Abstract
The Clauss assay is the assay most often used for measuring plasma fibrinogen levels. However, the FIBTEM-assay, determined using thromboelastometry (ROTEM) can also be used to estimate fibrinogen levels. A major advantage of the FIBTEM is that it can provide information about fibrinogen levels within minutes, while the Clauss assay needs 30–60 min before the result is available. The aim of this study was to investigate the correlation between fibrinogen levels measured by the Clauss assay and results from the FIBTEM-assay. We included 111 patients ≥18 years for whom both ROTEM analyses and a fibrinogen measurement using the Clauss assay were available. In addition, ROTEM and Clauss measurements from 75 healthy subjects were included. Spearman correlation was used to determine the association between the results of both assays. The patients included were mostly patients with major trauma or undergoing large surgery (e.g. cardiac surgery or liver transplantation). Strong correlations were found between FIBTEM clot firmness parameters and fibrinogen levels measured by the Clauss assay in patients (Spearman’s correlation coefficients (rs) above 0.80 (p < .001) for all subgroups) and healthy subjects (rs = 0.66, p < .001). The correlation between early FIBTEM parameters (clot firmness at 5 or 10 min) and the maximum clot firmness was almost perfect (rs above 0.96). Also, the correlation between the α-angle and FIBTEM parameters was strong (rs above 0.7). In conclusion, strong correlations were found between early FIBTEM parameters and fibrinogen levels.
Introduction
During acute settings accompanied by major blood loss (e.g. major trauma or complicated surgical procedures), it is important for clinicians that fibrinogen concentrations are available as quickly as possible, to guide adequate management. The risk of bleeding is increased in individuals when fibrinogen levels decrease during trauma or surgery and it is recommended to maintain them above 1.5 g/L [Citation1].
For the most commonly used fibrinogen assay, the Clauss assay, it takes 30–60 min before the result is known in a diagnostic laboratory [Citation2]. Another method that is regularly used in acute settings to rapidly estimate fibrinogen concentration is rotational thromboelastometry (ROTEM). A specific test of the ROTEM, the FIBTEM, provides information about the extrinsic pathway of coagulation, while eliminating the role of platelets. Therefore, information obtained with this assay gives an estimate of the contribution of fibrinogen to coagulation. Different parameters can be obtained from the FIBTEM test, of which the amplitude (or clot firmness) at 5 min (A5) or 10 min (A10) and the maximum clot firmness (MCF) are most used for estimating fibrinogen levels.
It is suggested that the A5 can already provide relevant information about the functional fibrinogen concentration [Citation3]. In addition, the α-angle might be a good indicator for the value of the A5 or A10 [Citation4]. Differences in the underlying mechanism of the Clauss assay and FIBTEM test can give discrepant results, especially in patients with dysfibrinogenemia or low levels of coagulation factors [Citation5,Citation6]. This is specifically relevant in trauma patients and patients undergoing large surgeries. Furthermore, in healthy individuals, heterogeneity in fibrinogen can potentially affect the results of both assays, resulting in discrepancies [Citation7]. The correlation between the FIBTEM and Clauss assay has been investigated before, however only in selected groups of patients [Citation8], and no information is available for this correlation in healthy individuals.
Therefore, the aim of this study was to determine correlations between fibrinogen concentration measured by the Clauss assay and FIBTEM clot firmness parameters in different patient groups in a real-life hospital setting and in healthy individuals.
Methods
Patients
Data from all patients aged ≥18 years for whom both ROTEM and Clauss assays were ordered as part of routine care in May or June 2019 in the Erasmus Medical Center Rotterdam were collected retrospectively for this study. Patients for whom no results were available for the ROTEM, Clauss assay or APTT were excluded, there were no other in- or exclusion criteria. Included patients were divided into the following groups: major bleeding or other trauma, liver transplantation or other liver surgery, cardiac surgery (mainly procedures involving heart valves or aorta) and other (mainly other surgical procedures). The following parameters were obtained from patients laboratory results: prothrombin time (PT), activated partial thromboplastin time (APTT), fibrinogen concentration (Clauss assay) and ROTEM results. Only one measurement of each patient was included, namely the first measurement in which the APTT was below 100 s, to exclude results strongly influenced by heparin. Based on the retrospective nature of this study, this study was not subject to the Medical Research Involving Human Subjects Act and a waiver for informed consent was granted for the patient group (MEC-2020-0507). As part of the Crescendo study (Clinical Relevance and Significance of New Diagnostic Options in patients with Unexplained Bleeding) healthy individuals were recruited between July 2016 and March 2018 among employees and students of the Erasmus MC University Medical Center [Citation9]. The Crescendo study was subject to the Medical Research Involving Human Subjects Act and approved by the Medical Ethics Committee of the Erasmus University Medical Center Rotterdam (MEC-2016-218). Written consent was obtained from each healthy participant. All healthy volunteers with results of both the ROTEM and Clauss assay (n = 75) available were included in the current study.
Fibrinogen assays
The Clauss assay was performed on a fully automated coagulation analyzer (Sysmex CS-5100 system, Siemens Healthcare Diagnostics, Breda, the Netherlands). FIBTEM measurements were performed on the ROTEM® Delta device, according to the manufacturer’s instructions (Werfen, Barcelona, Spain). The following ROTEM parameters were analyzed: clot firmness at 5 or 10 min (A5 and A10, respectively), maximum clot firmness (MCF) and the α-angle.
Statistical analysis
We used descriptive statistics to summarize baseline characteristics of the study group. Because of a skewed distribution, all data are presented as median with interquartile range (IQR). Correlations between the Clauss assay and ROTEM parameters were tested by non-parametric analyses, determining Spearman’s correlation coefficients. Kappa statistics was done to test the agreement in classification in three groups (low, normal, high) between the two assays. Receiver operating characteristic (ROC) curves were used to determine the best cut-off value of early FIBTEM parameters to predict low fibrinogen levels (below 1.5 g/L). All tests were two-tailed and a p-value below .05 was considered statistically significant. All statistical analyses were performed using IBM SPSS Statistics 25.
Results
A total of 111 patients of whom both ROTEM and Clauss assay results were available were included in this study, in addition to 75 healthy subjects. The median [IQR] age was 60.0 [49.0–69.0] for the patients and 32.0 [26.0–46.0] for the healthy subjects. 38% of the patients was female, while in the healthy subjects the fraction of women was 85%. The majority of patients of whom ROTEM measurements were available, were patients undergoing cardiac surgery (40%) or patients that experienced bleedings or other trauma (31%). Twelve percent of patients had a liver transplantation or liver surgery and 18% of patients were classified as ‘other’. In this last group, mainly patients undergoing surgical procedures, other than cardiac or liver surgery (for example laparotomy), were included. In the patient subgroups, the fraction of women was between 16% and 65% (). As expected, PT and APTT values were significantly higher in the patient groups compared to healthy subjects.
Table 1. Study group characteristics.
The correlation between the clot firmness at 5 or 10 min (A5 and A10) and the maximum clot firmness (MCF) of the FIBTEM was almost perfect in all patient subgroups and healthy individuals (rs above 0.96, p < .001) ( and Supplementary Table 1). In addition, the α-angle and the clot firmness parameters were strongly correlated in all subgroups ().
Figure 1. Correlation between the maximum clot firmness (MCF) of the FIBTEM assay and early FIBTEM parameters (clot firmness at 5 (A5) or 10 (A10) min).
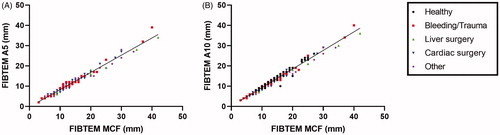
Table 2. Correlation between fibrinogen level (Clauss assay) or the FIBTEM α–angle and FIBTEM clot firmness parameters.
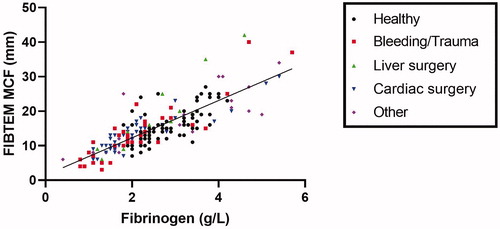
and show the correlations and Spearman’s correlation coefficients for fibrinogen levels measured by the Clauss assay and the FIBTEM clot firmness parameters. In both the patients groups as well as healthy individuals, strong correlations between the Clauss assay and FIBTEM were found: rs above 0.80, p < .001 for all patient subgroups and rs=0.66, p < .001 for healthy subjects. The correlation in the healthy individuals is somewhat lower, probably because of the smaller range of the fibrinogen levels.
Figure 2. Correlation between fibrinogen concentrations measured by the Clauss assay and maximum clot firmness (MCF) of the FIBTEM assay.
In addition, the agreement between the two assays in classifying fibrinogen levels in low, normal and high was calculated (). The fibrinogen measurements were divided in three groups; ≤1.5 g/L, between 1.5 and 3.5 g/L and >3.5 g/L. Also the levels of the FIBTEM parameters were categorized in three groups (lower than or equal to the normal range, in the normal range, or above the normal range as provided by the manufacturer of the ROTEM instrument) (). Especially for A10 and MCF, the agreement between the FIBTEM parameters and the Clauss assay was found to be strong (Κ above 0.5, p < .001) ().
Figure 3. Agreement between classification in low, normal and high levels according to the Clauss assay and FIBTEM parameters clot firmness at 5 (A5) or 10 (A10) min or maximum clot firmness (MCF).
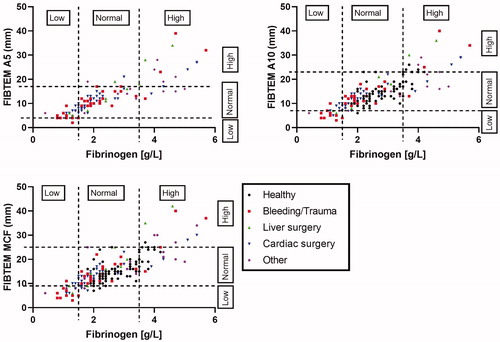
Table 3. Agreement between the Clauss assay and FIBTEM parameters in classification in low, normal or high fibrinogen.
Finally, we determined the optimal cut-off values of the FIBTEM parameters to identify fibrinogen levels below 1.5 g/L, based on our study population instead of the general reference values provided by the manufacturer. This was only done for the 111 patients, since there were no healthy subjects with fibrinogen levels below 1.5 g/L. In total, 27 out of 111 patients (24.3%) had fibrinogen levels below 1.5 g/L. The optimal cut-off value was ≤9.5 for both A5 and A10 values and ≤10.5 for MCF ().
Table 4. Optimal cut-off values of FIBTEM parameters for fibrinogen levels ≤1.5 g/L.
Discussion
We compared the results of fibrinogen levels measured by the Clauss assay with those of the FIBTEM assay and found high correlations in different patient groups and healthy individuals. We also found an almost perfect correlation between the MCF of the FIBTEM and the A5 or A10, which implicates that early parameters of the FIBTEM predict the final clot firmness. Finally, strong correlations were found between the α-angle and clot firmness parameters, implicating that faster clot formation also predicts higher clot firmness.
The correlation between the Clauss assay and the FIBTEM assay is strong for most patients (rs above 0.80, p < .001), however some individuals have discrepant values. This could have been caused by dysfibrinogenemia or low levels of coagulation factors caused by trauma or surgery. Previously, other studies have been performed to correlate FIBTEM measurements with fibrinogen levels measured by the Clauss assay in different patient groups [Citation8]. In women with postpartum hemorrhage, moderate to good correlations have been found between the Clauss assay and FIBTEM parameters A5, A10 or MCF [Citation10–12]. Also in children [Citation13], trauma patients [Citation14–16] and patients undergoing liver transplantation [Citation17–19] or cardiac surgery [Citation20,Citation21], moderate to good correlations have been found between fibrinogen concentrations and the different FIBTEM parameters. A potential confounder in the patients undergoing cardiac surgery in our study is heparinization, which might have interfered with the FIBTEM measurements. However, a heparin inhibitor is present in the FIBTEM measurement, neutralizing high heparin concentrations up to 1 U/ml. To exclude samples with heparin concentrations above 1 U/ml, all measurements for which APTT results were above 100 s were not included in the analyses. In addition, we do not observe a weaker correlation in patients undergoing cardiac surgery compared to the other subgroups, suggesting heparin did not influence the results. A strength of our study was that we retrospectively compared the results of both assays measured during normal clinical settings instead of selecting patients. This shows that the results are applicable to a wide range of patients. In addition, we included a large healthy population to compare the Clauss assay with FIBTEM parameters, which, to our knowledge, has not been reported before. The correlation between fibrinogen levels and FIBTEM parameters was slightly lower in healthy individuals compared to the patients. This is most likely caused by the much smaller range of fibrinogen levels in the healthy individuals.
When both assays were used to classify patients in low, normal or high levels of fibrinogen in this study, especially strong agreement was found in patients with bleedings or other trauma and patients undergoing liver transplantation or surgery. For patients undergoing cardiac surgery or other surgeries, the agreement was somewhat lower, which could have been due to the low number of patients in these groups. In addition, the reference values based on the manufacturer of the ROTEM instrument might not have been the best cut-off values to classify patients in low, normal or high fibrinogen levels, since the normal values potentially differ between different laboratories. Therefore, we determined the optimal cut-off values of the FIBTEM parameters, based on our study group, to predict whether fibrinogen levels are below 1.5 g/L. Multiple studies have looked at optimal threshold values of the early FIBTEM parameters A5 and A10 to quickly determine if fibrinogen levels are below a critical point. One study found a similar threshold for A5 as we did (9.5 mm) to determine fibrinogen levels below 1.5 g/L in trauma patients [Citation15], while other studies determined lower threshold levels (5, 6 or 7 mm) [Citation12,Citation20,Citation22,Citation23]. For A10, only one study in patients undergoing cardiac surgery determined the optimal cut-off value, which was similar to ours [Citation24]. It is important to work with the optimal cut-off values, because this prevents unnecessary supplementation of fibrinogen, while the risk of bleeding is reduced to a minimum.
In the Erasmus Medical Center, according to the massive blood loss protocol, fibrinogen concentrate is given to patients when A10 values are ≤9 mm. If A10 values are ≤7 mm or ≤5 mm, increased amounts of fibrinogen concentrate are given. The values currently used to guide transfusion of fibrinogen concentrate correspond well to the optimal cut-off value found in this study: 9.5 for fibrinogen levels below 1.5. As described above, the reported optimal cut-off values for FIBTEM parameters to determine low fibrinogen levels are quite variable across different studies. This might partially be caused by variation in assays and reagents per laboratory, and also the type of ROTEM used contributes to the variation [Citation11]. It is therefore of great importance for each laboratory to work with reference values specific for the device used .
A limitation of our study is the retrospective nature, which may have introduced bias and increases the risk of statistical errors. However, a selection bias was minimized by including all patients for which ROTEM and Clauss measurements were ordered in May and June 2019. Therefore, this is a good representation of patient population for which ROTEM measurements are needed and these results are relevant. In addition, results from these measurements are not very likely to be wrongly recalled, since the raw data of these tests are available in the patient laboratory results. Another limitation is the limited number of subject with very low or very high fibrinogen levels. However, it is important to realize that this study was performed in a real-life hospital setting, there was no selection of samples based on the fibrinogen level. In addition, the healthy individuals were more often female and on average much younger than the patients included in this study, which makes the groups less comparable to each other. However, the aim of this research was not to compare these groups, but to investigate the correlation between the two assays in both groups. Finally, fibrinogen concentrate might have been given to patients included in this study, especially after major blood loss or during large surgeries. We believe this does not have consequences for our results, since this will both affect the Clauss assay and ROTEM measurement.
In conclusion, early FIBTEM clot firmness parameters correlate well with final clot firmness as measured by the FIBTEM assay and to fibrinogen concentration as measured by the Clauss assay. This means that early FIBTEM parameters as well as the MCF might be used to evaluate fibrinogen concentrations, thus saving time in emergency situations.
| Abbreviations | ||
| A5 | = | clot firmness at 5 minutes |
| A10 | = | clot firmness at 10 minutes |
| APTT | = | activated partial thrombin time |
| Crescendo | = | Clinical Relevance and Significance of New Diagnostic Options in patients with Unexplained Bleeding |
| FIBTEM | = | fibrinogen part of the ROTEM |
| IQR | = | interquartile range |
| MCF | = | maximum clot firmness |
| PT | = | prothrombin time |
| ROC | = | receiver operating characteristic |
| ROTEM | = | rotational thromboelastometry |
Acknowledgements
The authors thank the technicians of the hemostasis laboratory for their technical assistance.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Levy JH, Welsby I, Goodnough LT. Fibrinogen as a therapeutic target for bleeding: a review of critical levels and replacement therapy. Transfusion. 2014;54(5):1389–1405.
- Whiting M. Impact, meaning and need for help and support: the experience of parents caring for children with disabilities, life-limiting/life-threatening illness or technology dependence. J Child Health Care. 2013;17(1):92–108.
- Curry NS, Davenport R, Pavord S, et al. The use of viscoelastic haemostatic assays in the management of major bleeding: a British Society for Haematology Guideline. Br J Haematol. 2018;182(6):789–806.
- Toffaletti JG, Buckner KA. Use of earlier-reported rotational thromboelastometry parameters to evaluate clotting status, fibrinogen, and platelet activities in postpartum hemorrhage compared to surgery and intensive care patients. Anesth Analg. 2019;128:414–423.
- Casini A, Neerman-Arbez M, Ariens RA, et al. Dysfibrinogenemia: from molecular anomalies to clinical manifestations and management. J Thromb Haemost. 2015;13(6):909–919.
- Theusinger OM, Schroder CM, Eismon J, et al. The influence of laboratory coagulation tests and clotting factor levels on Rotation Thromboelastometry (ROTEM(R)) during major surgery with hemorrhage. Anesth Analg. 2013;117(2):314–321.
- Nieuwenhuizen W. Biochemistry and measurement of fibrinogen. Eur Heart J. 1995;16 Suppl A (Suppl A):6–10.
- Ranucci M, Dedda DU, BaryshnikovaE. Trials and tribulations of viscoelastic-based determination of fibrinogen concentration. Anesth Analg. 2020;130:644–653.
- Veen CSB, Huisman EJ, Cnossen MH, et al. Evaluation of thromboelastometry, thrombin generation and plasma clot lysis time in patients with bleeding of unknown cause: a prospective cohort study. Haemophilia. 2020;26(3):e106–e115.
- de Lange NM, van Rheenen-Flach LE, Lance MD, et al. Peri-partum reference ranges for ROTEM(R) thromboelastometry. Br J Anaesth. 2014;112(5):852–859.
- Gillissen A, van den Akker T, Caram-Deelder C, et al. Comparison of thromboelastometry by ROTEM® Delta and ROTEM® Sigma in women with postpartum haemorrhage. Scand J Clin Lab Invest. 2019;79(1-2):32–38.
- Huissoud C, Carrabin N, Audibert F, et al. Bedside assessment of fibrinogen level in postpartum haemorrhage by thrombelastometry. BJOG. 2009;116(8):1097–1102.
- Haas T, Spielmann N, Mauch J, et al. Comparison of thromboelastometry (ROTEM(R)) with standard plasmatic coagulation testing in paediatric surgery. Br J Anaesth. 2012;108(1):36–41.
- Meyer AS, Meyer MA, Sorensen AM, et al. Thrombelastography and rotational thromboelastometry early amplitudes in 182 trauma patients with clinical suspicion of severe injury. J Trauma Acute Care Surg. 2014;76:682–690.
- Rourke C, Curry N, Khan S, et al. Fibrinogen levels during trauma hemorrhage, response to replacement therapy, and association with patient outcomes. J Thromb Haemost. 2012;10(7):1342–1351.
- Rugeri L, Levrat A, David JS, et al. Diagnosis of early coagulation abnormalities in trauma patients by rotation thrombelastography. J Thromb Haemost. 2007;5(2):289–295.
- Hashir A, Singh SA, Krishnan G, et al. Correlation of early ROTEM parameters with conventional coagulation tests in patients with chronic liver disease undergoing liver transplant. Indian J Anaesth. 2019;63(1):21–25.
- Song JG, Jeong SM, Jun IG, et al. Five-minute parameter of thromboelastometry is sufficient to detect thrombocytopenia and hypofibrinogenaemia in patients undergoing liver transplantation. Br J Anaesth. 2014;112(2):290–297.
- Jeong SM, Song JG, Seo H, et al. Quantification of both platelet count and fibrinogen concentration using maximal clot firmness of thromboelastometry during liver transplantation. Transplant Proc. 2015;47(6):1890–1895.
- Matzelle SA, Weightman WM, Gibbs NM. An audit of the diagnostic accuracy of rotational thromboelastometry for the identification of hypofibrinogenaemia and thrombocytopenia during cardiopulmonary bypass. Anaesth Intensive Care. 2018;46(6):620–626.
- Olde Engberink RH, Kuiper GJ, Wetzels RJ, et al. Rapid and correct prediction of thrombocytopenia and hypofibrinogenemia with rotational thromboelastometry in cardiac surgery. J Cardiothorac Vasc Anesth. 2014;28(2):210–216.
- David JS, Durand M, Levrat A, et al. Correlation between laboratory coagulation testing and thromboelastometry is modified during management of trauma patients. J Trauma Acute Care Surg. 2016;81(2):319–327.
- Bouzat P, Guerin R, Boussat B, et al. Diagnostic performance of thromboelastometry in trauma-induced coagulopathy: a comparison between two level I trauma centres using two different devices. Eur J Trauma Emerg Surg. 2019.
- Mace H, Lightfoot N, McCluskey S, et al. Validity of thromboelastometry for rapid assessment of fibrinogen levels in heparinized samples during cardiac surgery: a retrospective, single-center, observational study. J Cardiothorac Vasc Anesth. 2016;30(1):90–95.
