Abstract
The concentrations of several diagnostic markers have been found to increase dramatically in critically ill patients with a severe disturbance of normal physiological homeostasis, without indication of the diseases they are normally associated with. To prevent false diagnoses and inappropriate treatments of critically ill patients, it is important that the markers aiding the selection of second-line treatments are evaluated in such patients and not only in the healthy population and patients with diseases the markers are associated with. The levels of trypsinogen isoenzymes, the trypsin inhibitor serine peptidase inhibitor Kazal type 1 (SPINK1), hCG and hCGβ, which are used as pancreatitis and cancer markers, were analyzed by immunoassays from serum samples of 17 adult patients who have undergone surgery of the ascending aorta during hypothermic circulatory arrest (HCA) with optional selective cerebral perfusion. Highly elevated levels of trypsinogen-1, -2 and -3, SPINK1 and hCGβ were observed in patients after HCA. This was accompanied by increased concentrations of S100β and NSE. In conclusion, this study highlights the importance of critically evaluating the markers used for aiding selection of second line of treatments in critically ill patients.
Introduction
Critically ill patients often suffer from generalized hypoperfusion and hypoxemia, which may lead to complications, a systemic inflammation reaction, or even multiple-organ failure. Several markers, such as S100β and NSE for brain damage, are used to guide treatment [Citation1]. However, the levels of some diagnostic markers, like pancreatitis markers lipase and amylase, have been found to increase dramatically in critically ill patients, without indication of the diseases they are normally associated with [Citation2,Citation3]. The pancreas also produces several other proteins that are useful as diagnostic markers, but have not been evaluated during critical illness. These include different trypsinogen-isoenzymes, i.e. trypsinogens-1, -2 and -3, and serine peptidase inhibitor Kazal type 1 [SPINK1, also called tumor-associated trypsin inhibitor (TATI) and pancreatic secretory trypsin inhibitor (PSTI)] [Citation4]. In acute pancreatitis the circulating levels of these proteins are increased, making them useful as diagnostic markers. Especially trypsinogen-2 and the complex of its activated form, trypsin-2, with α1-antitrypsin (i.e. trypsin-2–API) have been found to accurately predict severe acute pancreatitis already at admission [Citation5]. In addition to pancreatitis, the expression of trypsin(ogen)s and SPINK1 are increased in several cancers, including pancreatic and ovarian cancers, and hepatocellular and cholangiocarcinomas, where they may also have a cancer-promoting role [Citation4,Citation6]. Pancreatic cancer is also associated with increased circulating levels of human chorionic gonadotropin (hCG) β-subunit (hCGβ), which is produced in many non-trophoblastic tumors and elevated levels of which are associated with adverse prognosis [Citation7]. Here we report highly elevated levels of trypsinogen-1, -2 and -3, SPINK1 and hCGβ in patients who have undergone surgery of the ascending aorta during hypothermic circulatory arrest (HCA).
Materials and methods
In this prospective, single-center cohort study, we recruited 17 adult patients (38–75 years of age, mean ± SD, 59 ± 10 years; 11 males and 6 females) who underwent elective (n = 10, 59%) or emergency (n = 7, 41%) surgery during cardiopulmonary bypass for aortic dissection (n = 10, 59%) or aneurysm (n = 7, 41%), and from whom serum and plasma samples were collected before the operation and 1, 2, 3, and 5–7 d after the operation. For some of the patients, samples were also available 8–10 d after the operation. All patients were operated under HCA, with optional selective cerebral perfusion. The patients were fully anticoagulated with heparin during the operation and the effect of heparin was reversed with protamine in the end of the operation. Patients with a preoperative neurological disease or symptoms were excluded. Twelve (71%) patients were classified as having a good postoperative primary neurological outcome as they survived the monitoring period with no significant neurological complications, while five had a poor outcome, defined by the appearance of new neurological symptoms or lesions on a brain CT scan, or operative death. Mild neurological complications were not classified as a poor outcome. The monitoring period lasted from the preoperative stage (moments before anesthesia and intubation) until extubation in the intensive care unit, or up to 48 h after surgery. All participants, or next of kin, gave their written informed consent. The study was approved by the Helsinki University Hospital Ethics Committee (HUS 133/E6/07).
The concentrations of trypsin(ogen)-1, -2 and -3 [Citation8,Citation9], trypsin-2–API [Citation10], SPINK1 [Citation11] and hCG and hCGβ [Citation12] were determined as described using in-house immunofluorometric assays (IFMAs). The trypsin(ogen)-assays detect both pro-forms and active forms of trypsins. hCG was determined by a commercial IFMA (AutoDelfia, Perkin-Elmer Wallac, Turku, Finland). The limits of detection for trypsinogen, trypsin-2–API and SPINK1 assays are <0.5 µg/l and intra- and inter-assay coefficients of variation (CV) <15%. For hCG and hCGβ assays the limits of detection are <2 pmol/l and the intra-assay CV <10% at concentrations above 10 pmol/l. SPINK1, trypsinogen-1 and -2, trypsin-2–API and hCGβ were analyzed within a week after the collection of the follow-up samples of individual patients. Trypsinogen-3 was analyzed in multiple batches, the samples being stored at −20 °C up to 9 months. hCG was analyzed in a single batch after a maximum of 1 year 9 months storage at −20 °C. In our experience, such storage times should not have a significant effect on the marker levels (unpublished and [Citation8–12]).
Serum S100β, serum neuron-specific enolase (NSE), plasma C-reactive protein (CRP) and plasma amylase were measured in accredited hospital laboratory (HUSLAB, Helsinki, Finland) immediately after the sampling, as part of clinical routine. S100 and NSE were quantitated with electrochemiluminometric assays on an automatic immunoanalyzer (Elecsys 2010, Roche Diagnostics, Mannheim, Deutschland). Detection limit of the S100 assay is 0.005 µg/l, and intra-assay and inter-assay CVs <3% and <4%, respectively, in the concentration range 0.09−2.6 µg/l. Detection limit of the NSE assay is 0.05 µg/l, and intra-assay and inter-assay CVs are <3% and <4%, respectively, in the concentration range 2.6−103 µg/l. CRP was quantitated with an immunoturbidimetric method and amylase with an enzyme kinetic method on Roche Modular analyzer (Roche Diagnostics, Basel, Switzerland). The detection limits are 0.12 mg/l, <3 U/l, respectively.
Wilcoxon signed-rank test was used to evaluate the differences between baseline and maximal levels of the markers and Mann–Whitney U test to evaluate differences in marker levels between different groups (IBM SPSS Statistics version 24; IBM SPSS, Armonk, NY). Asymptotic (two-tailed) p values are reported. The values are reported as median, and, in parenthesis, interquartile range (IQR).
Results
Significantly increased concentrations of trypsinogen-1, -2 and -3, trypsin-2–API, SPINK1 and hCGβ were observed in patients after HCA (, ). In all patients, the SPINK1 concentrations increased rapidly after the first day, reaching concentrations exceeding the upper reference limit 17-fold on average and in two patients (12%) over 50-fold. The concentrations remained increased until the end of follow-up at 8−10 d. Trypsinogen-1 and -3 concentrations peaked 1 d after HCA, after which they normalized within one day and started again to rise in 13 (76%) and 10 (59%) cases, respectively, exceeding the levels observed on day one. The concentrations of trypsinogen-2 and trypsin-2–API complex showed a similar pattern, although the peak on day one was less pronounced, if found at all. The hCGβ levels also increased, but mostly only after day three, when the hCG levels tend to decrease. These changes were accompanied by increased concentrations of S100β and NSE one day after HCA. Often the S100β and NSE levels normalized within a week after HCA. In all but one patient amylase levels also increased. However, in 11 (65%) patients this happened only 2 d after the operation or later. Serum CRP levels before and 3 d after the operation were available from ten patients (59%) and indicate a dramatic increase from 7.5 (3.0–60) to 226 (140–303) mg/l (p = .005).
Figure 1. Time course of the changes in concentrations of the markers after hypothermic circulatory arrest (median and interquartile range, n = 16–17 for days 0–7 and n = 8–9 for day 8–10, except for hCG n = 11 and 6, respectively). Day 0 is the baseline sample, collected right before anesthesia induction. Dashed lines show the upper reference limit of the markers. *The upper reference limit for hCG varies between 1 and 7 IU/l depending on age and gender (see ). One outlier value of trypsin-2–API has been removed.
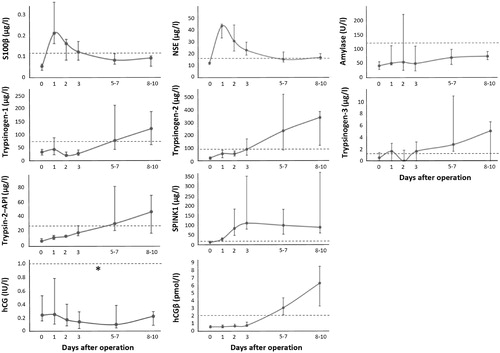
Table 1. The baseline and postoperative concentrations of the studied markers.
The baseline levels of NSE were higher in patients who underwent emergency surgery (n = 7) than in elective cases (n = 10) [15.3 (11.7–22.5) and 11.2 (9.6–12.2) µg/l, respectively, p = .019]. Similarly, the maximum SPINK1 levels during the follow-up were higher in patients who underwent emergency operation [375 (109–778) and 95.4 (51.7–145) µg/l, p = .010]. Otherwise, there were no differences between the studied markers in these two groups of patients. The levels of studied markers did not differ between the patients who had good or poor postoperative primary neurological outcome.
Discussion
The reason for the increased levels of the studied markers after aortic surgery and HCA, and whether they reflect any specific clinical condition(s) is unknown. There are, however, several possible explanations. All patients in this study underwent cardiopulmonary bypass and HCA, which lead to transient global ischemia and may lead to multi-organ failure with signs of neuronal, liver and kidney damage. Several patients also presented with blood–brain barrier dysfunction, as suggested by increased circulating levels of NSE and S100β, and with systemic inflammation or tissue damage as suggested by an increase in CRP concentrations following the operation. Since the levels of SPINK1 in circulation have been found to be strongly increased in severe inflammation and tissue damage [Citation13], it is likely that inflammation and tissue destruction explain the observed increase in SPINK1 concentrations to which extrapancreatic expression, potentially from the liver, may contribute to.
The trypsinogen-1, -2 and -3 and SPINK1 peak concentrations were similar to those observed in acute pancreatitis patients [Citation14]. However, the patients in this study were not reported to have pancreatitis, although this cannot be completely ruled out. This agrees with the very modest and transient increase in amylase. Of note, amylase levels have been found to be significantly elevated after coronary artery bypass surgery [Citation15] and in variety of other conditions. Since the expression of trypsin-isoenzymes has been found not only in the pancreas, but also in many other tissues, including brain and biliary tract [Citation6], disturbed function in these may also affect the levels of these markers.
Especially the early fluctuation in levels of studied markers may also be related to transient renal insufficiency and dialysis after the operation. Moreover, the patients suffered significant loss of blood and received autologous and homologous blood transfusions, which may cause some fluctuations in the marker levels. However, the increase in hCGβ is difficult to explain. We have found that hCGβ levels may be elevated in pancreatic and biliary cancer and several other cancers [Citation6], but only rarely in acute pancreatitis. The finding of elevated hCGβ concentrations in men without cancer is new. The source of this hormone subunit remains to be established.
In this small descriptive study, we did not find any differences in the markers regarding the primary neurological outcome of the patients. However, the baseline levels of NSE and maximal levels of SPINK1 during the follow-up were higher in patients who underwent emergency surgery as compared to elective cases. It is possible, although speculative, that the emergency cases had more pronounced brain hypoxia, which resulted in higher NSE levels. However, higher SPINK1 levels are hard to explain, especially as there was no difference in CRP levels.
In conclusion, our results highlight the importance of critically evaluating the markers used for aiding selection of second line of treatments in critically ill patients.
Acknowledgments
The authors would like to thank Ms. Annikki Löfhjelm, Ms. Maarit Leinimaa and Ms. Taina Grönholm for excellent technical assistance.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Kochanek PM, Berger RP, Bayir H, et al. Biomarkers of primary and evolving damage in traumatic and ischemic brain injury: diagnosis, prognosis, probing mechanisms, and therapeutic decision making. Curr Opin Crit Care. 2008;14(2):135–141.
- Cohen J, MacArthur KL, Atsawarungruangkit A, et al. Defining the diagnostic value of hyperlipasemia for acute pancreatitis in the critically ill. Pancreatol. 2017;17(2):176–181.
- Weaver DW, Busuito MJ, Bouwman DL, et al. Interpretation of serum amylase levels in the critically ill patient. Crit Care Med. 1985;13(7):532–533.
- Räsänen K, Itkonen O, Koistinen H, et al. Emerging roles of SPINK1 in cancer. Clin Chem. 2016;62(3):449–457.
- Hedström J, Kemppainen E, Andersén J, et al. A comparison of serum trypsinogen-2 and trypsin-2-alpha1-antitrypsin complex with lipase and amylase in the diagnosis and assessment of severity in the early phase of acute pancreatitis. Am J Gastroenterology. 2001;96(2):424–430.
- Nyberg P, Ylipalosaari M, Sorsa T, et al. Trypsins and their role in carcinoma growth. Exp Cell Res. 2006;312(8):1219–1228.
- Stenman UH, Alfthan A. Determination of human chorionic gonadotropin. Best Pract Res Clin Endocrinol Metab. 2013;27(6):783–793.
- Itkonen O, Kylänpää L, Zhang WM, et al. Reference intervals for and validation of recalibrated immunoassays for trypsinogen-1 and trypsinogen-2. Clin Chem. 2012;58(10):1494–1496.
- Oiva J, Itkonen O, Koistinen R, et al. Specific immunoassay reveals increased serum trypsinogen 3 in acute pancreatitis. Clin Chem. 2011;57(11):1506–1513.
- Hedström J, Leinonen J, Sainio A, et al. Time-resolved immunofluorometric assay of trypsin-2 complexed with alpha 1-antitrypsin in serum. Clin Chem. 1994;40(9):1761–1765.
- Janeiro E, Guimarães J, Stenman UH, et al. Validation and comparison of tumor-associated trypsin inhibitor (TATI) immunoassays. Clin Chim Acta Int J Clin Chem. 2012;413(15–16):1244–1248.
- Lempiäinen A, Stenman UH, Blomqvist C, et al. Free beta-subunit of human chorionic gonadotropin in serum is a diagnostically sensitive marker of seminomatous testicular cancer. Clin Chem. 2008;54(11):1840–1843.
- Lasson A, Borgström A, Ohlsson K. Elevated pancreatic secretory trypsin inhibitor levels during severe inflammatory disease, renal insufficiency, and after various surgical procedures. Scand J Gastroenterol. 1986;21(10):1275–1280.
- Rainio M, Lindström O, Penttilä A, et al. Serum serine peptidase inhibitor Kazal-type 1, trypsinogens 1 to 3, and complex of trypsin 2 and α1-antitrypsin in the diagnosis of severe acute pancreatitis. Pancreas. 2019;48:374–380.
- Algin HI, Parlar AI, Yildiz I, et al. Which mechanism is effective on the hyperamylasaemia after coronary artery bypass surgery? Heart Lung Circ. 2017;26(5):504–508.
