Abstract
A very quick and easy LC-MS/MS analysis method for 5-HIAA (5-hydoxyindoleacetic acid) has been developed. The method was fully validated and proved to work well in a clinical setting. Precision at the upper reference limit 123 nmol/L was 3,3% CV. Accuracy ranged from 96% at low levels (50–100 nmol/L) to 99.7% at high levels (500 nmol/L). A previously reported reference interval of 35–123 nmol/L was confirmed as valid based on analysis of 40 samples from voluntary blood donors.
Introduction
Urinary 5-hydroxyindoleacetic acid (5-HIAA) has been analyzed and employed as marker for neuro endocrine tumors (NETs) for many years with different techniques [Citation1–3]. However, 24-hour urine collection under strict dietary restrictions is prone to errors, and plasma or serum 5-HIAA has been studied as tumor marker for NETs with good results [Citation4–6]. Until now, only few methods have been published on analysis of serum or plasma 5-HIAA [Citation4,Citation5,Citation7–9], some of which are complicated using organic solvent extraction and evaporation [Citation4–5], employ special chromatography columns like Hilic [Citation7] or mixed-mode [Citation8], use microextraction plates [Citation7], ice-cold extraction of whole blood [Citation9], and showing chromatographic runtimes of 8 min [Citation7,Citation8] or more [Citation4,Citation9]. Since analysis of serum 5-HIAA was included in the Swedish national care program for neuroendocrine tumors, the demand for this analysis has increased dramatically. Here we present an ultra-fast LC-MS/MS method with injection-to-injection time of 3.6 min, combined with a quick and easy work-up procedure.
Materials and methods
Since methanol is the preferred LC-solvent in our lab, methanol was chosen as precipitation solvent for chromatographic compatibility reasons.
Reagents and materials
Water was 18.2 MΩ ultra-pure water produced in-house using an Elga Purelab Chorus 1 system.
Methanol from Merck, LiChrosolv for Liquid Chromatography
Formic acid from VWR, AnalaR Normapur
5-Hydroxyindole-3-acetic acid from Sigma (>98%)
5-Hydroxyindole-4,6,7-D3-3-acetic acid-D2, solid material from LGC Standards.
Calibrators were prepared in water at three levels (35, 125, 1000 nmol/L). Control samples were prepared from patient pools at two levels (approximately 60 and approximately 200 nmol/L). Calibrators and controls were stored at −86 °C.
Precipitating agent was 510 nmol/L 5-HIAA D5 in methanol stored in freezer (−20 °C).
Sample preparation robot: Tecan freedom evo with fixed tips.
Low 5-HIAA level serum was prepared by incubating 10 ml serum mixed with 0.2 ml 5% sodiumhypochlorite solution at 60 °C for 90 min. The resulting 5-HIAA level was 5-10 nmol/L. Repeating the procedure with fresh sodiumhypochlorite did not reduce the 5-HIAA-level further.
LC-MS/MS
The instrumentation consisted of a Waters Acquity UPLC I-Class system with binary pump and FTN-injector fitted with a sample organizer. A Waters Xevo TQS micro triple quadrupole mass spectrometer with electrospray was used for detection. A Waters Acquity HSS T3 (1.8 µm 100*2.1 mm) column with an in-line guard filter (0.2 µm) was employed for analysis.
Injection volume was 3 µl and injections are performed with a needle off-set of 10 mm from the bottom using a 1 ml 96-well plate. Mobile phase A was water with 0.1% formic acid and mobile phase B was methanol with 0.3% formic acid. Column flow rate was 0.4 ml/min with the following linear gradient: time 0 min, 100% A; 0.2 min, 100% A; 2.0 min, 50% B; 2.01 min, 90% B; 2.5 min, 90% B; 2.51 min, 100% A. Total run time was 3.0 min. Column oven was set at 45 °C and sample compartment was set at 6 °C.
Detection was carried out in positive mode and the electrospray interface was operated with a capillary voltage of 0.7 kV, source temperature of 150 °C, desolvation gas temperature of 600 °C, desolvation gas flow of 800 L/h and a cone gas flow of 20 L/hr. A cone voltage of 26 V was employed. For quantitation, MS/MS transition 192.0 > 146.0 (collision energy 14) was used and 192.0 > 91.0 (collision energy 36) for qualification. For the internal standard, transition 197.0 > 151.0 (collision energy 14) was monitored. Data was acquired and processed using Waters Mass Lynx 4.1 software.
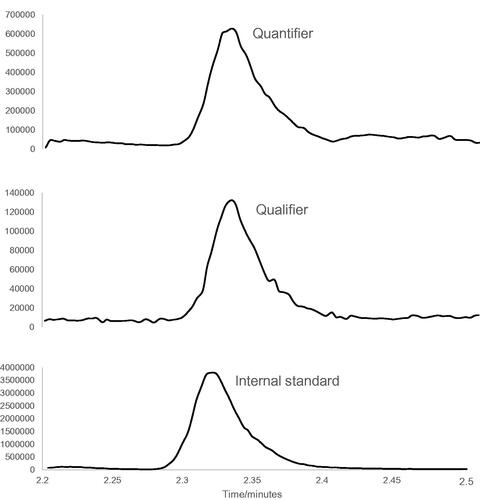
A typical chromatogram for a fairly low level patient sample is shown in .
Sample preparation
Samples, calibrators and controls are pipetted (100 µl) into a 1 ml 96-well plate. Precipitating agent (Internal standard in methanol, 400 µl) is added, and the plate is shaken 5 min at 1500 rpm. The 96-well plate is incubated in refrigerator (4 °C) for 1 h, and centrifuged (relative centrifugal force of 4 000 at 8 °C, 10 min) before analysis.
Analytical method validation
Validation was carried out in line with EMA guidelines, with small adaptations due to the fact that 5-hydroxyindoleacetic acid is an endogenous analyte.
Results
Selectivity
Patient serum samples were shown not to contain any obvious interferences to the endogenous 5-HIAA peak, as judged by peak shape. However, as low-level 5-HIAA serum prepared in-house was never totally free from 5-HIAA, selectivity was further examined by comparing ion-ratios of spiked low-level samples over a concentration range of 5-2000 nmol/L. Ion ratios for two qualifiers were constant over this range within ±20% with no obvious drift at low levels.
Carry-over
Carry over was estimated to less than 0.05% by spiking serum with 20,000 nmol/L 5-HIAA, and analyzing followed by water blank containing internal standard. The water blank was estimated to be <3 nmol/L, which makes carry-over insignificant in general for clinical samples.
Lower limit of quantification LLOQ
Low level of quantification was not established since it was judged to be less important, but as shown below, total CV at 45 nmol/L was demonstrated to be 9%. Furthermore, low-level 5-HIAA serum was spiked with analyte to a total concentration of 10 nmol/L. Eight such samples worked up in parallel gave a CV of 11%. Signal to noise was approximately 13 at this level.
Linearity and dilution integrity
Linearity was demonstrated in water as well as spiked in low 5-HIAA-level plasma in the range of 5-2000 nmol/L. Linearity was assessed using ten levels, showing back-calculated accuracy within ±10% when fitted with 1/x weighting to a linear function. When investigating dilution integrity, linearity was similarly demonstrated up to 20,000 nmol/L.
Six patient samples were spiked with 20,000 nmol/L 5-HIAA. Dilution with water (10 µl sample + 90 µl water) and analysis resulted in an average concentration of 2010 nmol/L with a CV of 7.5%.
Accuracy
Accuracy was evaluated by comparison to Huslab, Helsinki, Finland. Huslab employs a 96-well weak anion exchange plate work-up procedure, isotope labeled internal standard, calibrators prepared in water, a HILIC chromatography column for separation, and triple-quadrupole mass spectrometry for detection [Citation7].
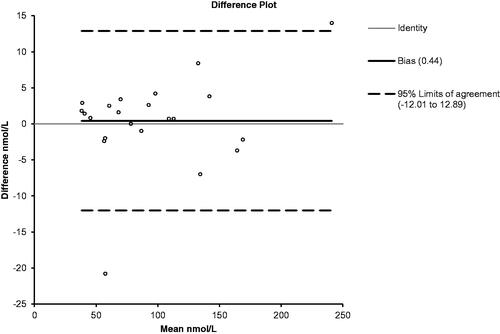
In all, 22 samples (range 35–240 nmol/L) were analyzed by both labs giving a mean difference of 0.44 nmol/L and agreement of 95–108% except one outlier. A difference plot is shown in .
Precision
Precision was determined by analyzing five samples at six different days by three different operators, at three levels. Total precision CV was 9.0% at 45 nmol/L; 3.3% at 130 nmol/L and 3.1% at 400 nmol/L. This is well in-line with long-term data from evaluation of control samples over more than one year where a total CV of 6.0% was found at 70 nmol/L and 3.4% at 230 nmol/L.
Recovery
Recovery during extraction was evaluated by spiking analyte to water and eight individual patient serum samples at 50 and 500 nmol/L. From analysis of analyte signal only, a slight loss of analyte was observed at approximately 10%. The internal standard however compensated well for this loss, as a similar analysis based on signal ratio (analyte/internal standard) showed recoveries of 96 and 100% at 50 and 500 nmol/L.
Matrix effects
Matrix effects were evaluated qualitatively by infusing internal standard solution during analysis of low-level serum as well as neat water. No significant ion suppression was observed. Matrix effects were also evaluated quantitatively by spiking (after precipitation) at 50 and 1000 nmol/L in eight patient samples with low 5-HIAA concentrations and comparing with water spiked similarly. Matrix suppression of an average of 10% was observed at low concentration, but no suppression was observed on high concentration samples. Internal standard compensated for ion-suppression such that no significant suppression was observed when signal ratio (analyte/internal standard) was evaluated. The average matrix suppression in that case was less than 2% over eight samples with a CV of 5.8 and 1.2% at 50 and 1000 nmol/L.
Stability
Stability of 5-HIAA is generally good, as previously reported by others [Citation6,Citation7]. Calibrators (water solutions) and control samples (patient serum pools) were stored at −80 °C and were shown to be stable for 12 months within ±10%. Calibrators were shown to be stable over three freeze-thaw cycles in seven days when kept at −18 °C.
Serum tubes were kept in refrigerator (<6 °C) before analysis. Samples were shown to be stable for 3 weeks.
Auto-sampler stability of worked up samples and calibrators was shown to be 48 h, with no significant drop in signal intensity.
Internal standard/precipitating solution was shown to be stable for 6 months at −20 °C.
Sample preparation
Methanol was chosen as extraction medium as it is compatible with the methanolic mobile phase which is the first choice at our lab. Acetonitrile as extraction medium was found to be incompatible with methanol mobile phase since it results in peak distortion at applicable injection volumes.
Clinical samples
Before sampling, patients fasted overnight (12 h) and were on serotonin dietary restrictions for 24 h, that is avoiding for example bananas and walnuts, which are reported to induce increased 5-HIAA levels [Citation10].
Over more than 3 years more than 3000 samples were analyzed. Only a few samples were found below 20 nmol/L and only approximately 1% of samples were >10000 nmol/L. The highest sample was measured at 35000 nmol/L.
Preclinical validation
Clinical samples were collected in clot-activator serum tubes. Tohmola [Citation7] has reported a discrepancy in 5-HIAA concentration between different sampling devices, where serum gel tubes gave significantly lower values than other sampling devices. As serum gel tubes is the most common serum tube type at the hospital it was therefore of interest to investigate this difference further. A comparison between clot-activator and gel tubes was made by taking blood from 20 volunteer blood donors in both tube types. The average difference in concentration was 0.55 nmol/L (mean 88 nmol/L, range 20–200 nmol/L, and agreement 92–110%) but was not statistically significant as evaluated using Student’s t-test (p = 0.54).
Reference interval
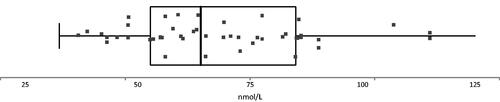
To test Tohmola’s previously reported reference interval of 35–123 nmol/L for all age groups and genders [Citation7], 40 blood donors were asked to leave a voluntary extra sample under the condition that they had not eaten serotonin rich food (banana, walnuts, etc) the last 24 h. Samples were deidentified immediately and were analyzed one day later. As it turned out, one sample out of 40 was showing elevated levels of 5-HIAA of 270 nmol/L, while all other samples were within the reported reference interval of 35-123 nmol/L, see . The two other highest samples showed approximately 115 nmol/L, and further studies will show if the upper limit could possibly be lowered somewhat.
Discussion
One small drawback with the presented method is the incubation step after precipitation. Incubation is actually not necessary for good extraction efficiency but reduces precipitation upon standing in autosampler. With no incubation, samples will appear cloudy within 2 h standing in cool autosampler. Even with 1 h incubation there will be some precipitation in samples when left overnight in autosampler, however without complications since crystals rather than cloudiness is the result. Plasma was however found to generate cloudy solutions within few hours when left standing in autosampler, making serum preferable.
In hindsight, the method could have been developed slightly differently. Less focus could, for example have been placed on achieving a low detection limit by simply reducing the injection volume. Addition of higher-level calibrators seems straightforward, and would allow for most samples being analyzed without sample dilution. After all, however the presented method works well and has been in routine use for nearly four years.
Disclosure statement
No potential conflicts of interests are reported.
References
- Bearcroft CP, Farthing MJ, Perrett D. Determination of 5-hydroxytryptamine, 5-hydroxyindoleacetic acid and tryptophan in plasma and urine by HPLC with fluorimetric detection. Biomed Chromatogr. 1995;9(1):23–27.
- Stephanson N, Helander A, Beck O. Alcohol biomarker analysis: simultaneous determination of 5-hydroxytryptophol glucuronide and 5-hydroxyindoleacetic acid by direct injection of urine using ultra performance liquid chromatography tandem mass spectrometry. J Mass Spectrom. 2007;42(7):940–949.
- Davis BA, Durden DA, Boulton AA. Simultaneous analysis of twelve biogenic amine metabolites in plasma, cerebrospinal fluid and urine by capillary column gas chromatography-high-resolution mass spectrometry with selected-ion monitoring. J Chromatog. 1986;374:227–238.
- Degg TJ, Allen KR, Barth JH. Measurement of plasma 5-hydroxyindoleacetic acid in carcinoid disease: an alternative to 24h urine collections? Ann Clin Biochem. 2000;37(5):724–726.
- Tellez MR, Mamikunian G, O’Dorisio TM. A single fasting plasma 5-HIAA value correlates with 24-hour urinary values and other biomarkers in midgut neuroendocrine tumors (NETs). Pancreas. 2013;42:405–410.
- Adaway JE, Dobson R, Walsh J, et al. Serum and plasma 5-hydroxyindoleacetic acid as an alternative to 24-h urine 5-hydroxyindoleacetic acid measurement. Ann Clin Biochem. 2016;53:554–560.
- Tohmola N, Itkonen O, Sane T, et al. Analytical and preanalytical validation of a new mass spectrometric serum 5-hydroxyindoleacetic acid assay as neuroendocrine tumor marker. Clin Chim Acta. 2014;428:38–43.
- Miller AG, Brown H, Degg T, et al. Measurement of plasma 5-hydroxyindole acetic acid by liquid chromatography tandem mass spectrometry–comparison with HPLC methodology. J Chromatog B. 2010;878(7-8):695–699.
- Danaceau JP, Anderson GM, McMahon WM, et al. A liquid chromatographic-tandem mass spectrometric method for the analysis of serotonin and related indoles in human whole blood. J Anal Tox. 2003;27(7):440–444.
- Tohmola N, Johansson A, Sane T, et al. Transient elevation of serum 5-HIAA by dietary serotonin and distribution of 5-HIAA in serum protein fractions. Ann Clin Biochem. 2015;52:428–433.