Abstract
Coagulation disturbances are common in severe COVID-19 infection. We examined laboratory markers in COVID-19 patients during the first wave of the pandemic in Finland. We analysed a wide panel of coagulation tests (IL ACL TOP 750/500®) from anonymously collected samples of 78 hospitalized COVID-19 patients in intensive care units (ICUs; n = 34) or medical wards (n = 44) at Helsinki University Hospital in April-May 2020. These coagulation data were supplemented with the laboratory information system results, including complete blood count and C reactive protein (CRP). Coagulation and inflammatory markers were elevated in most: FVIII in 52%, fibrinogen 77%, D-dimer 74%, CRP 94%, platelet count 37%. Anaemia was common, especially in men (73% vs. 44% in women), and overall weakly correlated with FVIII (women R2 = 0.48, men R2 = 0.24). ICU patients had higher fibrinogen and D-dimer levels (p < .01). Men admitted to the ICU also had higher platelet count, leukocytes and FVIII and lower haemoglobin than the non-ICU patients. None of the patients met the disseminated intravascular coagulation (DIC) criteria, but 31% had a D-dimer level of at least 1.5 mg/L. Presence of both anaemia and high D-dimer together with FVIII is independently associated with ICU admission. Antithrombin was reduced in 47% of the patients but did not distinguish severity. Overall, CRP was associated with coagulation activation. Elevated FVIII, fibrinogen and D-dimer reflected a strong inflammatory response and were characteristic of hospitalized COVID-19 patients. The patients were often anaemic, as is typical in severe inflammation, while anaemia was also associated with coagulation activity.
Introduction
Although the majority of coronavirus disease 2019 (COVID-19) cases present as mild respiratory tract infection, a proportion of patients develop severe disease with lung injury, acute respiratory distress syndrome and organ dysfunction. Coagulopathy in patients with severe COVID-19 is frequent and contributes to morbidity and mortality [Citation1,Citation2]. Distinct COVID-19-associated coagulopathy with a high inflammatory component has been identified [Citation3,Citation4].
During the early days of the pandemic, the International Society on Thrombosis and Haemostasis (ISTH) interim guidance on the recognition and management of coagulopathy in COVID-19 infection recommended measuring prothrombin time (PT), fibrinogen, D-dimer and platelet count in all patients presenting at the hospital. With major findings, that is, PT prolongation (<50% if expressed as activity), low fibrinogen (<2.0 g/L), 3- to 4-fold increase in D-dimer (≥1.5 mg/L) or low platelet count (<100 × 109/L) should be admitted to the hospital even without clinical concerns for the follow-up of possible rapid disease progression. Relating to coagulation dysregulation, platelet counts, PT ratio, fibrinogen, and D-dimer were suggested to be followed up once or twice daily. Upon bleeding complications, only supplementation therapies were recommended to target a PT ratio below 1.5, fibrinogen above 1.5 g/L and platelet count above 25–50 × 109/L [Citation5]. In COVID-19 infection, classic disseminated intravascular coagulation (DIC) with PT prolongation, consumption of fibrinogen, high D-dimer and decreased platelet count were later less frequently reported than in China [Citation2,Citation6]. In contrast, most patients exhibit inflammatory changes, shortened PT and high fibrinogen and high platelet count [Citation7,Citation8]. Leukocytes are increased, but lymphocytopenia is relatively common. A neutrophil-lymphocyte ratio of over 4.5 has been shown to predict severe infection [Citation9]. The presence of anaemia is common in COVID-19 infection, likely due to the inflammatory state [Citation10,Citation11].
The COVID-19 epidemic in Finland started in February 2020, with the first isolated cases in January. Major restrictions (remote working and schools, closure of nonessential public facilities) and a lockdown of Southern Finland were commenced in mid-March. The first wave of the epidemic subsided by July 2020. At that time, in Southern Finland, that is, in the Helsinki University Hospital district region, a total of 5350 COVID-19 infections were reported, representing 74% of the total national incidence [Citation12].
In this study, the laboratory profile of COVID-19 patients was examined during the first wave in April–May 2020. In April, a local guideline along the ISTH guidance recommended coagulation laboratory testing from all COVID-19 patients presenting to the hospital emergency room [Citation5]. Patients at infection and pulmonary wards and intensive care units (ICUs) received low molecular weight heparin prophylaxis, and we routinely tested their coagulation status. We aimed to study the patients’ coagulation profile and analysed disease severity (non-critically and critically ill, that is, non-ICU vs. ICU stay) in association with the laboratory findings. We also compared the study cohort with the big data of all COVID-19 patients in terms of laboratory results and disease severity in our hospital district.
Patients and methods
This is a retrospective observational study on COVID-19 infection using collected citrated plasma combined with a laboratory information system (LIS) database. We performed this study during April–May 2020 at Helsinki University Hospital, which serves Southern Finland (Uusimaa region). As thromboprophylaxis, all patients (unless contraindicated) received low molecular weight heparin (enoxaparin) at a prophylactic or intermediate dose, depending on risk factors of thrombosis.
As a background for the observations, we performed a general survey of all COVID-19 patients at Helsinki University Hospital during April-May 2020 using an LIS big data search. Positive COVID-19 test (SARS CoV-2 with PCR method), haemoglobin, C-reactive protein (CRP), D-dimer and fibrinogen results were analysed, and the disease severity, that is, whether the patient was treated in the medical ward or ICU was recorded.
For a comprehensive assessment of coagulation, we examined surplus citrated plasma samples from the patients in the medical wards and specific ICUs for COVID-19-infected patients only. From the LIS (My+, MyLab, Tampere, Finland), the patient’s other laboratory results from the time of sample collection were recorded using sample ID information. We randomly collected samples from 78 individual patients. From the LIS, we recorded the unit requesting the laboratory test (ICU or other wards), admittance to the hospital, possible admittance to the ICU, patient gender and age, and laboratory results. The samples and data were collected anonymously, and no further clinical details were available. The study was performed with institutional approval (HUS/157/2020 and HUS/211/2020).
Citrated plasma (3.2% Na-citrate, Becton Dickinson, New Jersey, USA) was double-centrifuged at 2500 g for 15 min and subsequently stored at −80 °C. Coagulation tests were performed in batches using ACL TOP® 500 and 750 analysers (Instrumentation Laboratory, Naples, Italy) with the following reagents: PT with Owren’s PT (Medirox®, Nyköping, Sweden), activated partial thromboplastin time (APTT), FVIII activity, fibrinogen with Clauss method, and antithrombin activity, all with HaemosIL® reagents (Instrumentation Laboratory, Naples Italy) and D-dimer concentration (HaemosIL D dimer HS 500, FEU units). A Sysmex® XN-9000 haematology analyser (Kobe, Japan) for K2-EDTA tubes was used for complete blood count (CBC). CRP and creatinine, including the estimated glomerular filtration rate (GFR) with the EPI formula, were analysed with a Siemens Atellica® Solution chemistry analyser (Siemens Healthineers, München, Germany) from Li-heparin tubes. We could not analyse patient outcomes based on a single laboratory assessment, but the severity of the disease was recorded (i.e. whether the patient was treated in the ICU during their hospital stay), and the groups were compared on the abovementioned variables.
For statistical analysis, Pearson correlations, Kruskal–Wallis test, Mann–Whitney U-test and Wilks’ lambda multivariate analysis were run using IBM SPSS Statistics version 25®. We used nonparametric tests since the data were not normally distributed. The p-value was regarded as significant at the levels p < .05.
Results
Big data survey
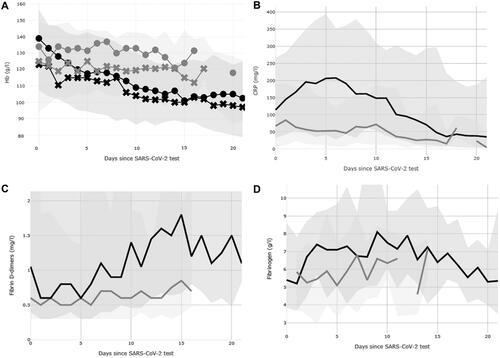
In the general big data survey, a maximum of 159 results were obtained from non-ICU wards, and 79 results were obtained from ICU wards. A clear trend of CRP peaking at day 5 was seen in the ICU, while haemoglobin decreased and D-dimer increased, during prolonged hospitalization. All four parameters correlated with one another. The correlation was best at day 10 after a positive COVID-19 test, with the highest R2 = 0.56 between CRP and fibrinogen and the lowest R2 = 0.07 between haemoglobin and CRP (Pearson correlation p < .01). This is in part due to selection bias, as only the sickest patients remained throughout the observation period (, Supplemental Table). For fibrinogen, the data were limited, with a maximum number of measurements of 28 for hospitalized patients and 22 for ICU patients.
Figure 1. General trends of haemoglobin (A), C reactive protein (B), D-dimer (C) and fibrinogen (D) in hospitalized patients in the ICU (black line, dark shaded area 10th to 90th percentile) and non-ICU wards (grey line, light shaded area 10th to 90th percentile). In Panel A, haemoglobin (Hb) results for men (circles) and women (crosses) are shown separately, with corresponding colours, black for ICU patients and grey for non-ICU wards. The number of measurements at each time point are shown in Supplemental Table.
Plasma samples
From the surplus plasma samples, a total of 78 different patients (44 men) with a mean age of 56 years (range 16–87) were analysed for the coagulation profile, and CBC, CRP, and creatinine (and GFR) results were obtained from the LIS during spring 2021, after all patients had been discharged or deceased. During their hospital stay, thirty-four patients were treated in the ICU, of which 23 (67%) were men and 44 (48% men) were treated in medical wards. Cross-sectional samples have obtained a median of 4 days from hospitalization in the non-ICU patients and at 13 days in ICU patients. The median length of ICU stay was 14 days (median in women 8 days, in men 16 days, Mann–Whitey U-test p = .03).
The majority of the laboratory results were abnormal ( and ). Coagulation and inflammatory markers were above the reference interval in most of the patients: FVIII (52%), fibrinogen (77%), D-dimer (74%), and CRP (in 94%), and the neutrophil-lymphocyte ratio was above a cut-off of 4.5 in 64%, consistent with the ongoing COVID-19 infection at the time of blood collection. Lymphocytopenia was also common (43% prevalence, ).
Table 1. Laboratory findings in hospitalized COVID-19 patients.
Table 2. Laboratory findings in hospitalized COVID-19 patients treated in medical wards (non-ICUs) and intensive care units (ICUs) according to gender. Only significant results are shown (Mann–Whitney p<0.05). The median time of sampling was 4 days after admission for non-ICU patients and 13 days for ICU patients.
Anaemia and red blood cell indices emerged as a major distinguishing feature among the patients treated in the ICU versus medical wards. Forty-seven patients (60%) exhibited anaemia: 16/34 (47%) women, but 32/44 (73%) men were anaemic (i.e. haemoglobin below the reference interval <117 g/L in women and <134 g/L in men). Patients who were admitted to the ICU during their hospital stay had lower haemoglobin levels (median 110 vs. 125 g/L) overall, and the prevalence of anaemia was higher (27/34, 79%) than in those not admitted to the ICU (21/44, 48%; ). Anaemic patients had only minor differences in their red cell indices, that is, higher red cell distribution width (RDW, median 14% vs. 13%) and higher mean cellular volume (MCV, median 94 vs. 91 fL) than in non-anaemic patients (data not shown), suggesting normocytic anaemia without significant red cell size variation in most cases.
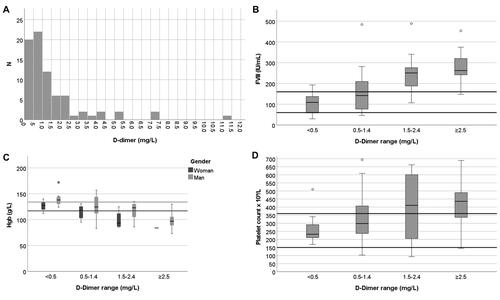
Anaemia was associated with coagulation variables. Anaemic patients had both higher FVIII and D-dimer levels than non-anaemic patients (). In addition to anaemia, D-dimer was another biomarker readily distinguishing the two patient groups. The patients with high D-dimer levels (≥1.5 mg/L) had higher FVIII activity (263 vs. 128 IU/mL), lower haemoglobin (100 vs. 124 g/L) and higher platelet count (436 vs. 274 × 109/L). Most patients (23/34, 68%) in the ICU had thrombocytosis and higher CRP (83 vs. 52 mg/L) (). Platelet count was increased in 29 patients and was significantly higher in patients admitted to the ICU than in those who were not admitted to the ICU (436 vs. 236 × 109/L, p < .001, ). FVIII levels also modestly correlated with haemoglobin concentration (in women R2 = 0.48 and in men R2 = 0.24; both Pearson correlation coefficient p < .001). Lymphocytopenia was common, but the neutrophil-lymphocyte ratio only poorly correlated with D-dimer (R2 = 0.15, p = .002) without any correlation with haemoglobin levels. D-dimer elevation was associated with a longer stay in the ICU (16 vs. 7 days), relating to more severe disease overall.
Overall, CRP correlated modestly with fibrinogen (R2 = 0.44; p < .001). The neutrophil-lymphocyte ratio correlated with all coagulation and inflammatory markers quite poorly (FVIII R2 = 0.09, Pearson correlation p = .025; fibrinogen R2 = 0.11, p < .009; D-dimer R2 = 0.10, p = .002; CRP R2 = 0.32, p < .001). Most of the markers were also more pathological in patients who were admitted to the ICU (n = 34) than in the others (Mann-Whitey U test p < .05; ). In our study, of the single laboratory variables, CRP, fibrinogen, antithrombin, PT, lymphocytes or neutrophil-lymphocyte ratio were not different in ICU vs. non-ICU patients. In a multivariate analysis, the presence of anaemia, high FVIII, fibrinogen, D-dimer and CRP, and platelet counts achieved greater significance for ICU than non-ICU status (Wilks’ lambda multivariate, 0.54, p < .001).
The gender of the patients showed differences not only in haemoglobin but also in D-dimer levels (median in women 0.7 mg/L, men 1.1 mg/L), while men and women were of similar age, mean 56 years. Anaemic men also had a higher platelet count (400 vs. 234 × 109/L) and lower mean cellular haemoglobin concentration (MCHC) in red cells (327 vs. 331 g/L) than non-anaemic men (p < .03).
According to the ISTH criteria, none of the patients presented with overt DIC findings [Citation8]. PT was abnormal (below 70%) in only 4 patients, and thrombocytopenia was rare (<150 × 109/L in 5 patients and below 100 × 109/L in a single patient). Fibrinogen was below the local reference interval of 2.0 g/L in only two patients, while in the majority (74%), it was above the local reference interval of 4.0 g/L. However, 74% of the patients had D-dimer at or over the reference 0.5 mg/L, and 31% exceeded 1.5 mg/L. Antithrombin was below the reference interval in 47% and significantly decreased (below 0.50 IU/mL) in 15% ().
Discussion
In this retrospective study with randomly selected samples from hospitalized COVID-19 patients, a wide array of coagulation and other laboratory tests were assessed. One of our main findings was a high prevalence of anaemia (61%). In the general population over the age of 44 years, the prevalence of anaemia is estimated to be 3% [Citation13]. In all COVID-19 patients at Helsinki University Hospital, haemoglobin tended to decrease with longer hospital stays. This is also correlated with the lower haemoglobin in ICU patients, in whom the samples were taken after longer hospital stays than patients in non-ICU wards. Anaemia also aligned with coagulation abnormalities: low haemoglobin was associated with high FVIII activity. This emphasizes the important interplay of red blood cells and haemostasis in various inflammatory processes [Citation14]. Anaemia is common among severely ill patients due to inflammation [Citation15]. However, anaemia alone increases total mortality, coronary disease and cancer mortality [Citation16]. Haemoglobin and lymphocyte levels have been previously described in a clinical study including all COVID-19 patients from mainland China between December 2019 and January 2020 [Citation17]. In our study, 41% of patients had lymphocytopenia, whereas its prevalence exceeded 80% in non-severe Chinese patients and 96% in severe patients. While in the Chinese study median haemoglobin differed by only 7 g/L between severe and nonsevere cases, our ICU patients had 17 g/L lower haemoglobin [Citation17]. Indeed, anaemia was more common than lymphocytopenia among our patients. The neutrophil-lymphocyte ratio and CRP level predicted more severe disease in a cohort from Wuhan, while in our study it did not predict ICU treatment [Citation18].
Low haemoglobin levels in severe COVID-19 infection have been reported. A recent meta-analysis including 63 studies found that severe COVID-19 cases have lower haemoglobin levels than moderate cases (weighted mean difference 4 g/L; [Citation10]). Anaemia at hospital admission correlates with a worse prognosis [Citation19–21]. However, many studies presenting coagulation laboratory results exclude the contribution of anaemia. In the 12 referenced original COVID-19 studies including laboratory test results, only 6 reported haemoglobin levels. Red blood cells exhibit major effects on haemostasis, including but not limited to vessel wall interaction, thrombin generation, FXIII interaction and inhibition of fibrinolysis [Citation14,Citation22–24].
Systemic inflammation and bacterial sepsis affect erythrocyte membrane composition, predisposing patients to anaemia due to accelerated clearance of erythrocytes [Citation11,Citation25]. Lower haemoglobin levels at baseline in kidney transplant patients predicted severe COVID-19 infection in a UK study [Citation26], whereas Chinese studies did not find differences in haemoglobin levels of severe vs. non-severe COVID-19 disease patients [Citation2,Citation6,Citation17]. Moreover, the RDW index can be abnormal in severe COVID-19 due to haemolysis, with increases in lactate dehydrogenase and ferritin [Citation27,Citation28]. In our patients, RDW was rarely abnormal. Red blood cell-derived microvesicles bind to platelets and promote primary haemostasis as well as coagulation on phosphatidylserine-positive surfaces [Citation29]. We did not study microvesicles, an important topic for future research. In extreme COVID-19 cases, red blood cell lysate contributes to the pathogenesis of COVID coagulopathy with increased lactate dehydrogenase and ferritin [Citation29,Citation30]. COVID-19 profoundly alters blood count, with the majority of COVID-19 patients having abnormal blood smears and alterations in platelet size [Citation31].
Concordantly with previous studies [Citation1], we measured elevated fibrinogen and D-dimer levels. We also observed these biomarkers longitudinally in the entire COVID-19 cohort in Helsinki University Hospital. In contrast, platelet counts did not generally decrease, and PT was rarely prolonged. Thus, our study, like others published after the first experiences, did not signature DIC. Only 6% of our patients were thrombocytopenic, whereas early pandemic studies have encountered low platelet counts (<150 × 109/L) in 70–95% of patients with severe COVID-19 [Citation1,Citation6]. Platelet counts tend to increase during the disease course, [Citation32], and as in ours, a Swedish study also found that anticoagulation use increases platelet counts and improves prognosis [Citation33]. In Finland prophylactic anticoagulation is consistently applied early on, which may improve the platelet counts. In contrast, high D-dimer levels appear to be the key uniform observation, associated with hypercoagulation and increased mortality; D-dimer over 1.0 mg/L at admission coincided with 18-fold higher mortality [Citation2]. Moreover, the incidence of venous thromboembolism is high in hospitalized COVID-19 patients, a rate of 18% in a recent meta-analysis [Citation34].
In addition, the higher the peak D-dimer level, the more severe lung injuries and more ICU admissions have been reported [Citation35]. CRP levels were also predictive of more severe lung injury, emphasizing the important crosstalk between inflammation and coagulation in severe COVID-19. The inflammatory markers CRP, FVIII, fibrinogen, and D-dimer also correlated with one another, as in a large US study [Citation36]. In bacterial sepsis, plasminogen activator inhibitor 1 (PAI-1) and thrombin activatable fibrinolysis inhibitor (TAFI), among others, inhibit fibrinolysis, associated with higher mortality, while D-dimer levels are not high [Citation37,Citation38]. In some COVID-19 patients, fibrinolysis may be inhibited as well, and patients with higher PAI-1 levels may require supplemental oxygen more frequently, irrespective of D-dimer or tissue plasminogen activator (tPA) levels [Citation39].
Tang and coworkers reported that antithrombin levels were similar at admission, but in patients who died, they were lower than those in survivors at day 7 after admission [Citation40]. Almost half of our samples had antithrombin levels below the reference interval but without an impact on the severity of COVID-19 (non-ICU vs. ICU), D-dimer level or anaemia status.
COVID-19-associated coagulopathy differs from classic DIC, while PT is not often prolonged, D-dimer is almost invariably markedly increased in severe COVID-19. Platelet counts (<100 × 109/L) and fibrinogen (<1.0 g/L) are rarely low [Citation8]. In fact, none of our results fulfilled the DIC criteria. In unique coronavirus-associated coagulopathy, inflammation predominates and tends to increase platelet counts, FVIII levels and fibrinogen concentrations [Citation4]. Similar to our study where inflammatory markers and FVIII, fibrinogen and D-dimer levels were elevated in ICU-admitted patients, a French study observed that the same markers were progressively higher in hospitalized patients than in outpatients [Citation41]. FVIII may also be associated with lung injury and is secreted from the local endothelium [Citation42]. Indeed, in our study, FVIII levels were high and correlated with fibrinogen and D-dimer and inversely correlated with haemoglobin, especially in ICU patients.
The main limitations of our study were the lack of clinical outcome data on the patients and the cross-sectional nature, without any sequential follow-up. In Finland, the total mortality of COVID-19 patients has been 4%, while the mortality of the critically ill patients has been 12% during ICU, and 16% during the hospitalisation period in April–May 2021 [Citation12,Citation43]. In contrast, before the COVID-19 pandemic, national ICU mortality was 6% and 10%, respectively. [Citation44]. The timing of the sample collection varied during the hospital stay; in ICU the samples were obtained later in the course of the disease than in those who were non-critically ill. Big data analysis confirmed the results of our plasma sample cohort with single occasion data. The major biomarkers, that is, haemoglobin, CRP, D-dimer, and fibrinogen, differed in relation to critical versus non-critical illness in the big data. This analysis followed the patients longitudinally after positive SARS-CoV2-test (). Laboratory marker levels were significantly associated with outcome, with higher levels in ICU- and hospital-admitted patients than in outpatients [Citation34]. Higher D-dimer and FVIII also predispose patients to an increased risk for thrombotic events at 30 days (OR 1.7–2.9) [Citation34]. Anaemia and high D-dimer levels are associated with poorer prognosis in COVID-19 patients and in general [Citation2,Citation15]. Our findings support these earlier studies, and emphasize the importance of the follow-up of inflammation and coagulation biomarkers in patient management.
Figure 2: Histogram of haemoglobin distribution among patients (A), boxplots of patient age (B), FVIII (C) and (D) D-dimer according to anaemia status. The highest D-dimer result of 57 mg/L is not shown. Variables were selected according to medians showing significant differences (Mann–Whitney U-test). The number of patients with anaemia was 48/78 (62%) in total, 32/44 men (72%), 16/34 women (47%), 27/34 ICU patients (79%), and 7/44 non-ICU patients (16%). Lines, reference intervals; thick line in box, median; box, first and third quartiles; whiskers, range; outlier ≥1.5 box lengths from median, circle; extreme outlier ≥3.0 box lengths from median, asterisk.
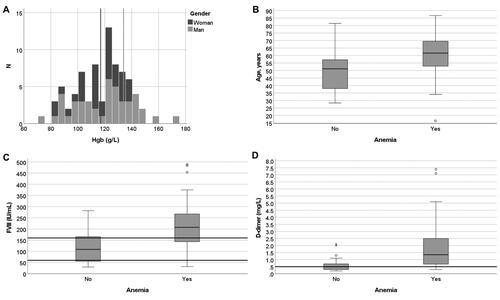
Figure 3. Histogram of D-dimer distribution among patients (A), boxplots of FVIII (B), haemoglobin (C), and platelet count (D), according to their D-dimer levels. One patient with a D-dimer value of 57 mg/L is not shown. Variables were selected according to medians showing significant differences (Mann–Whitney U-test for differences at a D-dimer level of 1.5 mg/L). Lines, reference intervals; thick line in box, median; box, first and third quartiles; whiskers, range; outlier ≥1.5 box lengths from median, circle; extreme outlier ≥3.0 box lengths from median, asterisk.
Conclusions
We observed strong inflammatory and coagulation activity profiles, in hospitalised COVID-19 patients, with high CRP and FVIII, fibrinogen and D-dimer. These increases are accentuated in the critically ill. We also found that anaemia is frequent, especially in men, exposing patients to worsening hypoxia, and risk of adverse events and poor prognosis. The clinical implications of the association of anaemia and coagulation biomarkers warrant further study.
Supplemental Material
Download PDF (60.1 KB)Acknowledgements
Liisa Tulikallio, Jaana Perholehto, Jolanta Lundgren and Riikka Kosonen are thanked for their assistance in sample collection and handling.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Levi M, Thachil J, Iba T, et al. Coagulation abnormalities and thrombosis in patients with COVID-19. Lancet Haematol. 2020;7(6):e438–e440.
- Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062.
- Becker RC. COVID-19 update: Covid-19-associated coagulopathy. J Thromb Thrombolysis. 2020;50(1):54–67.
- Iba T, Warkentin TE, Thachil J, et al. Proposal of the definition for COVID-19-Associated coagulopathy. JCM. 2021;10(2):191.
- Thachil J, Tang N, Gando S, et al. ISTH interim guidance on recognition and management of coagulopathy in COVID-19. J Thromb Haemost. 2020;18(5):1023–1026.
- Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in wuhan, China. Lancet. 2020;395(10223):497–506.
- Iba T, Levy JH, Levi M, et al. Coagulopathy in COVID-19. J Thromb Haemost. 2020;18(9):2103–2109.
- Toh CH, Hoots WK. The scoring system of the scientific and standardisation committee on disseminated intravascular coagulation of the international society on thrombosis and haemostasis: a 5-year overview. J Thromb Haemost. 2007;5(3):604–606.
- Li X, Liu C, Mao Z, et al. Predictive values of neutrophil-to-lymphocyte ratio on disease severity and mortality in COVID-19 patients: a systematic review and meta-analysis. Crit Care. 2020;24(1):647.
- Taneri PE, Gomez-Ochoa SA, Llanaj E, et al. Anemia and iron metabolism in COVID-19: a systematic review and meta-analysis. Eur J Epidemiol. 2020;35(8):763–773.
- Dinkla S, van Eijk LT, Fuchs B, et al. Inflammation-associated changes in lipid composition and the organization of the erythrocyte membrane. BBA Clin. 2016;5:186–192.
- Finnish Institute for Health and Welfare. [Internet]. Helsinki: Finnish Institute for Health and Welfare [cited 2021 Oct 13] Available from: https://thl.fi/en/web/thlfi-en.
- Eisele L, Durig J, Broecker-Preuss M, et al. Prevalence and incidence of anemia in the German Heinz Nixdorf recall study. Ann Hematol. 2013;92(6):731–737.
- Weisel JW, Litvinov RI. Red blood cells: the forgotten player in hemostasis and thrombosis. J Thromb Haemost. 2019;17(2):271–282.
- Weiss G, Ganz T, Goodnough LT. Anemia of inflammation. Blood. 2019;133(1):40–50.
- Kabat GC, Kim MY, Verma AK, et al. Association of hemoglobin concentration with total and cause-specific mortality in a cohort of postmenopausal women. Am J Epidemiol. 2016;183(10):911–919.
- Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708–1720.
- Yufei Y, Mingli L, Xuejiao L, et al. Utility of the neutrophil-to-lymphocyte ratio and C-reactive protein level for coronavirus disease 2019 (COVID-19). Scand J Clin Lab Invest. 2020;80(7):536–540.
- Benoit JL, Benoit SW, Oliveira MD, et al. Anemia and COVID-19: a prospective perspective. J Med Virol. 2021;93(2):708–711.
- Tao Z, Xu J, Chen W, et al. Anemia is associated with severe illness in COVID-19: a retrospective cohort study. J Med Virol. 2021;93(3):1478–1488.
- Tremblay D, Rapp JL, Alpert N, et al. Mild anemia as a single independent predictor of mortality in patients with COVID‐19. EJHaem. 2021;2(3):319–326. https://doi.org/10.1002/jha2.167 Online ahead of print
- Whelihan MF, Mann KG. The role of the red cell membrane in thrombin generation. Thromb Res. 2013;131(5):377–382.
- Walton BL, Byrnes JR, Wolberg AS. Fibrinogen, red blood cells, and factor XIII in venous thrombosis. J Thromb Haemost. 2015;13:S208–S215.
- Wohner N, Sotonyi P, Machovich R, et al. Lytic resistance of fibrin containing red blood cells. Arterioscler Thromb Vasc Biol. 2011;31(10):2306–2313.
- Kempe DS, Akel A, Lang PA, et al. Suicidal erythrocyte death in sepsis. J Mol Med. 2007;85(3):273–281.
- Sran K, Olsburgh J, Kasimatis T, et al. COVID-19 in kidney transplant patients from a large UK transplant center: exploring risk factors for disease severity. Transplant Proc. 2021;53(4):1160–1168.
- Akhter N, Ahmad S, Alzahrani FA, et al. Impact of COVID-19 on the cerebrovascular system and the prevention of RBC lysis. Eur Rev Med Pharmacol Sci. 2020;24(19):10267–10278.
- Gong J, Ou J, Qiu X, et al. A tool for early prediction of severe coronavirus disease 2019 (COVID-19): a multicenter study using the risk nomogram in Wuhan and Guangdong, China. Clin Infect Dis. 2020;71(15):833–840.
- Thangaraju K, Neerukonda SN, Katneni U, et al. Extracellular vesicles from red blood cells and their evolving roles in health, coagulopathy and therapy. Int J Mol Sci. 2020;22(1):153.
- Melki I, Tessandier N, Zufferey A, et al. Platelet microvesicles in health and disease. Platelets. 2017;28(3):214–221.
- Alnor A, Sandberg MB, Toftanes BE, et al. Platelet parameters and leukocyte morphology is altered in COVID-19 patients compared to non-COVID-19 patients with similar symptomatology. Scand J Clin Lab Invest. 2021;81(3):213–217.
- Amgalan A, Othman M. Hemostatic laboratory derangements in COVID-19 with a focus on platelet count. Platelets. 2020;31(6):740–745.
- Sjöström A, Wersäll J, Warnqvist A, et al. Platelet count rose while D-Dimer levels dropped as deaths and thrombosis declined-an observational study on anticoagulation shift in COVID-19. Thromb Haemost. 2021; Apr 8. doi:https://doi.org/10.1055/a-1477-3829. Online ahead of print.
- Kunutsor SK, Laukkanen JA. Incidence of venous and arterial thromboembolic complications in COVID-19: a systematic review and meta-analysis. Thromb Res. 2020;196:27–30.
- Trimaille A, Thachil J, Marchandot B, et al. D-Dimers level as a possible marker of extravascular fibrinolysis in COVID-19 patients. JCM. 2020;10(1):39.
- Al-Samkari H, Karp Leaf RS, Dzik WH, et al. COVID-19 and coagulation: bleeding and thrombotic manifestations of SARS-CoV-2 infection. Blood. 2020;136(4):489–500.
- Semeraro F, Colucci M, Caironi P, et al. Platelet drop and fibrinolytic shutdown in patients with sepsis. Crit Care Med. 2018;46(3):e221–e228.
- Semeraro F, Ammollo CT, Caironi P, et al. Low D-dimer levels in sepsis: good or bad? Thromb Res. 2019;174:13–15.
- Zuo Y, Warnock M, Harbaugh A, et al. Plasma tissue plasminogen activator and plasminogen activator inhibitor-1 in hospitalized COVID-19 patients. Sci Rep. 2021;11(1):1580.
- Tang N, Li D, Wang X, et al. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18(4):844–847.
- Rauch A, Labreuche J, Lassalle F, et al. Coagulation biomarkers are independent predictors of increased oxygen requirements in COVID-19. J Thromb Haemost. 2020;18(11):2942–2953.
- Shovlin CL, Angus G, Manning RA, Okoli GN, et al. Endothelial cell processing and alternatively spliced transcripts of factor VIII: potential implications for coagulation cascades and pulmonary hypertension. PLoS One. 2010;5(2):e9154.
- Finnish national update on ICU care on COVID [Internet]. Kuopio: Kuopio University Hospital; 2021 [cited Oct 13]. Available from: https://www.psshp.fi/documents/7796350/7841067/Tehohoidon+tilannekuva+02062021/ca3ff1b6-d41c-43d1-ad01-310630e42162
- Reinikainen M, Varpula T. [Finnish ICU-care]. Duodecim. 2018;134:161–163. Finnish.
