Abstract
In this study, we evaluated the performance of the flow cytometer-based Sysmex UF-5000 automated urine analyzer as a screening tool for ruling out urinary tract infections in elderly patients presenting at the emergency department. A total of 1119 unselected patient samples (including 544 samples from elderly patients) submitted for urine culture were included in this study. Samples were measured on UF-5000 and dipsticks and the results were compared with interpretation of culture results, which is the gold standard. We obtained a diagnostic sensitivity of 99% and specificity of 51% with a low rate of false negatives (0.2%) and a negative predictive value of 99% at 108 colony forming bacteria/L (CFB/L). A bacterial count ≥ 50x106/L or yeast like cells ≥ 25x106/L was used as the cutoff value. At this cutoff value, 30% of the urine cultures would have been redundant. This resulted in 35% false positive samples, mainly due to particle contamination or nongrowing bacteria. In comparison, at best, the dipsticks have a diagnostic sensitivity of 89%, a specificity of 52% and a negative predictive value of 92% at 108 CFB/L.
Introduction
Urinary tract infections (UTIs) are one of the most common clinically encountered infections in emergency department patients and the etiology is typically translocation from the gastrointestinal tract [Citation1]. The presence of bacteriuria is indicative of a UTIs, but its significance can range from asymptomatic carriage to severe infections. The risk of UTIs is increased following bladder catheterization or instrumentation and is more frequent in women [Citation2]. A diagnosis based on symptoms alone will overestimate the presence of UTIs by up to 33%, depending on chosen criteria, and may lead to unnecessary prescription of antibiotics [Citation3]. The definition of a symptomatic UTIs generally requires the presence of urinary tract-specific symptoms in the setting of significant bacteriuria, with a quantitative count of ≥108 colony forming bacteria per liter (CFB/L) in one urine specimen (corresponding to the conventional 105 colony-forming units per milliliter, CFU/mL) [Citation2,Citation4,Citation5]. A lower threshold (106–7 CFB/L) of bacterial growth caused by primary pathogens for diagnosing UTIs based on the patient’s age, sex, symptoms and urine collection method may be necessary in certain clinical situations [Citation5,Citation6]. Urine dipsticks are commonly used as initial screening to rule out any UTIs, despite a sensitivity between 45%, 67% and 80% at the cutoff ≥106 CFB/L, ≥107 CFB/L, ≥108 CFB/L, respectively [Citation7]. Urine culture is considered as gold standard for diagnosis [Citation3,Citation5]. However, this method is labor intensive, prone to false-negative or false-positive results, time-consuming and expensive.
A new generation of automatic flow cytometers for urine analysis has evolved as a rapid screening method. The intent is to reduce the number of samples submitted for culturing that exhibit nonsignificant or no growth [Citation8–10]. This method has shown increasing diagnostic accuracy in screening for bacteriuria, depending on the model used, instrument setting and patient groups [Citation9–13]. Recently, the latest third generation urine flow cytometer UF-5000 (Sysmex, Kobe, Japan) was introduced, featuring depolarized side scattered light for better discrimination of red blood cells and crystals and the ability to differentiate Gram negative bacteria. Evaluation of UF-5000 has thus far shown better accuracy than previous iterations in terms of bacterial quantification and stainability, as well as a high diagnostic accuracy in bacteriuria screening with a low rate of false negatives [Citation11,Citation14]. All methods are hampered by high frequency of contamination by bacteria that originate from the skin or genital area, and not from the urinary tract, occurring from specimen collection to analysis.
The aim of this study was to investigate whether UF-5000 could be used in primarily elderly patients at the emergency department as a fast clinical screening procedure to rule out clinically relevant UTIs allowing a reduction in unnecessary urine cultures. A secondary aim was to compare UF-5000 results with urine dipstick results.
Materials and methods
Sample selection and study protocol
This prospective cohort study was conducted on 30 separate days from November 2018 to March 2019. The study was performed at Holbaek Hospital, one out of six hospitals in the region; it is a 330 bed emergency hospital with 100,000 admissions per year with abdominal and orthopedic surgery, pediatric, gynecology and obstetrics and medical departments (cardiology, endocrinology, gastroenterology, geriatrics, rheumatology, and nephrology). At Holbaek Hospital approximately 16,000 dipstick tests are performed annually (of which 8,500 are performed in the emergency department). The microbiology service is centralized in Region Sjaelland located at Slagelse Hospital, an approximately 1.5 h drive away, performing approximately 110,000 urine cultures per year (for the whole region, including the primary sector). At Holbaek Hospital, all unscheduled adult patients arrive at a separate emergency department for initial evaluation with subsequent referral to other specialized departments or direct discharge.
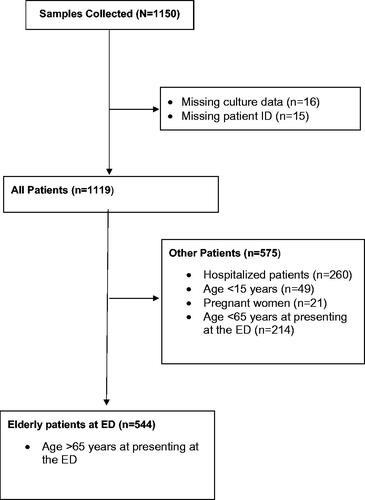
Investigation of urine was initiated by doctors, nurses, and secretaries on a broad indication. All specimens were collected into two plain sterile 10 ml polypropylene tubes (Sarstedt, Nümbrecht, Germany) for i) urine culture and ii) analysis on a flow cytometer and on dipsticks locally. Sample tubes were delivered to the laboratory and stored at 4–8 °C if analysis was delayed by >1 h. The way of collection of urine was noted (mid-stream, urinary catheters). Our primary focus was urine samples from elderly patients (>65 years) presenting at the emergency department, However, we received many samples from other patient groups. Accordingly, we divided the microbiological test results into two groups: i) the primary target: (Elderly patients at ED group)) and ii): All Patients group, ().
Figure 1. CONSORT flow diagram. Unselected urine samples from all patients with a microbiological test results were included in the All Patient group, while only elderly patients (≥65 years) presenting at the emergency department were included in the Elderly patients at ED group. Indications for requesting urine culture and symptoms were unknown. Some data were unavailable. Patient identification (ID), emergency department (ED)
Routine diagnostic procedures are not considered under the regulation for research projects; therefore, no approval from the local ethics committee or written informed consent was obtained.
Routine microbiological examination
One microliter of urine was inoculated onto a 5% blood agar and UTI chromagar plates (SSI Diagnostica, Hillerød, Denmark) and examined after incubation for 24 h at 35 °C in an ambient air atmosphere. The growth of pathogens was recorded in CFB/L. All urine samples with a bacterial count in excess of 106 CFB/L were further identified using matrix-assisted laser desorption/ionization – time of flight (MALDI-TOF; Bruker Daltonics, Billerica, MA, USA) and conventional biochemical tests. If more than two organisms were isolated, the sample was reported as ‘mixed flora’ without any further identification or quantification, except in cases concerning urine samples from nephrostomies or suprapubic sampling in which all species were identified. Positive urine cultures were classified according to growth with one or two microorganisms: i) ≥106 CFB/L, ii) ≥107 CFB/L, and iii) ≥108 CFB/L. Apparently contaminated samples (fecal contamination, skin-and mucosal flora) and mixed flora as concluded by an expert microbiologist, were classified as negative urine samples. Staphylococcus aureus, β-hemolytic streptococci, Enterobacterales, and Pseudomonas aeruginosa were considered likely pathogenic bacteria. Common skin commensal bacteria (e.g. coagulase-negative staphylococci (except for Staphylococcus lugdunensis and Staphylococcus saprophyticus), and Micrococcus species, (Corynebacterium species, Propionibacterium species) were regarded as contaminants.
Urine dipstick (clinitek)
Each urine sample was tested within one hour with a dipstick (Multistix 8 SG; Siemens, Erlangen, Germany) using an automated reader (Clinitek Status, Siemens) with manufacturer defined cutoff values for nitrate and leucocyte esterase. Quality was assured with a monthly internal quality control at two levels with Chek-stix (Siemens) and with the external quality control system 3055DK four times per year provided by the Danish Institute for External Quality Assurance for Laboratories in Health Care (DEKS).
Urine particle flow cytometer (UF-5000)
Urine particles were analyzed using the STAT mode function on the Sysmex UF-5000 according the manufacturers’ instructions. The following particle counts were collected: bacteria (BACT x106/L), leucocyte count (WBC x106/L), yeast like cells (YLC x106/L), epithelial cells (EC x106/L) and erythrocyte cells (RBC x106/L). Manufacturer control materials at two levels were used to determine the interassay coefficient of variation (CV%) for BACT, WBC and RBC counts (). A stability evaluation up to 24 h at 4 °C showed that WBC, BACT and RBC varied less than 20% from the original range.
Table 1. Inter assay coefficients of variation for bacterial, leucocyte, and erythrocyte counts on a Sysmex UF-5000.
Statistics, diagnostic performance and comparability
The results from the Sysmex UF-5000 cell counts were compared to the results from dipsticks and urine culture and to determine sensitivity, specificity, positive (PPV) and negative (NPV) predictive values. Receiver operating characteristic (ROC) curves and calculation of the area under the curve (AUC) were used to compare different UF-5000 cutoff values. A two-tailed value of p < .05 was considered statistically significant. Data analyses were performed using SPSS software Version 22.0 (IBM, Armonk, NY, USA).
Results
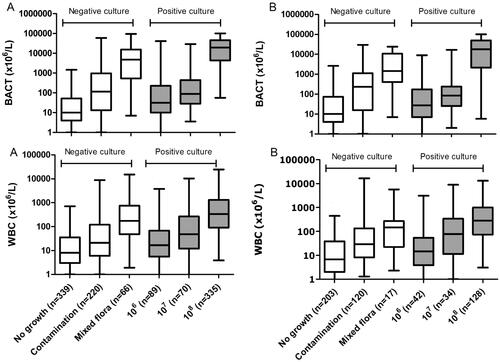
The urine samples originated from 449 (40%) males and 621 (56%) females, and and a total of 49 (4%) children were below the age of 15 years. The mean age and standard deviation (SD) of the patients were 68 (22) years. All 1119 urine specimens (All Patient group) were evaluated by UF-5000, urine dipstick and urine culture. Some 790 of the obtained urine samples were mid-stream urine and 329 were obtained from catheters. Nephrostomy and suprapubic aspiration samples were not included. The average time from specimen collection to analysis on the UF-5000 was 5.2 h. The culture results are presented in Supplementary Table 1. The distribution of the bacterial (BACT) and leucocyte (WBC) counts by the UF-5000 to the colony count defined is presented in .
Figure 2. Distribution of bacterial counts and leucocyte counts measured by UF-5000 according to the observed CFB/L by urine culture. A) All Patient group; B) Elderly patients at ED group (>65 years) presenting at the emergency department. Positive urine cultures were grown with ≤2 microorganisms: i) ≥106 CFB/L, ii) ≥107 CFB/L, and iii) ≥108 CFB/L. Negative urine cultures were samples with apparent contamination and sterile cultures or mixed flora (without quantification). Mixed flora was defined as growth of >2 microorganisms (without any further identification or quantification). Boxplots indicate the median and first and third quartiles, whiskers indicate the 2.5 and 97.5 percentiles. Bacterial count (BACT), the leucocyte count (WBC) from UF-5000.
The diagnostic performance of UF-5000 at different cutoff values for the BACT, WBC and YLC counts was compared to urine culture results at different bacterial growth levels, (Supplementary Table 2). At the UF-5000 cutoff point of >50 BACT ×106/L, six false negative cultures were observed at the ≥108 CFB/L level. Three of these samples contained Candida species while the remaining three contained gram-negative species. The median YLC count in samples containing Candida species was 47 × 106/L compared to 1.4 × 106/L in all other samples. Adding a YLC count cutoff point at 25 YLC ×106/L (experimentally determined) allowed most samples containing Candida species to be classified as positive by the UF-5000.
ROC curves were constructed to compare the different cutoff points of BACT, WBC, and YLC ×106/L for verifying an optimal NPV at different colony count levels (Table 3). The ROC and AUC with 95% confidence intervals (CIs) for the WBC, BACT, YLC, the combination of WBC and BACT (WBC*BACT), and the combination of BACT and YLC (BACT*YLC) are shown at the 106, 107 and 108 CFB/L levels in . The AUC was highest for BACT alone, at all three CFB/L levels. We found that the WBC count did not improve the diagnostic accuracy of the screening, not even in combination with BACT count. For obtaining optimal NPV (88–99%) we defined UF-5000 cutoff points of ≥10 BACT ×106/L OR ≥25 YLC ×106/L at the 106 CFB/L level, at ≥20 BACT ×106/L OR ≥ 25 YLC ×106/L, at the 107 CFB/L, and ≥50 BACT ×106/L OR ≥ 25 YLC ×106/L at the 108 CFB/L level ().
Figure 3. Receiver operating characteristic (ROC) curves, comparing UF-5000 results with bacterial culture classification. ROC curves were used to compare the cutoff points of bacterial count (BACT), leucocyte count (WBC), and yeast like cells (YLC) and the combined BACT*WBC and BACT*YLC count. The area under the curve (AUC) with 95% confidence intervals (CI) is shown for each parameter at all three culture levels. Positive urine cultures were grown with ≤2 microorganisms: i) ≥106 CFB/L, ii) ≥107 CFB/L, and iii) ≥108 CFB/L. Negative urine cultures were samples with apparent contamination and sterile cultures or mixed flora (without quantification). Mixed flora was defined as growth of >2 microorganisms (without any further identification or quantification). All samples were included (All Patient group, n = 1119).
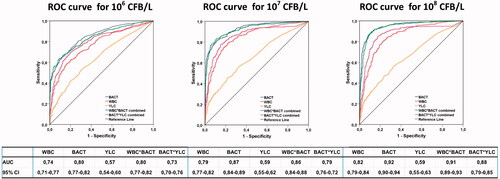
Table 2. Diagnostic performance of UF-5000 and dipsticks in comparison to urine culture.
The diagnostic performance of the combined BACT*YLC cutoff point at bacterial count levels of 106, 107 and 108 CFB/L are listed in . Both in the All Patient group and the Elderly patients at ED group at all colony count levels, UF-5000 had a better sensitivity and NPV than the dipstick; however, UF-5000 had a lower PPV with 387 (35%) false positive samples at 108 CFB/L (). Most of the false positive samples were samples containing skin-and mucosal flora and fecal contamination (n = 138). At the suggested cutoff, there were relatively few false negative samples, mainly Candida spp. and Gram-negative species at low colony counts. Adding these UF-5000 cutoff values for screening before culture would lead to a 19%, 27%, 36% reduction in samples for urine culture at levels 106, 107, and 108 CFB/L, respectively.
Discussion
Many of the patients presenting themselves at the emergency department are elderly with a broad variety of conditions where the indication for urine culture is loose, supported by the high prevalence of UTI and the occurrence of asymptomatic bacteriuria in 15–50% of the institutionalized elderly populations [Citation2,Citation4]. This leads to many unnecessary cultures and some unnecessary antibiotic treatments. A rapid test with high a NPV could improve the clinical performance. The gold standard for diagnosing urinary tract infections is the expert microbiologist interpretation of a culture of the pathogen. A clinical drawback of urine cultures is the slow turn-around-time of 18–24 h. Dipsticks are frequently used as a fast screening test, although with a modest sensitivity [Citation7]. We find nearly similar NPVs (75–92%) for any combination of dipstick results at 108 CFB/L.
In this study, we compared the results from the UF-5000 with the interpretation of clinically relevant culture results as the gold standard with an emphasis on establishing cutoff values for optimal NPV. Similar to other reports, we found that UF-5000 may potentially reduce 19–32% of urine cultures, depending on the case mix and colony count level used [Citation15]. At the suggested UF-5000 cutoff values, the proportion of false negatives at 106–108 CFB/L was 6.0–0.2%, compared to the 2% maximum allowable false negative rate conventionally used for ≥108 CFB/L [Citation2]. These results are comparable to other studies [Citation11,Citation16]. The combined use of the bacterial and yeast-like cell count on the UF-5000 in defining the cutoff allows the identification of most samples containing yeast. This could be useful as Candida spp. may contribute as fungal uropathogens in UTIs in selected cases [Citation17].
The lower specificity found in the present study may be due to differences in the inclusion criteria. In contrast to others, we mimicked real life and did not exclude mucus; high turbidity and hematuria samples and contaminated samples (fecal contamination, skin and mucosal flora and mixed flora) were included in all calculations. Therefore, 17% of the samples contained skin and mucosal flora, indicating the challenge of obtaining a clean midstream patient-collected sample [Citation18]. Contaminated cultures are frequent in the older population because of decreased self-care, poor hygiene, fecal contamination and cognitive disorders. This may explain the high rate of contamination present in our urine cultures. In this study, we classified contaminated samples as negative, based on the clinical microbiology interpretation and included them in the calculations of the NPV. As particles and bacteria from contaminated samples are inevitably counted as bacteria in the UF-5000 bacterial blue channel, a high rate of false positive samples is to be expected. The inborn low specificity when focusing on high negative predictive values has no consequence in a screening protocol where all UF-5000-positive samples are subsequently submitted for conventional culture.
Some of the differences in diagnostic performance among the studies may also be due to the criteria for requesting a urine culture and for the diagnosis of UTI. We investigated both outpatients and subsequently hospitalized patients, with the majority coming from elderly patients >65 years presenting at the emergency department (62%). Emergency department patients are more prone to UTIs (30). Compared to other studies, we had a different frequency of hospitalized patients (>98%) with a higher median age (74 years) [Citation11,Citation13,Citation16,Citation19]. Another factor that could contribute to false positive results on UF-5000 is carryover; however, in our case, this is not an issue, as samples were not analyzed in batch but in STAT mode [Citation20].
This study has several limitations. First, a high rate of samples (n = 220) were classified as contaminants, consisting mainly of samples with skin-and mucosal flora, making it difficult to interpret the results from the UF-5000. Second, our cutoff values may not be representative of other settings or populations, such as pediatric, hematological or pregnant patients where lower levels of bacteriuria may be of clinical relevance. Specific sex-, sample type- and/or age specific cutoffs may be necessary in specific subgroups and the option for culture regardless of the UF-5000 results in these settings to alleviate these concerns. However, observing nearly equivalent performance in both the Elderly patients at ED and All Patient groups indicated that UF-5000 cutoffs could be used in broader patient categories.
In conclusion, the major results from this study are establishing cutoff values for the UF-5000 instrument with a sufficient NPV at 108 CBU/L for ruling out clinically irrelevant urine cultures in elderly patients at the emergency department, potentially saving up to 36% of ordered urine cultures. The primary advance of UF-5000 is, however, fast results compared to urine culture. Because a result is quickly available after sampling, the results could better guide the prescription of antibiotics. The reagent cost of urine culture and UF-5000 is of the same order of magnitude, while dipsticks are much cheaper. The main benefit of using the instrument for screening for UTIs may be the increased patient flow, which is difficult to capitalize.
Supplemental Material
Download MS Word (40.1 KB)Acknowledgement
Sysmex Denmark generously donated the reagent for this study.
We thank the expert evaluation and help from the Department of Clinical Microbiology, University of Copenhagen, Slagelse Hospital.
Disclosure statement
Sysmex played no role in the design or interpretation of this study. The authors have nothing to declare.
References
- Stalenhoef JE, van Dissel JT, van Nieuwkoop C. Febrile urinary tract infection in the emergency room. Curr Opin Infect Dis. 2015;28(1):106–111.
- Bonkat G, Pickard R, Bartoletti R, et al. EAU guidelines on urological infections. European Association of Urology; Arnhem, the Netherlands: EAU Guidelines Office, 2017. p. 22–26. http://uroweb.org/guidelines/compilations-of-all-guidelines/
- Schmiemann G, Kniehl E, Gebhardt K, et al. The diagnosis of urinary tract infection: a systematic review. Dtsch Arztebl Int. 2010;107:361–367.
- Nicolle LE, Bradley S, Colgan R, et al. Infectious Diseases Society of America guidelines for the diagnosis and treatment of asymptomatic bacteriuria in adults. Clin Infect Dis. 2005;40(5):643–654.
- Aspevall O, Hallander H, Gant V, et al. European guidelines for urinalysis: a collaborative document produced by European clinical microbiologists and clinical chemists under ECLM in collaboration with ESCMID. Clin Microbiol Infect. 2001;7(4):173–178.
- European Confederation of Laboratory M. European urinalysis guidelines. Scand J Clin Lab Invest Suppl. 2000;231:1–86.
- Deville WL, Yzermans JC, van Duijn NP, et al. The urine dipstick test useful to rule out infections. A meta-analysis of the accuracy. BMC Urol. 2004;4(1):4.
- Van Dilla MA, Langlois RG, Pinkel D, et al. Bacterial characterization by flow cytometry. Science. 1983;220(4597):620–622.
- Manoni F, Valverde S, Antico F, et al. Field evaluation of a second-generation cytometer UF-100 in diagnosis of acute urinary tract infections in adult patients. Clin Microbiol Infect. 2002;8(10):662–668.
- Delanghe JR, Kouri TT, Huber AR, et al. The role of automated urine particle flow cytometry in clinical practice. Clin Chim Acta. 2000;301(1–2):1–18.
- De Rosa R, Grosso S, Lorenzi G, et al. Evaluation of the new Sysmex UF-5000 fluorescence flow cytometry analyser for ruling out bacterial urinary tract infection and for prediction of Gram negative bacteria in urine cultures. Clin Chim Acta. 2018;484:171–178.
- Previtali G, Ravasio R, Seghezzi M, et al. Performance evaluation of the new fully automated urine particle analyser UF-5000 compared to the reference method of the Fuchs-Rosenthal chamber. Clin Chim Acta. 2017;472:123–130.
- Wang J, Zhang Y, Xu D, et al. Evaluation of the Sysmex UF-1000i for the diagnosis of urinary tract infection. Am J Clin Pathol. 2010;133(4):577–582.
- Song D, Lee HJ, Jo SY, et al. Selection of unnecessary urine culture specimens using Sysmex UF-5000 urine flow cytometer. Ann Clin Microbiol. 2018;21(4):75–79.
- Manoni F, Fornasiero L, Ercolin M, et al. Cutoff values for bacteria and leukocytes for urine flow cytometer Sysmex UF-1000i in urinary tract infections. Diagn Microbiol Infect Dis. 2009;65(2):103–107.
- Kim SY, Park Y, Kim H, et al. Rapid screening of urinary tract infection and discrimination of gram-positive and gram-negative bacteria by automated flow cytometric analysis using sysmex UF-5000. J Clin Microbiol. 2018;56(8):e02004–17.
- Gajdacs M, Doczi I, Abrok M, et al. Epidemiology of candiduria and Candida urinary tract infections in inpatients and outpatients: results from a 10-year retrospective survey. Cent Eur J Urol. 2019;72:209–214.
- LaRocco MT, Franek J, Leibach EK, et al. Effectiveness of preanalytic practices on contamination and diagnostic accuracy of urine cultures: a laboratory medicine best practices systematic review and meta-analysis. Clin Microbiol Rev. 2016;29(1):105–147.
- Taylor RA, Moore CL, Cheung KH, et al. Predicting urinary tract infections in the emergency department with machine learning. PLoS One. 2018;13(3):e0194085.
- Andersen ES, Brasen CL, Christensen AF, et al. Carryover issues with UF-5000 urine flow cytometry - how did we miss it? Clin Chem Lab Med. 2020;58(4):e120–e122.
