Abstract
Leucocyte telomere length (LTL) has been associated with nonalcoholic fatty liver disease (NAFLD), but the evidence is imperfect. Furthermore, liver fibrosis has been shown to correlate with mortality and recent studies have also found associations with LTL and fibrosis suggesting that LTL may have additional prognostic value in liver diseases. Our objective was to study the association of LTL and NAFLD and evaluate the association of LTL in prognosis of NAFLD subjects. Study subjects (n = 847) were middle-aged hypertensive patients. All participants were evaluated for NAFLD and their LTL was measured at baseline. Outcomes were obtained from Finnish Causes-of-Death Register and the Care Register for Health Care in Statistics Finland to the end of 2014. An inverse association with NAFLD prevalence and LTL length was observed (p < .001 for trend). Shortest telomere tertile possessed statistically significantly more NAFLD subjects even with multivariate analysis (shortest vs. middle tertile HR 1.98 p = .006 and shortest vs. longest tertile HR 2.03 p = .007). For the study period, mortality of the study group showed statistically significant relation with telomere length in univariate but not for multivariate analysis. In subgroup analysis, LTL did not associate with prognosis of non-NAFLD subjects. However, LTL was inversely associated with overall mortality in the subjects with NAFLD in both univariate (HR 0.16 p = .007) and multivariate analysis (HR 0.20 p = .045). In middle-aged Caucasian cohort, shorter leucocyte telomeres associated independently with increased prevalence of NAFLD. Shorter LTL was not associated with mortality in non-NAFLD patients whereas it predicted mortality of NAFLD patients independently.
Introduction
The nonalcoholic fatty liver disease (NAFLD) covers a spectrum of liver conditions from simple steatosis to nonalcoholic steatohepatitis and varying degrees of liver fibrosis with cirrhotic end-stage liver disease as an endpoint. The pathophysiology of the disease is complex and not fully understood but at the center of it has been recognized to be insulin resistance and excessive free fatty acid supply to the liver leading to oxidative stress [Citation1]. The global prevalence of NAFLD is around 25% and increasing rapidly along with its comorbid conditions and mortality shown to be associated with the disease [Citation2–5]. With increasing prevalence of NAFLD there is urgent need to determine both better understanding of the underlying pathological drivers and biomarkers with prognostic value [Citation6].
Telomeres are the repetitive DNA sequences at the tip of chromosomes that are responsible for stabilizing and protecting chromosomes through various mechanisms. During mitosis, telomeres shorten due to problems in DNA replication at the very end of the linear chromosomes. Some cells, with high proliferative capacity, can counter some of the attrition of telomeres with the help of telomerase enzyme. Telomerase is a reverse transcriptase enzyme that adds repeat sequences in the end of telomeres. However, in most cells, the telomerase enzyme expression is limited, and when telomere length reaches critical point, a signaling cascade is initiated leading to cell apoptosis or senescence [Citation7]. Leucocyte telomere shortening has been linked to several diseases and traits many related to metabolic syndrome (MBO) as well as all-cause mortality in large cohorts [Citation7–10]. The causality of the associations is under debate, but a body of evidence suggests the relation can be bidirectional and, in some cases, reversible [Citation10]. The underlying pathological processes proposed to be involved in the reduction in leucocyte telomere length (LTL) are cumulative oxidative stress and inflammation which in turn is linked with insulin resistance [Citation11]. Thus, both NAFLD and LTL shortening share similar pathologic events.
The research done addressing LTL and NAFLD suggest a tendency for shorter LTL on NAFLD subjects [Citation12–17]. However, the association between NAFLD and LTL is yet debatable [Citation18]. Numerous associations between liver fibrosis and telomere length have been reported [Citation8,Citation19–21]. Interestingly, telomere shortening associated with liver fibrosis does not seem to limit only to hepatocytes and LTL has shown to shorten also [Citation8]. The same finding was observed in studies examining specifically NAFLD [Citation16,Citation17,Citation22]. Liver fibrosis has been identified as the strongest disease-related risk factor in NAFLD [Citation23–25]. It is therefore possible that prognostic data related to liver fibrosis is imprinted in peripheral blood LTL.
We hypothesize that LTL could be a prognostic factor of NAFLD. According to authors’ knowledge, no previous study has evaluated the relation of LTL in NAFLD with mortality in longitudinal set up. With respect to recent findings, we set to investigate the associations of LTL and middle-aged Caucasian cohort with ultrasound verified NAFLD as well as LTL and NAFLD related mortality in 21-year follow-up study.
Material and methods
Study design and patient population
This study is a part of the Oulu Project Elucidating the Risk Atherosclerosis-study (OPERA) which originally consisted of randomly selected Finnish hypertensive patients and their age- and sex-matched controls with a total of 1045 participants. Recruitment of the participants was done between years 1990 and 1993, when subjects were 40–62 years of age. Participants were clinically examined, and a wide range of laboratory tests were obtained including LTL. MBO was diagnosed according to International Diabetes Federation 2006 criteria [Citation26]. A homeostatic model assessment of insulin resistance (HOMA-IR) was calculated (formula: fasting insulin × fasting glucose/22.5) [Citation27]. Standardized questionnaires were completed with the help of two specialized nurses. Questionnaires covered past medical history, medication, family history, physical activity, smoking and alcohol consumption. Anthropologic metrics, such as waist, height, weight, were measured. Presence of single nucleotide polymorphisms known to be a genetic risk factor for NAFLD were examined as follows: patatin-like phospholipase domain-containing 3 (PNPLA3) variant rs738409, the transmembrane 6 superfamily member 2 (TM6SF2) variant rs58542926 and membrane-bound o-acyltransferase domain containing 7 (MBOAT7) rs641738 [Citation28–31]. DNA extraction was done from frozen plasma samples with high-salt method and analyses were performed with TaqMan assays (Life Technologies, Carlsbad, CA).
For the purposes of this study 76 participants with alcohol consumption exceeding limits of NAFLD (≥210 g a week in men and ≥140 g a week in women) were excluded resulting in total of 969 subjects. Ultrasound of the liver was offered for all participants. However, for 11 of the 969 patients ultrasound of the liver was failed to acquire reducing eligible subjects for analyses to 958. For 111 patients, the baseline relative telomere length value was not available either due to missing sample or failure in sample analysis. Thus, the final study population, eligible for our study, included 847 patients of which 394 (46.5%) were males and 453 (53.5%) females. Of these patients, 431 (50.9%) were hypertensive and 416 (49.1%) were not. Mean age for males was 51.2 (range 40.2–61.0) and for females 51.2 (range 41.2 − 62.0).
Viral hepatitis does not require exclusion for NAFLD studies in Finnish population due to very low prevalence [Citation2]. Other reasons for fatty liver were considered rare. Thus, NAFLD diagnosis was made with exclusion of excessive alcohol consumption as described above and detection of fatty liver by ultrasound.
Ultrasound evaluation
Ultrasound examination was used to detect fatty liver. Steatosis evaluation was based on liver-kidney contrast assessment [Citation32]. Ultrasound examinations were performed by trained radiologist with 10 years of experience of abdominal ultrasound examinations. Of 224 (26.4%) of the final study population had liver steatosis detectable by ultrasound.
Leukocyte telomere length measurements
The DNA used to measure LTL was obtained from peripheral blood samples taken at baseline up using a multiplex quantitative real-time polymerase chain reaction (qRT-PCR) method [Citation33]. Telomeric DNA sequence (T) was measured using polymerase chain reaction (PCR) amplification and compared with single-copy gene (S) providing the relative LTL in T/S ratio for each subject. Amplification of the telomere sequence was done using primers (TelG: ACA CTA AGG TTT GGG TTT GGG TTT GGG TTT GGG TTA GTG T and TelC: TGT TAG GTA TCC CTA TCC CTA TCC CTA TCC CTA TCC CTA ACA). Albumin was used as a single copy gene with primers developed by Cawthon (Albugcr2: cgg cgg cgg gcg gcg cgg gct ggg cgg CCA TGC TTT TCA GCT CTG CAA GTC and Albdgcr2: gcc cgg ccc gcc gcg ccc gtc ccg ccg AGC ATT AAG CTC TTT GGC AAC GTA GGT TTC). Lowercase bases in albumin primers are non-templated sequences that, in a very high melting temperature, result into a PCR product that enables the measurement of T and S signals from the same PCR product. A standard curve spanning 0.5 − 25 ng of DNA was formed from a reference sample and run-in triplicates in each plate. The mean R2 values of standard curves for T were 0.991 (SD 0.003) and 0.995 (SD 0.002) for S. If coefficient variation was more than 15% the samples were rerun. The mean coefficient variation value for all T/S values was 5.31%.
Outcome classification
Information on deaths was obtained from Finnish Causes-of-Death Register and the Care Register for Health Care in Statistics Finland to the end of 2014.
Statistical methods
The statistical analyses of this study were performed with IBM SPSS software version 27 (IBM Corp, Armonk, NY). For purposes of examination of the differences of baseline characteristics of the study population a categorical variable was formed from LTL with division into tertiles. For continuous variables, a mean and standard error was calculated. For categorical values frequency was reported. Statistical significances for baseline characteristics were calculated using chi-square for categorical values and analysis of variance for continuous variables. For multivariate analysis, an analysis of covariance was performed. Total mortality was assessed as cumulative proportional probability of death and analyzed using Kaplan–Meier survival curves. Separately, for each Kaplan–Meier analysis of subgroups (NAFLD and non-NAFLD) a new tertile divided categorical variable of LTL was formed. Statistical significances of the Kaplan–Meier survival curves were calculated using Log-rank test. The association between LTL and risk of death during follow-up time was estimated with Cox proportional hazards model. p Values <.05 were considered statistically significant.
Results
Associations with NAFLD and LTL
The baseline leucocyte telomeres were shorter in those with NAFLD (p for trend <.001, ). Shorter telomeres associated significantly with increasing age (p<.001), female sex (p<.001), higher body mass index (BMI) (p=.017), coronary heart disease (CHD) (p <.001), higher low-density lipoproteins (LDL) (p=.038), higher total cholesterol (p=.012), HOMA-IR defined insulin resistant (p=.032) and with TM6SF2 non-risk allele carriers for NAFLD (p = 0.038) (). When comparing each LTL tertile, the shortest telomere tertile had statistically significantly more NAFLD subjects when compared to other tertiles (shortest vs. middle tertile: HR 1.66 (95%CI 1.15–2.39, p=.007) and shortest vs. longest tertile: HR 1.98 (95%CI 1.36–2.89, p<.001)). The correlation was significant even when adjusting for age, sex, smoking, BMI, CHD, HOMA-IR and TM6SF2 risk allele carriers ().
Figure 1. Proportion of NAFLD in each telomere tertile. Tertile one represents the shortest telomeres and tertile three the longest telomeres. The unadjusted univariate analysis of proportion of NAFLD in each tertile showed statistically significant difference when comparing the shortest telomere tertile against other tertiles. Reference category was selected to be the tertile with longer telomeres. Multivariate model adjusting for age, sex, BMI, CHD, LDL, HOMA-IR and TM6SF2 as follows: Tertile 1 vs. 3: p = .007 HR 2.03 (95% CI 1.22–3.38); Tertile 1 vs. 2: p = .006 HR 1.98 (95% CI 1.22–3.23); Tertile 2 vs. 3: p = .636 HR 1.13 (95% CI 0.68–1.87). CI: confidence interval; BMI: body mass index; CHD: coronary heart disease; LDL: low-density lipoproteins; HOMA-IR: homeostatic model of assessment of insulin resistance; TM6SF2: transmembrane 6 superfamily member 2.
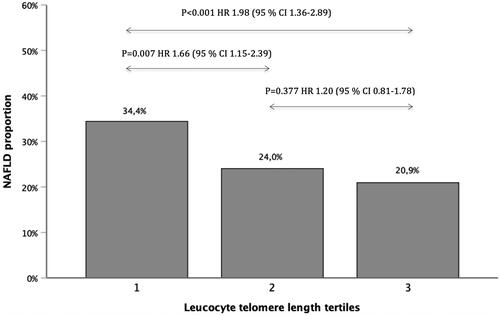
Table 1. Characteristics of the study population according to telomere length.
Associations with LTL and mortality
By the end of 2014, with a mean period of 21 years after enrollment, 185 deaths (27.9% of the study group) occurred. 67 deaths occurred in the NAFLD group (29.9% of NAFLD patients) and 118 in the non-NAFLD group (18.9% of non-NAFLD patients). Thus, NAFLD was associated with increased all-cause mortality HR 1.67 (95% CI 1.24–2.26, p=.01). However, in multivariate analysis, NAFLD was not associated with increased mortality when adjusted for sex, BMI, CHD, diabetes, smoking, age, LTL, MBO and history of stroke, gamma-glutamyltransferase (GGT) (). Baseline longer telomeres were associated with overall decreased mortality risk HR 0.41 (95% CI 0.20–0.84, p = 0.014) but association disappeared after adjustment (). However, relative LTL correlated with overall mortality in the subpopulation of NAFLD even after adjustment HR 0.20 (95% CI 0.04–0.98 p=.045) ().
Table 2. Cox regression analysis for the hazard of death for the whole study group.
Table 3. Cox regression analysis for hazard of death in non-NAFLD and NAFLD subjects.
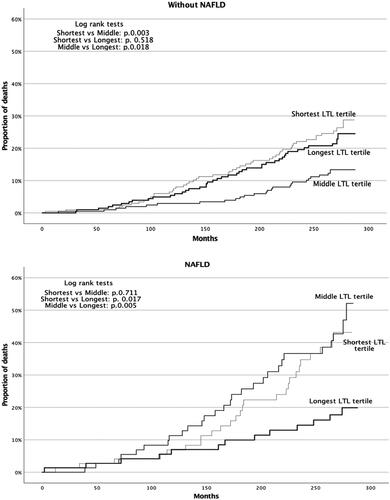
Kaplan–Meier analysis for subgroups of both NAFLD and non-NAFLD showed differing outcomes in mortality in telomere length divided into tertiles. For NAFLD, the survival benefit was evident for the longest telomere tertile followed by shortest tertile and middle tertile with following p values: shortest vs. middle tertile p=.711, shortest vs. longest tertile p=.017, middle vs. longest tertile p=.005. For non-NAFLD population, the Kaplan–Meier survival analysis favored the middle tertile followed by the longest tertile and shortest tertile in that order. p Values for Kaplan–Meier analysis in non-NAFLD population were: shortest vs. middle tertile p=.003, shortest vs. longest tertile p=.518 and middle vs. longest tertile p=.018 ().
Discussion
To our knowledge, this is the first study to investigate the association of mortality and LTL in NAFLD. In our study, LTL in middle-aged participants was associated independently with both prevalence of NAFLD and predicted mortality in NAFLD subjects in a 21-year follow up. Association with mortality in non-NAFLD subgroup was not statistically significant. The difference in mortality in NAFLD subjects was between the longest versus both middle and the shortest tertile.
The inverse association with NAFLD and LTL has been observed in Chinese population and a few studies with population of Western ethnicity with limited generalizability due to small sample sizes or with method in defining NAFLD [Citation12–17]. Two of the studies done in non-Asian ethnicity, that defined NAFLD on basis of alanine aminotransferase elevation with or without combining it to obesity, observed association between LTL and NAFLD, on certain age groups and ethnicity. In a study by Laish et al. similar association, as in our study, was found with LTL and NAFLD in 22 NAFLD patients [Citation12].
LTL is reduced in conditions with oxidative stress and insulin resistance, which, in turn, are involved with pathogenesis and progression of NAFLD as pointed before [Citation11,Citation34]. Sharing the same general pathologic events makes the association of NAFLD and LTL shortening plausible. In addition, several of the comorbidities accompanying NAFLD are known to shorten LTL as well [Citation6,Citation10]. With these comorbidities, there is a complex interplay with NAFLD, and it is therefore difficult to determine to which extent are the actual histopathologic events in the liver, other than fibrosis, responsible for association with shortened of LTL [Citation35]. As liver fibrosis has been associated to shorter LTL itself, it is possible that the association with NAFLD and LTL is actually induced by the individuals with fibrotic livers among our NAFLD participants. This would also explain the mortality increase as suggested in our hypothesis. The hypothesis bases on the earlier observations on association with LTL and liver fibrosis but we cannot verify this in our study group as we are unable to assess the baseline fibrosis. Genetic mutations in the telomerase complex, responsible for maintaining telomere length, have been shown to induce liver fibrosis when accompanied by appropriate environmental factors [Citation8]. These findings have raised a question whether short telomeres act as drivers of fibrosis in other liver diseases such as NAFLD [Citation18].
On the other hand, if LTL is not associated with fibrosis in our cohort, and fibrosis is not the observed driver for mortality, explanation may lie again with the comorbid conditions. By accumulation of these comorbidities, of several associated with shorter LTL solely and some associated with increased mortality, the expected result is further attrition of LTL and observed mortality increase. It is also possible that one of these comorbidities is responsible for the increased mortality in interplay with NAFLD and either LTL directly or the underlying pathology involved with shorter LTL. For example, certain gene defects participating in maintenance of LTL length have shown to associate with increased risk of coronary artery disease (CAD) [Citation10]. It is, therefore, possible that shortened LTL in NAFLD subjects aggravate CAD eventually adding up to mortality. The intriguing finding of TM6SF2 risk allele association with longer telomeres also points toward MBO related NAFLD and CAD to be the issue with the adverse outcomes. TM6SF2 rs641738 has been shown to be associated with liver steatosis and fibrosis of the liver but also protective from CAD [Citation30,Citation36]. Now TM6SF2 risk allele carriers have longer telomeres, increased risk for fibrosis of the liver but less CAD suggesting CAD may be the stronger driver for LTL shortening than liver fibrosis. PNPLA3 and MBOAT7 did not correlate with LTL even though they are also associated with increased risk of liver fibrosis. Without exact knowledge on baseline fibrosis, we cannot analyze further the primary factor behind shorter LTL and mortality in NAFLD subjects.
Of interest is that NAFLD has not been a covariate in studies addressing LTL and mortality. A recent large meta-analysis, resulting in an association with mortality and LTL, covered 25 different studies [Citation9]. In those 25 studies, covariates covered usually age, sex and metabolic traits and diseases such as BMI, CHD and diabetes. NAFLD is a very common condition in general population and accompanied by increased independent all-cause mortality risk as stated before. It is, therefore, possible that some of the mortality increase seen in the studies addressing LTL and mortality may be explained, at least up to a certain point, by the association with NAFLD and LTL seen in our study.
The strength of our study is the vastly examined middle-aged cohort resembling overall Finnish population. The study population consisted of hypertensives and controls with nearly equal proportions. The prevalence of hypertension in Finnish population aged over 30 years in 2000 is about 50% (males 53% and females 46.5%) [Citation37]. Baseline age of the subjects resembles that of everyday practice in hepatology clinics and is appropriate for a long surveillance period and examinations in the factors for mortality. Thoroughly examined metabolic factors, history of illnesses, anthropometric data, NAFLD risk genes, laboratory examinations and history of substance use (alcohol, tobacco) of the subjects offer reliable data on study of covariates. As often in the clinical practice, study used direct ultrasonographic assessment of the liver to visualize echogenicity to determine liver steatosis. This is to be considered as strength of the study, as it has not been done earlier with this magnitude in NAFLD and LTL association studies but as a weakness in overall as ultrasound is not very sensitive in mild steatosis and does not offer reliable quantitation [Citation32].
Unfortunately, we could not assess baseline fibrosis with calculators without Asat-value which was not obtained at the beginning of the study early 1990s. Stratifying NAFLD with fibrosis grades would have given additional information on relations with both LTL with NAFLD and LTL with mortality. The qRT-PCR-method is generally accepted for measuring LTL in cohort studies, but it measures the average LTL irrespective of cell type [Citation33,Citation38]. Given the complex pathology in NAFLD certain type of leucocytes may interact differently within the spectrum of the NAFLD disease and the attrition of cell type may vary [Citation1]. Furthermore, it is unknown whether events in the fatty liver itself are the cause of the attrition of leucocyte telomeres or is the reason another organ or organ system. Of note is also that for this 21-year period, we had to rely on baseline LTL measurement. Although LTL does offer a window for cumulative burden of oxidative stress in a history of one subject, a series of LTL measurements during follow up would have given extended view of LTL and its prognostic value in NAFLD subjects.
We found an independent association with LTL and NAFLD in middle-aged Caucasian cohort. Within NAFLD subjects, shorter LTL was independently associated with overall mortality. Thus, LTL has the potential to be a useful prognostic marker for NAFLD subjects. The mortality of non-NAFLD patients did not associate with LTL. Future studies need to confirm the association on LTL and NAFLD as well as LTL and liver fibrosis. Confirmative studies on mortality with NAFLD should include liver fibrosis grading and in optimal setting, longitudinal LTL measurements and outcome classifications with sufficient sample size.
Acknowledgments
The authors thank Markku Päivänsalo, radiologist, for liver ultrasound examinations and Ms Saija Kortetjärvi, Ms Heidi Häikiö and Ms Leena Ukkola for the excellent technical assistance. Elina Malo, MSc and Meiju Saukko, MSc, are thanked for their co-operation in organizing mortality data.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Parthasarathy G, Revelo X, Malhi H. Pathogenesis of nonalcoholic steatohepatitis: an overview. Hepatol Commun. 2020;4(4):478–492.
- Younossi ZM, Koenig AB, Abdelatif D, et al. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64(1):73–84.
- Estes C, Razavi H, Loomba R, et al. Modeling the epidemic of nonalcoholic fatty liver disease demonstrates an exponential increase in burden of disease. Hepatology. 2018;67(1):123–133.
- Adams LA, Anstee QM, Tilg H, et al. Non-alcoholic fatty liver disease and its relationship with cardiovascular disease and other extrahepatic diseases. Gut. 2017;66(6):1138–1153.
- Liu Y, Zhong G, Tan H, et al. Nonalcoholic fatty liver disease and mortality from all causes, cardiovascular disease, and cancer: a Meta-analysis. Sci Rep. 2019;9(1):11124–11110.
- Cotter TG, Rinella M. Nonalcoholic fatty liver disease 2020: the state of the disease. Gastroenterology. 2020;158(7):1851–1864.
- Kirchner H, Shaheen F, Kalscheuer H, et al. The telomeric complex and metabolic disease. Genes-Basel. 2017;8(7):176.
- Calado RT, Brudno J, Mehta P, et al. Constitutional telomerase mutations are genetic risk factors for cirrhosis. Hepatology. 2011;53(5):1600–1607.
- Wang Q, Zhan Y, Pedersen NL, et al. Telomere length and all-cause mortality: a Meta-analysis. Ageing Res Rev. 2018;48:11–20.
- Blackburn EH, Epel ES, Lin J. Human telomere biology: a contributory and interactive factor in aging, disease risks, and protection. Science. 2015;350(6265):1193–1198.
- Demissie S, Levy D, Benjamin EJ, et al. Insulin resistance, oxidative stress, hypertension, and leukocyte telomere length in men from the Framingham heart study. Aging Cell. 2006;5(4):325–330.
- Laish I, Mannasse-Green B, Hadary R, et al. Telomere dysfunction in nonalcoholic fatty liver disease and cryptogenic cirrhosis. Cytogenet Genome Res. 2016;150(2):93–99.
- Zhang M, Hu M, Huang J, et al. Association of leukocyte telomere length with non-alcoholic fatty liver disease in patients with type 2 diabetes. Chin Med J. 2019;132(24):2927–2933.
- Ping F, Li Z, Lv K, et al. Deoxyribonucleic acid telomere length shortening can predict the incidence of non-alcoholic fatty liver disease in patients with type 2 diabetes mellitus. J Diabetes Investig. 2017;8(2):174–180.
- Wojcicki JM, Rehkopf D, Epel E, et al. Shorter leukocyte telomere length in relation to presumed nonalcoholic fatty liver disease in Mexican-American men in NHANES 1999–2002. Int J Hepatol. 2017;2017:8435178.
- Kim D, Li AA, Ahmed A. Leucocyte telomere shortening is associated with nonalcoholic fatty liver disease‐related advanced fibrosis. Liver Int. 2018;38(10):1839–1848.
- Zhang J, Zang S, Yang W, et al. Shortened leucocyte telomere length is associated independently with fibrosis stage in non-alcoholic fatty liver disease. Int J Clin Exp Med. 2017;10(7):11204–11212.
- Barnard A, Moch A, Saab S. Relationship between telomere maintenance and liver disease. Gut Liver. 2019;13(1):11–15.
- Carulli L. Telomere shortening as genetic risk factor of liver cirrhosis. World J Gastroenterol. 2015;21(2):379–383.
- Nault J, Ningarhari M, Rebouissou S, et al. The role of telomeres and telomerase in cirrhosis and liver cancer. Nat Rev Gastroenterol Hepatol. 2019;16(9):544–558.
- Donati B, Valenti L. Telomeres, NAFLD and chronic liver disease. Int J Mol Sci. 2016;17(3):383.
- Dong K, Zhang Y, Huang JJ, et al. Shorter leucocyte telomere length as a potential biomarker for nonalcoholic fatty liver disease-related advanced fibrosis in T2DM patients. Ann Transl Med. 2020;8(6):308.
- Angulo P, Kleiner DE, Dam-Larsen S, et al. Liver fibrosis, but no other histologic features, is associated with long-term outcomes of patients with nonalcoholic fatty liver disease. Gastroenterology. 2015;149(2):389–397.
- Ekstedt M, Hagström H, Nasr P, et al. Fibrosis stage is the strongest predictor for disease-specific mortality in NAFLD after up to 33 years of follow-up. Hepatology. 2015;61(5):1547–1554.
- Dulai PS, Singh S, Patel J, et al. Increased risk of mortality by fibrosis stage in nonalcoholic fatty liver disease: systematic review and Meta-analysis. Hepatology. 2017;65(5):1557–1565.
- International Diabetes Federation. The IDF consensus worldwide definition of the metabolic syndrome. 2006. Available from: https://www.idf.org/e-library/consensus-statements/60-idfconsensus-worldwide-definitionof-the-metabolic-syndrome.html
- Matthews DR, Hosker JP, Rudenski AS, et al. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–419.
- Sookoian S, Pirola CJ. Meta-analysis of the influence of I148M variant of patatin-like phospholipase domain containing 3 gene (PNPLA3) on the susceptibility and histological severity of nonalcoholic fatty liver disease. Hepatology. 2011;53(6):1883–1894.
- Liu YL, Reeves HL, Burt AD, et al. TM6SF2 rs58542926 influences hepatic fibrosis progression in patients with non-alcoholic fatty liver disease. Nat Commun. 2014;5:4309.
- Dongiovanni P, Petta S, Maglio C, et al. Transmembrane 6 superfamily member 2 gene variant disentangles nonalcoholic steatohepatitis from cardiovascular disease. Hepatology. 2015;61(2):506–514.
- Mancina RM, Dongiovanni P, Petta S, et al. The MBOAT7-TMC4 variant rs641738 increases risk of nonalcoholic fatty liver disease in individuals of European descent. Gastroenterology. 2016;150(5):1219–1230.
- Li Q, Dhyani M, Grajo JR, et al. Current status of imaging in nonalcoholic fatty liver disease. World J Hepatol. 2018;10(8):530–542.
- Cawthon RM. Telomere length measurement by a novel monochrome multiplex quantitative PCR method. Nucleic Acids Res. 2009;37(3):e21.
- Méndez-Sánchez N, Arrese M, Zamora-Valdés D, et al. Current concepts in the pathogenesis of nonalcoholic fatty liver disease. Liver Int. 2007;27(4):423–433.
- Gehrke N, Schattenberg JM. Metabolic inflammation-A role for hepatic inflammatory pathways as drivers of comorbidities in nonalcoholic fatty liver disease? Gastroenterology. 2020;158(7):1929–1947.
- Pirola CJ, Sookoian S. The dual and opposite role of the TM6SF2-rs58542926 variant in protecting against cardiovascular disease and conferring risk for nonalcoholic fatty liver: a Meta-analysis. Hepatology. 2015;62(6):1742–1756.
- Antti J, Veikko S, Arpo A. Terveys, toimintakyky ja hyvinvointi suomessa 2011. Tampere, Finland: National Institute of Health and Welfare; 2011. p. 7–69.
- Mather KA, Jorm AF, Parslow RA, et al. Is telomere length a biomarker of aging? A review. J Gerontol A Biol Sci Med Sci. 2011;66(2):202–213.