Abstract
Objective
The amino-terminal peptide of type III procollagen (PIIINP) is a byproduct of type III collagen synthesis that exhibits promise as a biomarker of fibrosis, specifically in monitoring hepatic fibrosis in methotrexate treated patients. The Advia Centaur® PIIINP assay is developed for track-based automated laboratory systems and is suitable for large volume analysis. Reference intervals in children and younger adults have been published previously. Here we measured PIIINP to determine reference ranges, specifically including elderly patients, for whom such are currently lacking.
Methods
Samples were collected from subjects ranging from 20 to 98 years of age. Blood donors and clinical samples from primary care patients were used for reference interval calculation. Samples were analysed using the Advia Centaur® PIIINP assay. After exclusion of samples high in alanine transaminase (AST), aspartate transaminase (ALT), and C-reactive protein (CRP) 386 samples were used in the reference interval calculation.
Results and conclusion
We determined the following reference interval for the Advia Centaur® PIIINP assay: the lower limit of the reference interval (2.5% percentile with 95% CI) was 4.42 (4.20–4.65) µg/L and the upper limit of the reference interval (97.5% percentile 95% CI) 16.0 (15.04–17.02) µg/L.No significant differences in mean PIIINP concentrations were found between men and women. While differing mean PIIINP concentrations were seen among subjects in different age groups, the differences were small and partitioning of reference range was determined not to be necessary.
Background
PIIINP is the amino-terminal peptide of type III procollagen, released from the precursor peptide during the synthesis and deposition of type III collagen, a fibrillar collagen that is abundant in the skin and a variety of internal organs. Increased amounts of type III collagen are present in many systemic diseases linked to tissue fibrosis and excessive scarrings such as systemic sclerosis, liver and kidney fibrosis, and its serum concentration has shown promise as a biomarker of increased collagen turnover and/or accumulation as a consequence of MTX treatment [Citation1]. Elevated levels of PIIINP have also been observed in inflammatory diseases such as pulmonary fibrosis [Citation2], acromegaly [Citation3], and rheumatoid arthritis (RA) [Citation4]. Serial measurements of PIIINP reduce the need for liver biopsy in dermatology patients on long-term methotrexate for psoriasis, as patients with repeated normal levels of PIIINP are unlikely to have developed significant fibrosis. If the PIIINP concentration remains low upon serial testing, the risk of developing liver fibrosis is negligible [Citation5,Citation6]. The ADVIA Centaur® PIIINP assay (Siemens Healthcare Diagnostics Inc., Tarrytown, NY, USA) is a commercially available, automated analytical method for measuring PIIINP. From an operational point of view, it is advantageous in comparison to many other PIIINP methods that are more manually labour intense and less automated.
This study aimed to define and verify clinically utilizable reference intervals of PIIINP using the ADVIA Centaur® PIIINP assay. Studies establishing reference ranges for PIIINP using the ADVIA Centaur® PIIINP assay have previously been published [Citation7], based on blood donors up to the age of 69. In this study, we aimed to validate this reference interval and to extend it to also include persons above the age of 69.
Materials and methods
Samples
The regional ethics committee board in Uppsala approved this study (2020-03756). Sample collection took place between 2019 and 2021. In total, 453 serum samples were obtained from primary care patients, where TSH analysis was requisitioned, and blood donors. Only samples with a TSH result within local reference ranges (0.55–4.8 mIE/L) were included. The serum was collected in vacutainer tubes (Vacuette®, Greiner Bio-One GmbH, Austria) and left to coagulate for at least 30 min prior to centrifugation for 7 min (at 2400 g) within 1 h after blood collection, and stored at 4 °C for 24 h or at −20 °C until further analysis. All samples were analyzed for alanine transaminase (AST), aspartate transaminase (ALT), and C-reactive protein (CRP). Transaminase levels exceeding the reference range of the laboratory (AST: >0.75 µkat/L for men or >0.60 µkat/L for women and ALT: >1.1 µkat/L for men or >0.75 µkat/L for women), or elevated CRP (> 5.0 mg/L) were used as exclusion criteria.
Methods
The study was performed at the accredited laboratory at Örebro University Hospital, Department of Laboratory Medicine, Clinical Chemistry, Örebro, Sweden. The PIIINP concentration was determined using the ADVIA Centaur® PIIINP assay on an ADVIA Centaur XPT platform. The ADVIA Centaur PIIINP assay is a fully automated, two-site sandwich immunoassay using direct chemiluminometric technology. The assay uses two monoclonal mouse antibodies, an acridinium ester-labeled anti-PIIINP antibody and a biotin-labeled anti-PIIINP antibody in the ancillary well reagent. The Solid Phase contains streptavidin-coated paramagnetic particles. During data collection, calibration and analysis were performed according to the manufacturer’s instructions using four different reagents and calibrator lots. Controls were obtained from the manufacturer. Within-series precision was determined using pooled patient samples at a low (8 µg/L) and a high (84 µg/L) serum PIIINP concentration, with a CV% of 1.2% and 1.9% respectively. Total precision was determined using manufacturer controls at a low (2 µg/L) and high (11.5 µg/L) PIIINP concentration, 3.9%, and 3.1%, respectively. PIIINP is part of the UK NEQAS pilot program for liver fibrosis markers. During the study period, the Advia Centaur XPT PIIINP method performed within the expected range of registering within its group in all 4 dispatches, with a + 4.2% bias and 3.6% variability reported. Method validation was performed comparing the ADVIA Centaur results of clinical samples with a RIA method (Cisbio, Codolet, France) performed at an external laboratory.
Statistical analysis
All statistical analysis was performed using Analyse-it 5.11 software (Analyse-it Ltd., Leeds, United Kingdom) for Microsoft Excel (Microsoft Corp. Redmond, WA, USA) and SPSS version 25 (IBM Corp., Armonk, NY). Outlier detection was performed using the interquartile range model [Citation8]. Histograms were used to visualize biomarker distribution and skewed distributions were Box-Cox transformed. Calculation of the reference interval was performed according to CLSI guidelines [Citation9]. Accordingly, evaluation of the need for subgroup partitioning of the material was done employing Sinton et al. methodology [Citation10], examining differences in means between subgroups, as well as Harris and Boyd methodology [Citation11], which examines the proportion of subclass results that exceeds a combined reference interval. We calculated the 2.5th and 97.5th percentiles to establish the reference intervals [Citation12].
Results
453 samples were collected in total, 87 from blood donors (ages 20–76) and 366 from primary care patients. A significant difference in mean PIIINP concentrations was observed between blood donors and primary care subjects (7.88 vs 8.87 µg/L, p = .044). 67 samples were excluded because of an increased AST, ALT, or CRP. PIIINP significantly correlated with CRP (p < .001), but not with AST (p = .10) or ALT (p = .24). To determine whether to partition the reference intervals into different subgroups we calculated a 2.5% and 97.5% reference interval for males and females and for different age groups (). No significant mean difference was found between men and women. Among the different age subgroups, a significant mean difference of age groups 20–29, 50–59, and >80 in comparison with the total population was observed, but the mean difference was low, falling short of the required difference suggested by Sinton et al., that it exceeds 25% of the total interval [Citation10]. Evaluation of the need for partitioning employing Harris and Boyd methodology, 2 subjects in the below 30 years of age subgroup (4.4% of the age group) had a PIIINP concentration below the lower limit of the reference interval of the combined group. In the 80 years or above age group, 2 individuals (5% of the age group) had PIIINP concentrations above the higher limit of the reference interval. The distribution of results is visualized in . The distribution of PIIINP concentrations was positively skewed. Roughly normal distribution was obtained by Box-Cox transformation ().
Figure 1. Histograms of the PIIINP measurements. Bin size 2. (A) Non-transformed frequencies. Vertical full lines indicate the calculated reference interval (4.42–16.0 µg/L), the vertical dotted lines indicated the 95% C.I. (B) Box-Cox transformed frequencies.
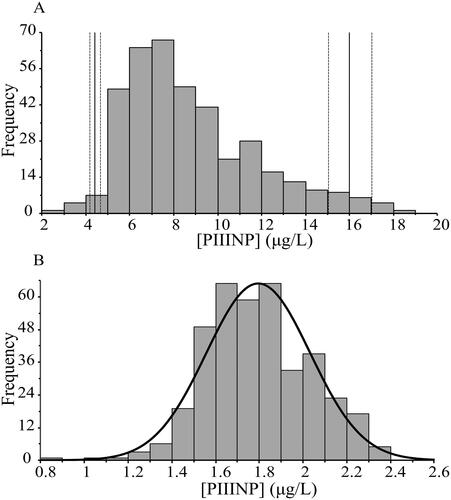
Figure 2. Procollagen III, N-terminal Propeptide (PIIINP) serum levels in different age groups. Box plots showing the median, the 25th and 75th quartiles, and the 2.5% and 97.5% quantiles values.
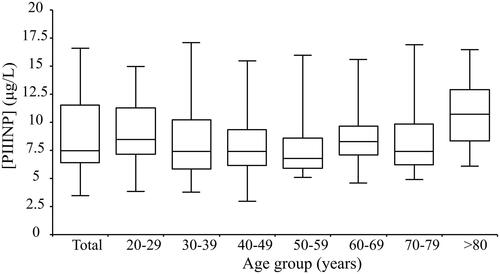
Table 1. PIIINP concentration by age group and sex.
The reference interval in the combined group was determined to be as follows: the lower limit of the reference interval (2.5% percentile with 95% CI) was 4.42 (4.20–4.65) µg/L and the upper limit of the reference interval (97.5% percentile with 95% CI) 16.0 (15.04–17.02) µg/L.
Discussion
In this study, we calculated a reference interval for PIIINP using the ADVIA Centaur® PIIINP assay on an ADVIA Centaur XPT platform. Siemens has developed the PIIINP essay as part of the Enhanced Liver Fibrosis examination (ELF) blood test, which combines three serum biomarkers shown to correlate to the level of liver fibrosis assessed by liver biopsy [Citation13].
The only previously published reference interval for the ADVIA Centaur® PIIINP assay [Citation7] relies on the sampling of blood donors below the age of 69. As MTX treatment among psoriasis patients above this age is fairly common, the aim of this study was to establish an interval including patients over 69. To obtain samples from an older age group, we mainly used sample remnants from primary care patients referred for thyroid-stimulating hormone (TSH) analysis rather than blood donors, since the number of blood donors in the oldest age group is often too small to collect enough samples in a reasonable time frame. As this includes a risk of inclusion of unhealthy patients with fibrosis, we excluded samples with aberrant TSH, AST, or ALT results with respect to local laboratory reference intervals. As an additional precaution, samples with CRP concentration >5.0 mg/L were omitted, to exclude patients with the concomitant inflammatory reaction of significance. CRP is accepted in clinical use as a major, although rather non-specific marker of inflammation. Low-grade inflammatory activity, as measured with high sensitivity CRP (hs-CRP) methods, has been associated with risk for atherosclerosis and cardiovascular disease [Citation14], as well as non-alcoholic fatty liver disease (NAFLD) and nonalcoholic steatohepatitis (NASH) [Citation15,Citation16]. In the latter case, liver transaminases were elevated as well, but a study correlating hs-CRP levels to PIIINP-levels is warranted.
The result from this study indicates a significantly wider reference interval for the ADVIA Centaur® PIIINP assay than previously reported by Knudsen et al. [Citation7]. This is likely due to the difference in populations upon which the intervals are based. Blood donor sampling is traditionally used to guarantee that the subjects included are healthy, but the typical homogeneity of blood donors may also risk resulting in a reference interval that is too narrow. In concordance with Knudsen et al., we observed no significant difference between sexes. However, Knudsen et al. observed differences in PIIINP concentrations between subjects below and above 40 years of age and determined separate reference intervals for these subgroups. While we found statistically different mean concentrations between age groups 20–29, 50–59, and >80 years of age and the combined population, we observed the differences to be small, less than the 25% suggested by Sinton et al. A tendency towards higher concentrations among the youngest and the oldest age groups was observed in and . While Knudsen et al. based their results exclusively on blood donors, they constituted a minority in our population. Blood donors being younger and displaying lower mean PIIINP concentrations likely contributes towards the higher upper reference limits we measured compared to Knudsen et al. Taking the above into account, we did not find support for partitioning of the reference interval into subgroups to be meaningful.
In conclusion, the PIIINP assay on the Siemens ADVIA Centaur® instrument has high precision, automated and easy to use. The reference interval proposed in this study is suitable for monitoring patients on MTX treatment for psoriasis. Furthermore, the wider reference interval should also be suitable for follow-up of patients older than 69, in order to avoid unnecessary invasive liver biopsies.
Acknowledgements
The authors are grateful for the laboratory work performed by Annelis Andersson, Emma Oliv, and Petrus Fintling during the data collection of this study.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Warren RB, Weatherhead SC, Smith CH, et al. British association of dermatologists' guidelines for the safe and effective prescribing of methotrexate for skin disease 2016. Br J Dermatol. 2016;175(1):23–44.
- Lammi L, Ryhanen L, Lakari E, et al. Type III and type I procollagen markers in fibrosing alveolitis. Am J Respir Crit Care Med. 1999;159(3):818–823.
- Piovesan A, Terzolo M, Reimondo G, et al. Biochemical markers of bone and collagen turnover in acromegaly or Cushing's syndrome. Horm Metab Res. 1994;26(05):234–437.
- Hakala M, Aman S, Luukkainen R, et al. Application of markers of collagen metabolism in serum and synovial fluid for assessment of disease process in patients with rheumatoid arthritis. Ann Rheum Dis. 1995;54(11):886–890.
- Boffa MJ, Smith A, Chalmers RJ, et al. Serum type III procollagen aminopeptide for assessing liver damage in methotrexate-treated psoriatic patients. Br J Dermatol. 1996;135(4):538–544.
- Maurice PD, Maddox AJ, Green CA, et al. Monitoring patients on methotrexate: hepatic fibrosis not seen in patients with normal serum assays of aminoterminal peptide of type III procollagen. Br J Dermatol. 2005;152(3):451–458.
- Knudsen CS, Heickendorff L, Nexo E. Measurement of amino terminal propeptide of type III procollagen (PIIINP) employing the ADVIA centaur platform. Validation, reference interval and comparison to UniQ RIA. Clin Chem Lab Med. 2014;52(2):237–241.
- Tukey JW. Exploratory data analysis. Addison-Wesley Pub; 1977.
- Boyd JC. Defining, Establishing, and Verifying Reference Intervals in the Clinical Laboratory; Approved Guidelines, CLSI document C28-A3. 2010.
- Sinton TJ, Cowley DM, Bryant SJ. Reference intervals for calcium, phosphate, and alkaline phosphatase as derived on the basis of multichannel-analyzer profiles. Clin Chem. 1986;32(1 Pt 1):76–79.
- Harris EK, Boyd JC. On dividing reference data into subgroups to produce separate reference ranges. Clin Chem. 1990;36(2):265–270.
- Box GaC D. An analysis of transformations. J R Stat Soc. 1964;26:211–252.
- Sanyal A, Cusi K, Hartman ML, et al. Cytokeratin-18 and enhanced liver fibrosis scores in type 1 and type 2 diabetes and effects of two different insulins. J Investig Med. 2018;66(3):661–668.
- Libby P, Ridker PM, Maseri A. Inflammation and atherosclerosis. Circulation. 2002;105(9):1135–1143.
- Foroughi M, Maghsoudi Z, Khayyatzadeh S, et al. Relationship between non-alcoholic fatty liver disease and inflammation in patients with non-alcoholic fatty liver. Adv Biomed Res. 2016;5:28.
- Yoneda M, Mawatari H, Fujita K, et al. High-sensitivity C-reactive protein is an independent clinical feature of nonalcoholic steatohepatitis (NASH) and also of the severity of fibrosis in NASH. J Gastroenterol. 2007;42(7):573–582.
