Abstract
Background
Circulating soluble urokinase plasminogen activator receptor (suPAR) is a marker of inflammation with prognostic value for elevated risk of morbidity and mortality. It has not yet been shown how the inflammatory process induced by cardiac surgery affects suPAR concentrations postoperatively
Methods
In a prospective observational study, plasma suPAR levels were measured in 30 patients undergoing cardiac surgery with cardiopulmonary bypass (CPB), pre-, peri, post-operatively, and 3–5 days after surgery. Fifteen patients underwent coronary artery bypass grafting (CABG) and 15 underwent complex procedures with longer CPB duration. Concentrations of suPAR at each time point were compared to the preoperative levels and compared between the two groups.
Results
In both groups, plasma suPAR concentrations were significantly higher on the first postoperative day (3.27 (interquartile range (IQR) 2.75–3.86) µg/L compared to baseline (2.62 (1.98–3.86)) µg/L, p < .001. There were no significant differences in suPAR concentrations between the groups at any time point. Preoperatively, the median suPAR concentration was 2.57 (2.01–3.60) µg/L in the CABG group versus 2.67 (1.89–3.97) µg/L in the complex group (p = .567). At ICU arrival 2.48 (2.34–3.23) µg/L versus 2.73 (2.28–3.44) µg/L in CABG and complex patients, respectively (p = .914). There was no difference in suPAR concentrations between the groups on postoperative day 1 (3.34 (2.89–3.89) versus 3.19 (2.57–3.62) p = .967) or 3–5 days after surgery (2.72 (1.98–3.16) versus 2.96 (2.39–4.28) p = .085.
Conclusions
After a transient rise on the first postoperative day, the suPAR levels returned to the preoperative levels by the third postoperative day. There was no significant difference in suPAR levels between the routine CABG and complex group with longer CPB time.
Introduction
In order to reduce mortality and customize the treatment offered to each patient requiring cardiothoracic surgery, several risk-stratification scores have been developed, including European System for Cardiac Operative Risk Evaluation (Euroscore) I, Euroscore II, and the Society of Thoracic Surgeons (STS) score [Citation1]. In addition to scores based on clinical data, several biomarkers have been tested for their ability to predict surgical outcomes, but the performance of these biomarkers has varied [Citation2,Citation3]. Most studies have aimed to improve preoperative risk stratification, but only limited research is available evaluating postoperative risk assessment.
Soluble urokinase plasminogen activator receptor (suPAR) is the soluble form of the cell membrane-bound protein uPAR. It is produced as the result of inflammatory processes and was initially found to be a marker of cancer progression [Citation4]. In recent years, it has been shown that elevated suPAR levels are associated with the incidence of several acute and chronic diseases including cardiovascular diseases, renal failure, type II diabetes and cancer [Citation5–8]. Furthermore, it has been shown to perform well as a prognostic marker in septic and critically ill patients [Citation6,Citation9,Citation10].
Cardiothoracic surgery and the use of cardiopulmonary bypass (CPB) cause an inflammatory response which induces an acute phase reaction leading to elevated levels of several inflammatory and infection biomarkers, including c-reactive protein (CRP) procalcitonin (PCT), interleukin (IL) -6, and heparin binding protein (HBP) [Citation11–14]. Studies on suPAR in the setting of cardiothoracic surgery are scarce, but recent reports have identified suPAR as a preoperative predictor of postoperative complications in patients with aortic stenosis and, in addition, suPAR has been shown to predict acute kidney injury after cardiac surgery [Citation15–17]. However, none of these studies have fully examined the perioperative dynamics of suPAR levels and available studies have been limited to patients undergoing routine surgery. Therefore, these reports have not accounted for any potential effects on suPAR levels caused by more complex surgical procedures.
The aim of this study was to analyze the perioperative kinetics of plasma suPAR concentrations up to 5 days after surgery and the impact of surgical complexity on suPAR levels.
Methods
Study design
This study is a post-hoc analysis of suPAR concentrations in serial plasma samples taken from a single center, prospective, observational study of patients undergoing cardiac surgery [Citation18]. The study was approved by the Ethics Committee for Clinical Research at Lund University, Sweden and informed consent was obtained from each study subject included (reference number 2019-00210).
The study design, patient enrollment, sample and data collection of this cohort have been described in detail previously [Citation18]. In this study, we compared suPAR levels between patients undergoing elective coronary artery bypass grafting surgery and patients undergoing complex cardiac surgery.
Patients over the age of 18 undergoing elective surgery were eligible for enrollment in the study unless they met any of the exclusion criteria: preoperative treatment with antibiotics, or preoperative CRP >10 mg/L.
This study included a total of 30 patients undergoing surgery at Skåne University hospital in Lund, Sweden enrolled between May 2019 and January 2020: 15 patients undergoing CABG and 15 patients undergoing complex cardiac surgery. Due to logistical limitations, the study subjects were not consecutively enrolled, but were included when resources for sample collection and handling were available. Concentrations of suPAR at the pre-defined time points were regarded as primary end-points.
Definitions
In this study, complex surgery was regarded as surgery with an expected CPB duration of >120 min. We defined in-hospital mortality as any death during hospital stay. Cardiac failure was defined as requirement of inotropes for more than 24 h postoperatively and myocardial infarction was defined as ST-elevation on EKG or a postoperative CKMB level of >50 mg/L. Stroke was defined as clinical loss of neurologic function confirmed by an ischemic lesion on computed tomography (CT).
Sample collection
Blood samples were collected at the following time points: (1) at anesthesia induction; (2) at ICU arrival (corresponding to 1 h after surgery); (3) 1 day post-surgery (approximately 12–18 h after surgery), and (4) 3–5 days post-surgery. The preoperative sample was drawn from the radial arterial line and the postoperative samples were collected using the central venous line. The blood samples were centrifuged for 10 min at 4400 × g, and plasma was separated and stored at −80 °C.
Enzyme linked immunosorbent assay of inflammatory markers
Plasma suPAR was measured using enzyme linked immunosorbent assay (ELISA) (suPARnostic®, ViroGates, Birkerød, Denmark). Samples were prepared according to the manufacturer’s directions and analyzed in singlets. All samples were found within the range of the standard curve and no further dilutions were necessary.
Plasma HBP was analyzed in duplicate using an HBP ELISA (Axis-Shield Diagnostics, Dundee, UK) and IL-6 and Myeloperoxidase (MPO) were analyzed using Quantikine ELISA kits (Bio Techne, Minneapolis, MN) according to the manufacturer’s directions.
Surgical procedures
All patients in the cardiothoracic surgery group received standard anesthetic treatment according to department guidelines [Citation18]. This included propofol, a volatile anesthetic agent (isoflurane or sevoflurane) throughout the procedure if necessary, and supplementation with fentanyl and a rocuronium as a neuromuscular blocking agent as required. All procedures included standard median sternotomy followed by CPB and intermittent cold blood cardioplegic arrest. The CPB was primed with a total 1.5 L Ringer’s Acetate. The complex group included a variety of aortic procedures with the exception of one patient, who received a left ventricular assist device (Heartmate III). The CPB system consisted of non-coated polyvinyl chloride and silicone tubing, a Stöckert S5 roller pump, a hard-shell open venous reservoir, and an FX25 Terumo oxygenator.
Statistical analysis
Categorical variables are given as numbers and percentages. Proportions were compared using the Chi-square test and if the number of cases was less than 5, Fisher’s exact test was used. Continuous variables are presented as medians and interquartile ranges (IQR) and groups were compared using the Mann–Whitney U test. The Wilcoxon signed rank test was applied for related sample comparisons. The correlations between suPAR concentration, duration of CPB, and concentration of other biomarkers were analyzed using Spearman correlation. Statistical analyses were performed using standard software (SPSS IBM Corp. Released 2017. IBM SPSS Statistics for Mac, Version 26. Armonk, NY: IBM Corp) and graphs were created using GraphPad Prism version 8.3.1 (GraphPad Software, La Jolla, CA). A p-value <.05 was considered statistically significant and all p values were adjusted for multiple comparisons using the Holm–Šidák method.
Results
Study population
Between May 2019 and February 2020, 812 different cardiac surgery procedures with CPB were performed. Forty-nine patients were screened and 30 patients included, of whom 15 patients underwent elective CABG surgery (CABG surgery group) and 15 patients who underwent complex cardiac procedures. Among the latter, all were elective aortic procedures except for one patient, who received a Heartmate III (complex surgery group).
Baseline and intraoperative data
Baseline and intraoperative data of the study population were similar between the two groups with the exception of surgery time [170 (147–205) versus 298 (252–352) min, p < .001], CPB time [65 (48–97) min versus 162 (152–205) min, p < .001] and duration of cross clamping [42 (35–57) min versus 97 (69–150) min, p = .002] being significantly shorter in the CABG group compared to the complex group ().
Table 1. Pre- and intraoperative characteristics of the study population.
Postoperative data, complications, and mortality
There were no group differences in rates of postoperative complications (). CABG patients had a peak CRP of 131 (98–207) mg/L compared to 189 (147–2321 mg/L in the complex group (p = .098). Peak WBC was 12 (9–14) × 109/L in the CABG group versus 14 (13–19) × 109/L in complex patients (p = .011). One patient in the complex group suffered an intraoperative rupture of the aorta with excessive bleeding and prolonged surgery with postoperative heart failure. Despite the use of an intra-aortic balloon pump, the patient could not be weaned of CPB and therefore veno-arterial extracorporeal membrane oxygenation (ECMO) was instituted.
Table 2. Postoperative characteristics of the study population presented by the group.
Biomarker measurements
First we analyzed the dynamics of plasma suPAR in all patients, regardless of surgery complexity, and found a statistically significant increase in plasma suPAR concentration on the first postoperative day (3.27 (2.75–3.86)) µg/L compared to preoperative levels (2.62 (1.98–3.86)) (p < .001) () µg/L. There were no significant differences between suPAR levels at the other time points compared to the preoperative levels.
Table 3. Concentration of suPAR at each specific time point presented for all patients (A), by each patient group (B and C), presentation of p values for comparisons of suPAR levels between the CABG and complex group at each timepoint (D) and the p value for differences between suPAR levels at aesthesia induction versus each timepoint for all patients (E), CABG patients (F) and the complex group (G).
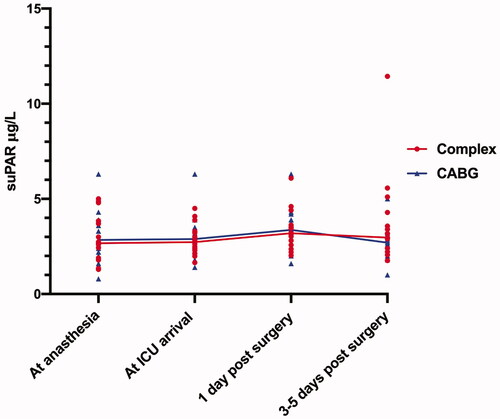
Next, we compared plasma suPAR concentrations at the predefined time points in patients undergoing CABG and patients undergoing complex procedures ( and ). There were no statistically significant differences in suPAR concentrations between the groups at any time point. Preoperatively, the suPAR concentration was 2.57 (2.01–3.60) µg/L in the CABG group versus 2.67 (1.89–3.97) µg/L in the complex group (p = .567). At ICU arrival, suPAR levels were 2.48 (2.34–3.23) µg/L versus 2.73 (2.28–3.44) µg/L in CABG and complex patients, respectively (p = .914). Also, there was no difference in suPAR concentrations between CABG and complex patients on postoperative day one (3.34 (2.89–3.89) µg/L versus 3.19 (2.57–3.62) µg/L, p = .967) or 3–5 days after surgery (2.72 (1.98–3.16) µg/L versus 2.96 (2.39–4.28) µg/L, p = .085.
Figure 1. Concentration of suPAR at each time point in the two patient groups (CABG surgery and complex procedures).
The patient that required ECMO treatment died on the ninth postoperative day and had a preoperative suPAR concentration of 4.87 µg/L and 6.09 µg/L 1 day after surgery and 11.44 µg/L on postoperative day 3, while still on ECMO. The sample at ICU arrival was not collected. Meanwhile, the patient that received a HeartMate III had preoperative concentration of 2.73 µg/L, 2.62 µg/L at ICU arrival and 3.04 µg/L and 2.96 µg/L, on postoperative days 1 and 3, respectively.
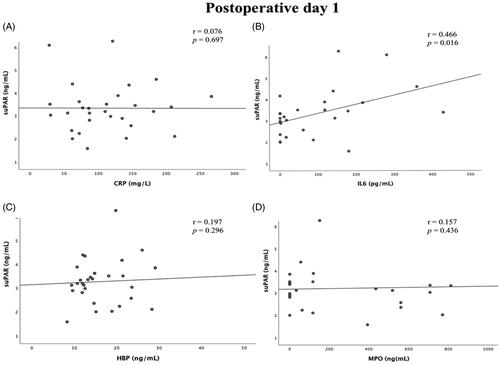
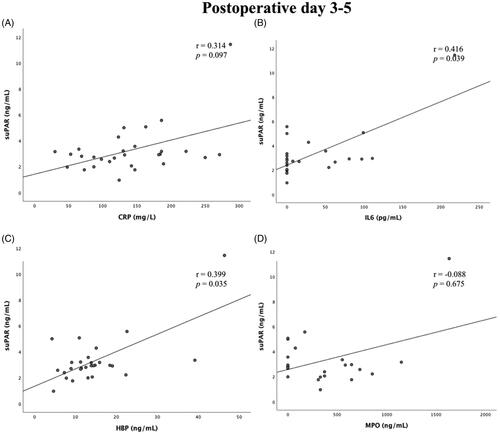
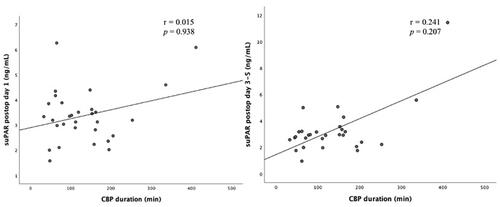
There was no correlation between suPAR concentration on day 1 or days 3–5 after surgery and duration of CPB (r = 0.015, p = .938 and r = 0.241, p = .207, respectively) (). There was a weak but statistically significant correlation between suPAR and IL-6 on postoperative days 1 and 3–5 (r = 0.466, p = .016 and r = 0.416, p = .039, respectively) and also between suPAR and HBP on postoperative days 3–5 (r = 0.399, p = .035) Apart from this, there was no statistically significant correlation between postoperative suPAR concentrations and concentrations of Myeloperoxidase (MPO) and CRP ( and ).
Figure 2. Correlation between suPAR levels on postoperative day 1 (left) and days 3–5 (right) and time on CPB.
Discussion
In this observational study, we analyzed the dynamics of suPAR during the perioperative course of cardiac surgical procedures and compared suPAR levels in patients undergoing routine CABG to those of patients undergoing more complex cardiac surgery with longer CPB time. Although we detected a rise in the suPAR levels from the preoperative timepoint to the first postoperative day, this rise was transient and limited, only statistically significant when both patient groups were pooled. Furthermore, there was no statistically significant difference in suPAR levels between the two patient groups at any timepoint, indicating that surgical complexity has no major effect on postoperative suPAR levels.
Although the prognostic value of preoperative suPAR levels in general surgical patients has been proven [Citation19,Citation20], less has been described about the dynamics of suPAR peri- and postoperatively. To our knowledge, there is only one other study on cardiac surgery patients treated with CPB, in which Gozdzic et al. [Citation17] showed that suPAR levels remained unchanged after CABG. In our study, the directly postoperative levels of suPAR did not differ significantly from baseline but rose significantly at the first postoperative day and returned to the preoperative levels at the third postoperative day. This delayed rise in suPAR levels is surprising given that suPAR is released rapidly by neutrophils and other immune cells following activation [Citation21,Citation22] and could be expected to rise as part of the inflammatory response that is typical following CPB [Citation11]. The lack of rise of suPAR in the initial postoperative measurements may be explained once we take into account hemodilution which is common in cardiac surgery and is caused by the volume of fluid given as prime volume of CPB, use of cardioplegia and as a modality for treatment of hypovolemia [Citation23]. From studies of renal function measurements, it is known that hemodilution and hydration may lead to falsely low biomarker measurements early in the postoperative period [Citation24,Citation25]. As the patients’ fluid distribution and fluid balance is normalized [Citation26], the suPAR levels may rise which could explain a higher suPAR level on the first postoperative day.
Although we found a significant increase in suPAR concentration from baseline to postoperative day 1, the elevation was modest, with median suPAR concentration increasing only from 2.62 to 3.27. Critical conditions in other settings are associated with suPAR levels much higher than those observed in our patients on postoperative day 1. For instance, it has been shown that a suPAR concentration exceeding 9 µg/L is associated with a 30-day mortality of 20% in patients with acute myocardial infarction [Citation27] and suPAR values are significantly higher in non-survivors of acute myocardial infarction compared to survivors (4.9 µg/L versus 3.9 µg/L, respectively) [Citation28]. Furthermore, in patients presenting at the emergency department, a suPAR concentration of 5.6 µg/L predicted admission to the intensive care unit and a concentration of 6.8 µg/L predicted 30-day mortality [Citation27]. Thus, the suPAR levels observed in association with critical illness in other studies are much higher than the moderate elevation caused by surgery using CPB, and so it is unlikely to interfere with the use of suPAR as a prognostic marker of severe complications following cardiothoracic surgery.
Interestingly, we demonstrated that suPAR levels are similar between routine CABG patients with relatively short CPB time and in the more complex cardiac surgery procedures with longer surgery and CPB times. We would expect that, if CPB affects suPAR levels postoperatively, there should be a more pronounced suPAR level rise in patients undergoing a more complex procedure with prolonged surgery and CPB time, however, we did not observe such differences. This further supports our finding that surgical trauma and exposure to CPB has limited effect on the levels of suPAR, so the predictive value of suPAR is not likely to be significantly affected by the surgery. This argument may be further supported by the fact that we did not find any significant correlation with postoperative CRP levels and this was in line with the findings of Gozdzic et al. [Citation17]. Furthermore, we did not see any correlation between postoperative suPAR levels and the neutrophil proteins HBP and MPO suggesting that, even though neutrophils are a major source of circulating suPAR during critical illness [Citation22], the increase in plasma suPAR is not associated with the neutrophil activation caused by CPB.
There are some limitations to our study as, first, it is a single center study with a limited study sample. As data on fluid status were not available, we could not adjust for the effect of hemodilution on suPAR levels. Furthermore, we included one patient that received an LVAD and one patient that underwent ECMO treatment in the complex group. Although these patients add to the heterogenicity of the complex group, we believe that it is of value to show that a rise in suPAR concentration was not automatically generated by the use of a circulatory assist device, but rather by the critical clinical state of the ECMO patient. These limitations welcome further future studies to bring more clarity on the effects of surgical trauma on the dynamics of plasma suPAR levels.
Summary and conclusion
Plasma concentrations of suPAR were not affected by surgical complexity but were significantly higher on the first postoperative day after cardiothoracic surgery with CPB when compared to preoperative values. However, the increase in suPAR levels was moderate and returned to the pre-procedural levels by the third postoperative day. Overall, surgery using cardiopulmonary bypass does not seem to have lasting effects on suPAR and therefore might not interfere with its use as a postoperative biomarker.
Disclosure statement
The ELISA-kits for suPAR analyses (suPARnostic®) were provided free of charge by ViroGates, Birkerød, Denmark.
Additional information
Funding
References
- Hote M. Cardiac surgery risk scoring systems: in quest for the best. Heart Asia. 2018;10(1):e011017.
- Polineni S, Parker DM, Alam SS, et al. Predictive ability of novel cardiac biomarkers ST2, galectin-3, and NT-ProBNP before cardiac surgery. J Am Heart Assoc. 2018;7:14.
- Brown JR, Jacobs JP, Alam SS, et al. Utility of biomarkers to improve prediction of readmission or mortality after cardiac surgery. Ann Thorac Surg. 2018;106(5):1294–1301.
- Brunner N, Nielsen HJ, Hamers M, et al. The urokinase plasminogen activator receptor in blood from healthy individuals and patients with cancer. APMIS. 1999;107(1):160–167.
- Hayek SS, Sever S, Ko YA, et al. Soluble urokinase receptor and chronic kidney disease. N Engl J Med. 2015;373(20):1916–1925.
- Donadello K, Scolletta S, Covajes C, et al. suPAR as a prognostic biomarker in sepsis. BMC Med. 2012;10:2.
- Borne Y, Persson M, Melander O, et al. Increased plasma level of soluble urokinase plasminogen activator receptor is associated with incidence of heart failure but not atrial fibrillation. Eur J Heart Fail. 2014;16(4):377–383.
- Sidenius N, Sier CF, Ullum H, et al. Serum level of soluble urokinase-type plasminogen activator receptor is a strong and independent predictor of survival in human immunodeficiency virus infection. Blood. 2000;96(13):4091–4095.
- Donadello K, Scolletta S, Taccone FS, et al. Soluble urokinase-type plasminogen activator receptor as a prognostic biomarker in critically ill patients. J Crit Care. 2014;29(1):144–149.
- Koch A, Voigt S, Kruschinski C, et al. Circulating soluble urokinase plasminogen activator receptor is stably elevated during the first week of treatment in the intensive care unit and predicts mortality in critically ill patients. Crit Care. 2011;15(1):R63.
- Paparella D, Yau TM, Young E. Cardiopulmonary bypass induced inflammation: pathophysiology and treatment. An update. Eur J Cardiothorac Surg. 2002;21(2):232–244.
- Arkader R, Troster EJ, Abellan DM, et al. Procalcitonin and C-reactive protein kinetics in postoperative pediatric cardiac surgical patients. J Cardiothorac Vasc Anesth. 2004;18(2):160–165.
- Pesonen E, Passov A, Salminen US, et al. Heparin binding protein in adult heart surgery. Ann Thorac Surg. 2019;107(4):1154–1159.
- Kragsbjerg P, Holmberg H, Vikerfors T. Serum concentrations of interleukin-6, tumour necrosis factor-alpha, and C-reactive protein in patients undergoing major operations. Eur J Surg. 1995;161(1):17–22.
- Mossanen JC, Pracht J, Jansen TU, et al. Elevated soluble urokinase plasminogen activator receptor and proenkephalin serum levels predict the development of acute kidney injury after cardiac surgery. Int J Mol Sci. 2017;18(8):1662.
- Hodges GW, Bang CN, Eugen-Olsen J, et al. SuPAR predicts postoperative complications and mortality in patients with asymptomatic aortic stenosis. Open Heart. 2018;5(1):e000743.
- Gozdzik W, Adamik B, Gozdzik A, et al. Unchanged plasma levels of the soluble urokinase plasminogen activator receptor in elective coronary artery bypass graft surgery patients and cardiopulmonary bypass use. PLoS One. 2014;9(6):e98923.
- Sterner N, Fisher J, Thelaus L, et al. The dynamics of heparin-binding protein in cardiothoracic surgery – a pilot study. J Cardiothorac Vasc Anesth. 2021;8: 2640–2650.
- Stephens RW, Nielsen HJ, Christensen IJ, et al. Plasma urokinase receptor levels in patients with colorectal cancer: relationship to prognosis. J Natl Cancer Inst. 1999;91(10):869–874.
- Biccard BM, Devereaux PJ, Rodseth RN. Cardiac biomarkers in the prediction of risk in the non-cardiac surgery setting. Anaesthesia. 2014;69(5):484–493.
- Pliyev BK. Activated human neutrophils rapidly release the chemotactically active D2D3 form of the urokinase-type plasminogen activator receptor (uPAR/CD87). Mol Cell Biochem. 2009;321(1–2):111–122.
- Gussen H, Hohlstein P, Bartneck M, et al. Neutrophils are a main source of circulating suPAR predicting outcome in critical illness. J Intensive Care. 2019;7:40.
- Chores JB, Holt DW. Colloid oncotic pressure, monitoring its effects in cardiac surgery. J Extra Corpor Technol. 2017;494:249–256.
- Swaminathan M, Phillips-Bute BG, Conlon PJ, et al. The association of lowest hematocrit during cardiopulmonary bypass with acute renal injury after coronary artery bypass surgery. Ann Thorac Surg. 2003;76(3):784–791. Discussion 92.
- Lassnigg A, Schmidlin D, Mouhieddine M, et al. Minimal changes of serum creatinine predict prognosis in patients after cardiothoracic surgery: a prospective cohort study. J Am Soc Nephrol. 2004;15(6):1597–1605.
- Maes T, Meuwissen A, Diltoer M, et al. Impact of maintenance, resuscitation and unintended fluid therapy on global fluid load after elective coronary artery bypass surgery. J Crit Care. 2019;49:129–135.
- Rasmussen LJ, Ladelund S, Haupt TH, et al. Soluble urokinase plasminogen activator receptor (suPAR) in acute care: a strong marker of disease presence and severity, readmission and mortality. A retrospective cohort study. Emerg Med J. 2016;33(11):769–775.
- Lyngbaek S, Marott JL, Møller DV, et al. Usefulness of soluble urokinase plasminogen activator receptor to predict repeat myocardial infarction and mortality in patients with ST-segment elevation myocardial infarction undergoing primary percutaneous intervention. Am J Cardiol. 2012;110(12):1756–1763.