcinoma. Patients were studied during hypothyroidism, i.e. after thyroidectomy, and after thyroid hormone supplementation. Plasma BCAA (sum of valine, leucine and isoleucine) and alanine were measured by nuclear magnetic resonance spectroscopy. During hypothyroidism (median thyroid-stimulating hormone 81 (IQR 67–120.5) mU/L), plasma BCAA were lower (255 (IQR 222–289) µmol/L) compared to a euthyroid reference population (n = 5579; 377 µmol/L (2.5th to 97.5th percentile 258–548), p < 0.001). After 20 weeks of thyroid hormone supplementation (thyroid-stimulating hormone 0.03 (IQR 0.01–0.14 mU/L) plasma BCAA had increased (328 (IQR 272–392) µmol/L, p = .001), but plasma alanine concentrations were unaltered (p = .50). Changes in body weight in response to thyroid hormone supplementation were correlated with changes in plasma BCAA (r = 0.721 p = .001, but not with changes in cholesterol or glucose (p > .80). In conclusion, plasma BCAA concentrations are lower during short-term profound hypothyroidism in humans, and increase in response to thyroid hormone supplementation. Changes in BCAA and in body weight after reversal of the hypothyroid state appear to be interrelated.
Introduction
Branched chain amino acids (BCAA) are non-linear aliphatic side-chain containing amino acids and include the essential amino acids valine, leucine and isoleucine [Citation1]. Evidence has accumulated recently that BCAA are not only required for protein synthesis but may also have a critical role in intracellular metabolism with adverse consequences for insulin resistance and mitochondrial dysfunction [Citation2,Citation3]. Plasma BCAA levels correlate positively with insulin resistance [Citation4] and are increased in the metabolic syndrome [Citation1]. Furthermore, plasma BCAA levels may predict (subclinical) atherosclerotic cardiovascular disease as well as the development of type 2 diabetes mellitus and hypertension [Citation1,Citation5–7].
Thyroid dysfunction has profound effects on cellular metabolism with adverse effects on the cardiovascular system [Citation8,Citation9]. Thyroid dysfunction also has adverse effects on glucose homeostasis and may predict the development of Type 2 diabetes mellitus [Citation10]. Notably, hypothyroidism was shown to impair the endogenous rate of appearance, oxidation and non-oxidative disposal of the amino acid leucine, indicating that thyroid function status plays a role in protein metabolism [Citation11]. Mild hyperthyroidism, on the other hand, may increase leucine flux, suggestive of accelerated protein degradation [Citation12].
Collectively, these data underline that it is relevant to determine whether hypothyroidism affects circulating BCAA levels. Hypothyroidism consequent to total thyroidectomy for differentiated thyroid carcinoma offers a unique condition to investigate the effects of short-term profound hypothyroidism on circulating biomarkers in humans as evidenced by robust effects on lipoprotein metabolism and energy expenditure [Citation13–15]. No data are currently available regarding the possible effect of thyroid dysfunction on plasma BCAA regulation.
The present study was, therefore, initiated to determine reversible effects of short-term profound hypothyroidism consequent of total thyroidectomy for differentiated thyroid carcinoma on plasma BCAA levels.
Materials and methods
Participants and study design
Reporting of this study follows the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines [Citation16]. This observational study was performed among patients with differentiated thyroid carcinoma (DTC). Newly diagnosed DTC patients (18–75 years old) were eligible to participate in the study. Pregnant woman and patients with cerebrovascular/coronary events, atrial fibrillation or heart failure were not allowed to participate. We also excluded subjects with suspected distant metastases. The study was approved by the Medical Ethics Committee of the University Medical Center Groningen (registration number 2015/116). This study was registered at the Netherlands Trial Register (NTR ID 7228). All participants gave written informed consent.
This study comprised two outpatient study visits. The first study visit took place under circumstances of severe short-term hypothyroidism, i.e. 4–6 weeks after total or completing thyroidectomy (the latter when a hemithyroidectomy was performed initially) at the day before ablative radioactive iodine treatment. This procedure was performed at high thyroid-stimulating hormone (TSH) levels to enhance uptake of radioactive iodine in any potentially remaining thyroid tissue. Shortly after radioactive iodine treatment, liothyronine (n = 13; 75 µg daily) or levothyroxine (n = 4; 150–200 µg daily) supplementation was started, aimed to achieve TSH suppression. All study measurements were repeated after 20 weeks of thyroid hormone supplementation. This treatment protocol followed Dutch guidelines of DTC treatment [Citation17].
At both study visits, height (m), weight (kg), blood pressure (mmHg) and heart rate (bpm) were measured. Body mass index (BMI) was calculated as weight divided by length squared (kg/m2). Venous blood was obtained after an overnight fast.
To compare laboratory data of the study population with the general North European population a set of reference intervals for plasma total BCAA, valine, leucine and isoleucine was obtained from the PREVEND cohort (second screening round, 2001–2003) [Citation18]. We selected individuals without a history of thyroid disease with a TSH level (0.27–4.2 mU/L), a free thyroxine (fT4) level (12–22 pmol/L) and a free triiodothyronine (fT3 3.1–6.8 pmol/L) each within the euthyroid reference range [Citation15]. Additional exclusion criteria for this reference population were the use of thyroid hormones, anti-thyroid drugs, amiodarone or lithium carbonate [Citation15]. This population consisted of 5579 subjects (2889 [52%] men and 2690 [48%] women) with a median age of 53 (interquartile range [IQR] 43–62) years. Of these, 316 (5.7%) had type 2 diabetes (no insulin treatment), 333 (6.0%) had a previous history of cardiovascular disease, 1131 (20.2%) used anti-hypertensive drugs and 489 (8.8%) used lipid lowering drugs. In this reference, population TSH was 1.57 (IQR 0.51–3.67) mU/L and fT4 was 15.7 (IQR 12.5–19.8) pmol/L.
Laboratory methods
Plasma glucose and total cholesterol were measured using routine methods. TSH was assayed using the Roche Modular E170 Analyzer (Roche Diagnostics, Mannheim, Germany). Ethylenediaminetetraaceticacid dipotassium (EDTA-K2) tubes were used to collect venous blood samples. Plasma samples were prepared by ultracentifugation and were immmediately frozen at −80 °C. Plasma samples were sent frozen to LipoScience/LabCorp (Morrisville, NC). Valine, leucine, isoleucine and alanine concentrations were measured using a Vantera Clinical Analyzer (LabCorp., Morrisville, NC), a fully automated, high-throughput, 400 MHz proton (1H) nuclear magnetic resonance (NMR) spectroscopy platform. Plasma samples were prepared on board the instrument, and automatically delivered to the flow probe in the NMR spectrometer’s magnetic field. The validation of the use of NMR for quantification of BCAAs has been described previously [Citation1]. Total BCAA concentrations were calculated as the sum of valine, leucine and isoleucine. Coefficients of variation for inter- and intra-assay precision range from 1.8 to 6.0, 1.7 to 5.4, 4.4 to 9.1, 8.8 to 21.3% and 1.3 to 2.8%, for plasma total BCAA, valine, leucine, isoleucine and alanine, respectively. In another data set we have previously documented that BCAA quantified from the same samples using NMR and liquid chromatography-mass spectrometry/mass spectrometry are highly correlated (r2 = 0.97, 0.95 and 0.90 for valine, leucine and isoleucine) [Citation1]. BCAA and alanine were assayed in one run. Total variability of BCAA (biological variability plus assay variability) was determined in 18 healthy subjects of whom fasting venous blood was obtained in the fasting state between 8 and 10 a.m. during five consecutive weeks and amounted to 11.8%. Similar laboratory methods for measurement of BCAAs were used for the DTC patients and the control population.
Statistical analysis
SPSS 23 (version 23.0, IBM Corp., Armonk, NY) was used for data analysis. Data were presented as the median (IQR), and categorical data in numbers and percentages. Reference values in the euthyroid population-based PREVEND cohort are given as median and 2.5th to 97.5th percentile. Changes in variables between hypothyroidism and thyroid hormone supplementation were determined by paired samples Wilcoxon signed rank tests. Comparison of plasma BCAA with the reference population was done by Mann–Whitney U tests. Relationships between changes in variables were determined by Spearman rank correlation coefficients. Two-sided p-values <0.05 were considered to be significant.
Results
Seventeen participants were included in this study. The median age of the participants was 46 (IQR 36–50) years. Sixteen participants were women (94%). Fourteen participants (82%) were diagnosed with papillary thyroid carcinoma, of which seven had lymph node metastases; the other three (18%) were diagnosed with follicular thyroid carcinoma. None of the participants had distant metastases. The most important medication prescription included insulin for Type 1 diabetes in one participant, prednisolone 5 mg daily, previously diagnosed with polymyalgia rheumatica (dose unchanged during follow-up) in another participant and oral contraceptives in eight women. None of the participants were current smokers and eight (47%) were former smokers. Nine participants (53%) consumed alcohol. Their average weekly intake of alcohol varied between 0.25 and 8 drinks, and was unchanged during the follow-up period.
At hypothyroidism median, TSH was 81.0 (IQR 67.0 − 120.5) mU/L and fT4 was 2.3 (IQR 1.7 − 3.5) pmol/L, whereas TSH had decreased to 0.03 (IQR 0.01 − 0.14) mU/L with thyroid hormone supplementation (p < 0.001). Clinical and laboratory variables are shown in . Pulse rate was lower, whereas body weight was higher during hypothyroidism. Hypothyroidism also resulted in a higher plasma total cholesterol and a lower plasma glucose (). Plasma total BCAA, valine, leucine and isoleucine were each lower during hypothyroidism than the values found in the reference population (total BCAA 377 (258–548) µmol/L; valine 209 (143–290) µmol/L; leucine 126 (81–193) µmol/L; isoleucine 43 (18–80) µmol/L (p ≤ 0.001 for each). After thyroid hormone supplementation, plasma total BCAA had increased which was due to a significant increase in valine and isoleucine (). Notably, the plasma alanine concentration was not different from its value in the reference population (321 (197-486) µmol/L; p = .30) and remained virtually unaltered during hypothyroidism (p = .50).
Table 1. Clinical and laboratory variable of 17 patients with differentiated thyroid carcinoma during hypothyroidism and after 20 weeks of thyroid hormone supplementation.
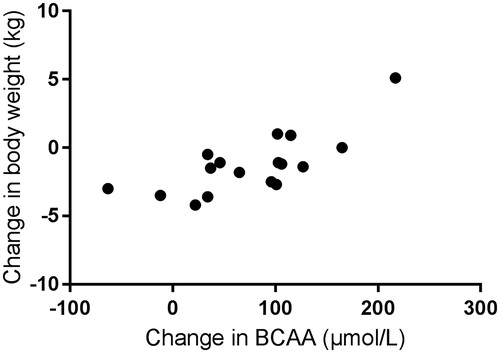
Changes in body weight after thyroid hormone supplementation were correlated with changes in plasma total BCAA (r = 0.721, p = .001; ). Changes in plasma glucose and cholesterol were unrelated to changes in plasma total BCAA (r = 0.011, p = .97 and r = 0.046, p = .86, respectively).
Discussion
The present preliminary study shows for the first time that plasma concentrations of BCAA are lower during short-term profound hypothyroidism as compared to levels obtained from a large euthyroid reference population. Twenty weeks of thyroid hormone supplementation, resulting in low-range TSH levels, significantly increased plasma total BCAA which was due to increases in valine, the most abundant BCAA, and in isoleucine. On the other hand, plasma levels of the amino acid alanine, a non BCAA, were not decreased during hypothyroidism and did not change after thyroid hormone supplementation. We, therefore, suggest that thyroid function status may affect circulating BCAA levels.
Proteolysis is likely to be impaired in hypothyroidism, whereas protein breakdown is accelerated in hyperthyroidism [Citation11,Citation19]. Moreover, thyroid hormones may inhibit cellular amino acid uptake in vitro [Citation20]. Such mechanisms may be involved in plasma BCAA alterations during hypothyroidism. Additionally, yet to be more precisely determined effects of thyroid hormone status on BCAA regulation could also involve alterations in the gut microbiome which is a recently recognized important determinant of BCAA bioavailability [Citation19,Citation21]. Of potential interest, we did not observe a change in plasma alanine during hypothyroidism. Mild hyperthyroidism did not affect alanine flux, contrasting effects on leucine turnover [Citation12]. However, the specificity of hypothyroidism to decrease plasma total BCAA (and the individual amino acids valine and isoleucine) as compared to other amino acids remains to be more precisely established.
Body weight expectedly decreased during thyroid hormone supplementation in the current study in line with a reservable decrease in energy expenditure during hypothyroidism [Citation12]. Paradoxically, a positive correlation between changes in plasma BCAA and changes in bodyweight in response to thyroid hormone supplementation was observed. In contrast, the anticipated decreases in plasma total cholesterol [Citation11,Citation12] and the modest increases in glucose after reversal of hypothyroidism were unrelated to changes in BCAA. Combined, these findings suggest that body weight, plasma lipids, glucose and BCAA regulation are at least in part differently affected by thyroid dysfunction. The mechanisms responsible for the interrelationship between changes in body weight and changes in plasma BCAA await further study.
The current study has limitations that need to be ascertained. In line with previous reports that made use of profound hypothyroidism after total thyroidectomy for DCT, a rather small group of subjects participated in this study [Citation13–15]. In agreement with these earlier reports [Citation13,Citation14], plasma cholesterol was profoundly elevated during hypothyroidism, as compared with its concentration during thyroid hormone supplementation. Furthermore, most participants were women, limiting extrapolation of our findings to both sexes. As we followed the Dutch guidelines for DCT management [Citation17,Citation22], we could not include a control group of prolonged untreated hypothyroidism. However, plasma BCAA and alanine levels during hypothyroidism were compared with a large euthyroid reference group from the same region of The Netherlands. Moreover, variations in plasma BCAA over time, measured in a separate euthyroid control group, appeared to be smaller than the changes found in response to thyroid hormone supplementation.
Conclusion
The plasma total BCAA concentration is lower during short-term profound hypothyroidism in humans. BCAA and body weight changes after reversal of the hypothyroid state appear to be interrelated. From a clinical perspective, we propose the thyroid dysfunction should be taken into account when evaluating the effects of BCAA on cardiometabolic disorders.
Author contributions
TvdB and RPFD developed and formulated the research questions, have full access to the study data and take responsibility for its integrity and the data analysis. TvdB and RPFD wrote the manuscript. TvdB, EGG, JDL, MAC and TPL contributed to the acquisition of data, contributed to discussion, draft revision and edited the manuscript. All authors approved the final version of the manuscript.
| Abbreviations | ||
| BCAA | = | branched chain amino acids |
| BMI | = | body mass index |
| CV | = | coefficient of variation |
| DTC | = | differentiated thyroid carcinoma |
| FT4 | = | free thyroxine |
| IQR | = | interquartile range |
| NMR | = | nuclear magnetic resonance |
| NTR | = | Netherlands Trial Register |
| STROBE | = | Strengthening the Reporting of Observational Studies in Epidemiology |
| TSH | = | thyroid stimulating hormone. |
Disclosure statement
MAC is an employee of LabCorp. The other authors declare that they have no conflicts of interest.
Additional information
Funding
References
- Lynch CJ, Adams SH. Branched-chain amino acids in metabolic signalling and insulin resistance. Nat Rev Endocrinol. 2014;10(12):723–736.
- Wolak-Dinsmore J, Gruppen EG, Shalaurova I, et al. A novel NMR-based assay to measure circulating concentrations of branched-chain amino acids: Elevation in subjects with type 2 diabetes mellitus and association with carotid intima media thickness. Clin Biochem. 2018;54:92–99.
- Batch BC, Shah SH, Newgard CB, et al. Branched chain amino acids are novel biomarkers for discrimination of metabolic wellness. Metabolism. 2013;62(7):961–969.
- Batch BC, Hyland K, Svetkey LP. Branch chain amino acids: biomarkers of health and disease. Curr Opin Clin Nutr Metab Care. 2014;17(1):86–89.
- Connelly MA, Wolak-Dinsmore J, Dullaart RPF. Branched chain amino acids are associated with insulin resistance independent of leptin and adiponectin in subjects with varying degrees of glucose tolerance. Metab Syndr Relat Disord. 2017;15(4):183–186.
- Tobias DK, Lawler PR, Harada PH, et al. Circulating branched-chain amino acids and incident cardiovascular disease in a prospective cohort of US women. Circ Genomic Precis Med. 2018;11:e002157.
- Flores-Guerrero JL, Groothof D, Connelly MA, et al. Concentration of branched-chain amino acids is a strong risk marker for incident hypertension. Hypertension. 2019;74(6):1428–1435.
- Flores-Guerrero J, Osté M, Kieneker L, et al. Plasma branched-chain amino acids and risk of incident type 2 diabetes: results from the PREVEND prospective cohort study. JCM. 2018;7(12):513.
- Danzi S, Klein I. Thyroid disease and the cardiovascular system. Endocrinol Metab Clin North Am. 2014;43(2):517–528.
- Jabbar A, Pingitore A, Pearce SHS, et al. Thyroid hormones and cardiovascular disease. Nat Rev Cardiol. 2017;14(1):39–55.
- Chaker L, Ligthart S, Korevaar TIM, et al. Thyroid function and risk of type 2 diabetes: a population-based prospective cohort study. BMC Med. 2016;14(1):8.
- Rochon C, Tauveron I, Dejax C, et al. Response of leucine metabolism to hyperinsulinemia in hypothyroid patients before and after thyroxine replacement. J Clin Endocrinol Metab. 2000;85(2):697–706.
- Tsalikian E, Lim VS. L-triiodothyronine at a slightly over physiologic dose increases leucine flux, which suggests an increase in protein degradation in normal subjects. J Lab Clin Med. 1989;114:171–175.
- Dullaart RPF, Hoogenberg K, Groener JEM, et al. The activity of cholesteryl ester transfer protein is decreased in hypothyroidism: a possible contribution to alterations in high-density lipoproteins. Eur J Clin Invest. 1990;20(6):581–587.
- Sigal GA, Tavoni TM, Silva BMO, et al. Effects of short-term hypothyroidism on the lipid transfer to high-density lipoprotein and other parameters related to lipoprotein metabolism in patients submitted to thyroidectomy for thyroid cancer. Thyroid. 2019;29(1):53–58.
- Wolf M, Weigert A, Kreymann G. Body composition and energy expenditure in thyroidectomized patients during short-term hypothyroidism and thyrotropin-suppressive thyroxine therapy. Eur J Endocrinol. 1996;134(2):168–173.
- Vandenbroucke JP, von Elm E, Altman DG, et al.; STROBE Initiative. Strengthening the reporting of observational studies in epidemiology (STROBE): explanation and elaboration. Int J Surg. 2014;12(12):1500–1524.
- Schildkliercarcinoom richtlijn. National Guideline Thyroid Carcinoma. Oncoline 2015. Available from: http://oncoline.nl/schildkliercarcinoom.
- Gruppen EG, Kootstra-Ros J, Kobold AM, et al. Cigarette smoking is associated with higher thyroid hormone and lower TSH levels: the PREVEND study. Endocrine. 2020;67(3):613–622.
- Morrison WL, Gibson JNA, Jung RT, et al. Skeletal muscle and whole body protein turnover in thyroid disease. Eur J Clin Invest. 1988;18(1):62–68.
- Prasad PD, Leibach FH, Mahesh VB, et al. Relationship between thyroid hormone transport and neutral amino acid transport in JAR human choriocarcinoma cells. Endocrinology. 1994;134(2):574–581.
- Brechmann T, Sperlbaum A, Schmiegel W. Levothyroxine therapy and impaired clearance are the strongest contributors to small intestinal bacterial overgrowth: Results of a retrospective cohort study. World J Gastroenterol. 2017;23(5):842–852.