Abstract
The prognosis of unstable angina pectoris (UAP) differs from non-ST-segment elevation myocardial infarction, and percutaneous coronary intervention (PCI) is considered to improve outcomes of UAP. This study aimed to assess the prognostic value of uric acid to albumin ratio (UAR) for long-term mortality in UAP patients after PCI. Our study retrospectively enrolled 2298 patients hospitalized because of UAP in a tertiary hospital. Divided by medium UAR, the patients were classified into two groups. Baseline demographics, clinical features and laboratory characteristics were obtained from medical records. Post-discharge follow-up was performed either in outdoor clinic or through phone call. The primary endpoint in this study was cardiac death, while all-cause death and rehospitalization were designated as the secondary endpoints. The median follow-up time was 672 days. Among all patients, 58 (2.5%) died, 28 of which died of cardiac deaths (1.2%), and 467 were re-hospitalized (20.3%). Cardiac mortality and all-cause mortality were found to be significantly higher in the high UAR group than in the low UAR group (p = 0.007, p < 0.001), and Kaplan–Meier analysis showed patients with higher UAR may suffer from worse outcomes (p = 0.020). UAR, PCI history, and age were identified as independent predictors of cardiac mortality by multivariate Cox regression. A UAR value of >8.35 was demonstrated as an ideal cut-off point to predict post-PCI cardiac mortality (p <0.001). Overall, it is indicated that baseline UAR was independently correlated with long-term cardiac mortality in patients with UAP treated by PCI.
Introduction
Non-ST-segment elevation acute coronary syndrome (NST-ACS) is a subtype of severe coronary heart disease associated with high morbidity and mortality, despite absence of the ST-elevation on electrocardiogram (ECG) [Citation1]. Unstable angina pectoris (UAP) and non-ST-segment elevation myocardial infarction (NSTEMI) are usually discussed in similar contexts because clinical and ECG features are indistinguishable between two disease conditions. However, the severity of ischemia is considered as the major difference, and the patients with NSTEMI experience tissue ischemia severely enough to cause myocardial damage leading to detectable elevations of myocardial injury biomarkers. Patients with elevated cardiac biomarkers or diagnosed with NSTEMI are always categorized as high-risk with the need of early revascularization, such as percutaneous coronary intervention (PCI). In contrast, UAP often endorses better prognosis, and in a three-year study, post-PCI mortality and the risk of new-onset myocardial infarction were found to be lower in UA than in NSTEMI [Citation2]. Nonetheless, mortality in patients with UAP remains markedly higher than in patients with stable angina [Citation2], and the long-term risk of UAP needs to be evaluated with caution.
The Global Registry of Acute Coronary Events (GRACE) risk score is recommended as a useful tool for risk evaluation in NST-ACS [Citation3]. However, because the risk levels of UAP and NSTEMI are different, there is still lack of an index or a system to specifically evaluate the prognosis of UAP. Besides, the GRACE score is sometimes inconvenient because of complicated indexes included, limiting its application in clinical practice. Recent studies reported that high uric acid level and hypoalbuminemia were related to adverse clinical outcomes in patients with acute coronary syndrome (ACS) [Citation4,Citation5]. However, the prognostic value of the combination of the two markers is unknown, especially in the UAP population. To address this issue, this study investigated a simple clinical index, the uric acid-to-albumin ratio (UAR) and its predictive value for long-term cardiac mortality in UAP patients treated with PCI.
Methods
Study design and population
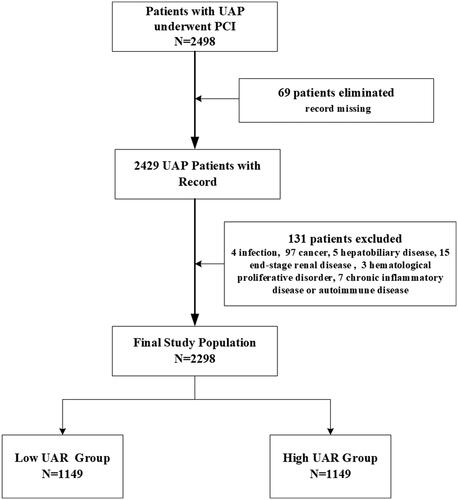
A total of 2498 consecutive cases of UAP admitted to the department of cardiology, Beijing Friendship Hospital, from January 2013 to December 2018, were enrolled retrospectively. The protocol was approved by the ethical committee of Beijing Friendship Hospital. Patients were included in the study if they fulfilled the following criteria, which was consistent with previous studies [Citation6] and clinical guidelines [Citation1]: (1) diagnosed with UAP based on typical chest pain at rest or on minimal exertion, with evidence of myocardial ischemia (ECG changes, including persistent or transient ST-segment depression, T-wave inversion or pseudo-normalization of T waves, or normal ECG) but without persistent ST-segment elevation. (2) PCI procedures were performed. Among these patients, 200 were excluded due to clinical complications, including active infectious diseases, untreated malignancies, ongoing hepatobiliary disorders, end-stage renal disease, hematologic proliferative disorders, chronic inflammatory diseases, and autoimmune diseases, or due to the absence of laboratory data on admission (). Finally, a total of 2298 patients were included and were divide into two groups by medium UAR value.
Clinical data collection
Medical records of each patient were carefully reviewed. Past medical history of previous PCI, hypertension, diabetic mellitus, dyslipidemia, stroke, end-stage renal disease, cancer, etc. were gathered to identify possible risk factors and exclusive criteria. Besides, the newly diagnosed comorbidities, especially those related to exclusion criteria, were also identified via collecting discharge notes from medical records, as we previously published [Citation7]. Patient vitals, such as the first records of systolic and diastolic blood pressure, heart rate, and body mass index (BMI) after admission were collected for further analysis. After standard dual antiplatelet therapy, all patients received PCI performed with standard techniques and appropriate strategies under the discretion of attending physicians. During hospitalization and after discharge, all patients were reminded and received secondary prevention such as antiplatelet medications, statins, β-blockers, and angiotensin-converting enzyme inhibitors in accordance with the guidelines, unless contraindicated.
Follow-up and clinical endpoints
All patients were informed and scheduled for follow-up on the first, third, and six post-discharge months, as well as the first and the second years. Earlier follow-up visits were also conducted if there were emerging symptoms or unsuspected conditions. Follow-up data were collected from medical records, outpatient services, and telephone calls by the investigators, including medical treatments, adverse events, rehospitalizations, deaths, and causes of death. According to previous studies, cardiac deaths, which served as the primary endpoint in this study, were defined as deaths from acute myocardial infarction, heart failure, arrhythmia, and unexplained sudden deaths [Citation7], whereas all-cause deaths, defined as deaths from any cause during follow-up, as well as rehospitalizations, were designated as secondary clinical endpoints.
Laboratory analysis and UAR calculation
Peripheral blood samples were obtained promptly after hospitalization. Uric acid and serum albumin level were measured within an hour using the AU5800 biochemistry analysis system (Beckman Coulter Company). The UAR was calculated as uric acid level (μmol/L) divided by albumin level (g/L) from the same sample drawn on admission. Other blood biochemistry parameters and routine complete blood count were examined by automatic analyzers. Variables related to fat and glucose metabolism such as total cholesterol, low density lipoprotein cholesterol, high density lipoprotein cholesterol, triglycerides, glycated hemoglobin (HbA1c) were measured from fasting blood samples collected on the morning after admission. Left ventricular ejection fraction (LVEF) was calculated by the Simpson method before PCI by experienced ultrasound physicians, for quantitative assessment of the left ventricular systolic function.
Statistical analysis
Qualitative variables were expressed as percentages and quantitative variables as mean ± standard deviation or median (interquartile range). For continuous variables, assumption of normality was tested with Kolmogorov–Smirnov tests, and differences between the two groups were analysed with Student T test, Chi-square test, or Mann–Whitney U test, when appropriate. Univariate Cox regression analysis was employed to identify factors related to cardiac mortality. For variables tested significant in the univariate regression analysis and those of clinical importance, multivariate Cox regression analysis was adopted to identify independent predictors of cardiac mortality. Receiver operating characteristic curve analysis was performed to define the cut-off value of UAR for long-term cardiac mortality. To evaluate added predictive ability of UAR to the GRACE score, the C-index, net reclassification improvement (NRI), and integrated discrimination improvement (IDI) were calculated and analysed with Hosmer–Lemeshow test. Statistical significance was defined as a p value of <0.05. Event-free survival curves were created via Kaplan–Meier method. Statistical analyses were performed on SPSS (version 24.0, Chicago, Illinois).
Results
All 2298 recruited patients were divided into two groups according to the UAR on admission, with overall median UAR being 8.38. Higher proportions of male patients and smokers, as well as a lower proportion of diabetic patients and higher BMI, haemoglobin, platelet distribution width, creatinine, alanine aminotransferase, triglyceride, uric acid, and high sensitivity C-reactive protein (HsCRP) levels, were observed in the high UAR group, compared to the low UAR group, with more details on baseline clinical, laboratory, and demographic characteristics summarized in .
Table 1. Basic demographic, clinical, and laboratory factors of the binary UAR groups.
A total of 58 patients (2.7%) were deceased during follow-up, including cardiac deaths in 28 patients (1.3%). Cardiac mortality and all-cause mortality in the high UAR group were significantly higher compared to the low UAR group (cardiac mortality, 2.0% vs. 0.7%, p = 0.007; all-cause mortality, 3.5% vs. 1.9%, p < 0.001; ). Although readmission rate was higher in the high UAR group than in the low UAR group, the trend was of no statistical significance.
Table 2. Clinical outcomes and follow-up rate of the binary UAR groups.
Ten risk factors, including those exhibiting statistical significance in univariate analysis and those of vital clinical importance (age, gender, PCI history, systolic blood pressure, heart rate, BMI, haemoglobin, hsCRP, HbA1c, and UAR), were further analysed with multivariate regression, where creatinine was not included due to its close correlation to uric acid level. A significant association between high UAR levels and the adjusted risk of long-term cardiac mortality was revealed (odds ratio [OR] 1.255, 95% confidence interval [CI] 1.076–1.463, p = 0.004, ) on multivariate analysis. In addition, age and PCI history were also recognized as independent risk factors for cardiac mortality. A UAR value of 8.35 was demonstrated to be a valid cut-off for risks of long-term cardiac mortality in patients with UAP after PCI by receiver operating characteristic curve (ROC) analysis (area under the curve, AUC = 0.70, 95% CI 0.59–0.81, p < 0.001; ). Kaplan–Meier analysis for event-free survival revealed higher occurrence of cardiac mortality in the high UAR group (p = 0.020; ).
Figure 2. (A) Receiver operating characteristic curve of uric acid to albumin ratio value. (B) Kaplan–Maier curves for cardiac death between the binary groups.
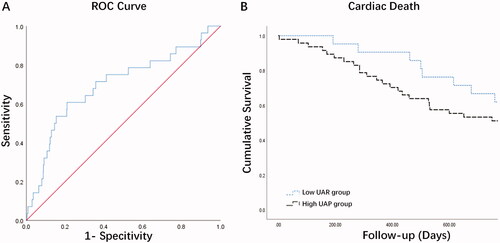
Table 3. Effects of multiple factors on cardiac mortality in univariate and multivariate COX regression.
UAR significantly enhanced the predictive value of the GRACE score for cardiac mortality. The C-index for UAR with GRACE score was increased compared with the C-index for GRACE score (0.74, 95% CI 0.61–0.82 vs. 0.69, 95% CI 0.58–0.80, p=.001). Adding UAR significantly improved the reclassification of patients beyond the GRACE score (NRI: 0.186, 95% CI 0.011–0.452; p = 0.030). Besides, addition of UAR also improved IDI. (0.011, 95% CI 0.003–0.022; p = 0.006).
Discussion
In this study, the UAR value on admission was demonstrated to be an independent predictor of long-term cardiac mortality after PCI in patients with UAP. To our knowledge, there have been few studies focusing on the association of UAR with long-term prognosis in patients with UAP after PCI.
Among cardiovascular diseases, ACS endorses the highest mortality, while UAP accounts for approximately one-fourth of ACS events [Citation8]. In most previous studies, UAP and NSTEMI were considered together as one single factor [Citation9,Citation10], leading to the lack of analysis specifically for the prognosis of UAP and thus a limited understanding of this issue. Besides, the majority of previous studies on selective invasive strategy have high crossover rates from control to interventional groups, so there is still a considerable uncertainty in the prognosis of UAP after invasive treatment [Citation2,Citation11]. As suggested by previous data, UAP is associated with better outcomes than NSTEMI, but with noticeably worse outcomes than stable angina [Citation2,Citation12]. Therefore, to specifically address the concerns on the post-interventional prognosis of UAP, further risk evaluation is required, which is helpful with the treatment strategies and the allocation of medical resources. Serum uric acid and albumin are clinical routines that could be acquired timely and easily with low extra health cost, and their combination could become a feasible index for assessing the risk of cardiac mortality in patients with UAP as indicated by our study. Furthermore, as clinical physicians, we may be faceted with circumstances where imaging or more laboratory test are not readily available, provided biochemical tests are performed, UAR would be adopted as an effective predictive method for risk stratification. This finding is useful especially for particular clinical settings where access to medical technical support is insufficient, for example, underdeveloped areas or where frequent follow-ups are needed.
In our study, elevated serum uric acid level was found to be associated with cardiac mortality. Although uric acid has been recognized as an antioxidant in experimental studies, it is also known that uric acid induces inflammation in vascular endothelial and smooth muscle cells, in addition to intracellular oxidative stress, leading to endothelial dysfunction [Citation13]. High uric acid level is significantly related to coronary artery calcification in the early stage of chronic kidney disease, but not in advanced stages [Citation14]. These findings illuminate possible pathophysiological mechanisms in the association between uric acid and the presence, severity, and prognosis of coronary artery disease (CAD). In the setting of clinical practice, serum uric acid level was further revealed in multiple studies to aid in the secondary prevention of stable CAD, by assisting the evaluation of the severity and complexity of coronary lesions in chronic coronary syndrome [Citation15,Citation16], as well as the estimation of total and cardiovascular mortality [Citation17]. Consistently, in UAP patients, the association between uric acid and cardiac biomarkers and its predictive value in short-term prognosis were reported in previous studies [Citation4,Citation18], which also supported the results of our study.
Low serum albumin level is recognized as a prognostic factor for new-onset heart failure and in-hospital mortality in patients with ACS, and its correlation with status of malnutrition and inflammation might be possible mechanisms [Citation5,Citation19,Citation20]. In patients undergoing PCI, hypoalbuminemia together with high uric acid, leads to a synergistic adverse effect on the risk of long-term major adverse cardiovascular events [Citation21]. As a result, albumin level is often incorporated in bivariate biomarkers, for example, the C-reactivity protein to albumin ratio (CAR). As a novel inflammatory marker, CAR can reliably evaluate CAD severity in NSTEMI patients and predict no-reflow phenomenon and short-term prognosis in patients with ST-segment elevation myocardial infarction [Citation22,Citation23]. In comparison, UAR is another novel compositive index, reflecting metabolic dysfunction, inflammation, and malnutrition, whose role in clinical practice has been examined only by a very limited number of studies, revealing its predictive value for short-term mortality in patients with acute kidney injury [Citation24]. In our study, high UAR level was found to be associated long-term cardiac mortality in UAP patients undergoing PCI. Similarly, a recent study demonstrated that high UAR level was a reliable marker in the prediction of the extent of CAD (syntax score) in NSTEMI patients [Citation25]. Although the aforementioned study was conducted in the NSTEMI population rather than UAP, it might indirectly provide explanation for the finding that UAR was able to predict the prognosis.
In ACS patients, impaired renal function is definitely a risk factor for long-term mortality. Although creatinine level was found to be significantly associated with cardiac mortality in our study, it was not included in the multivariate regression analysis because of its close correlation to uric acid. Moreover, gender was not demonstrated significantly related to cardiac mortality in the current study. However, because gender was found to affect the selection of treatment regimen and prognosis in our previous study with a larger sample size authored by Xu Min [Citation26], it was still included in the multivariate regression analysis. After the adjustment of risk factors, UAR remained an independent prognostic factor for long-term cardiac mortality.
Limitations
The UAR, a simple composite marker that could be quickly calculated, reflects the status of metabolism dysfunction and inflammation. Similarly, monocyte to high-density lipoprotein ratio, neutrophil-to-lymphocyte ratio, platelet-leukocyte aggregates, red cell distribution width and CAR are all indexed related to inflammation. These kinds of markers including UAR possess the advantages of objective laboratory variables, and they can be easily acquired from initial serum biochemical analyses routinely performed in ACS population in clinical practice. Nonetheless, the clinical outcomes of UAP are most likely determined by many factors such as advanced age, syntax score, treatment strategy of PCI, diabetic mellitus, renal failure, etc. In a word, the more key variables a risk model involves, the more precisely it predicts. Some mature scoring systems have already been developed, such as the GRACE score that takes multiple factors into account [Citation3]. Limited by the current data, we did not compare the prognostic value of UAR between patients with UAP and those with NSTEMI. Because a weakness of the study is retrospective design, future perspective studies are expected to provide more information in these aspects.
Conclusion
High baseline UAR was identified as an independent predictor of two-year cardiac mortality in patients with UAP receiving PCI. Our results suggested that more intensive follow-up management should be considered for UAP patients with high UAR value because of its close association with cardiovascular mortality. Further studies are still required to confirm and illuminate clinical implications of this finding.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Collet J-P, Thiele H, Barbato E, et al. 2020 ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: the task force for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J. 2020;42(14):1289–1367.
- Piątek Ł, Janion-Sadowska A, Piątek K, et al. Long-term clinical outcomes in patients with unstable angina undergoing percutaneous coronary interventions in a contemporary registry data from Poland. Coron Artery Dis. 2020;31(3):215–221.
- Chan Pin Yin D, Azzahhafi J, James S. Risk assessment using risk scores in patients with acute coronary syndrome. JCM. 2020; 9(9):3039.
- Magnoni M, Berteotti M, Ceriotti F, et al. Serum uric acid on admission predicts in-hospital mortality in patients with acute coronary syndrome. Int J Cardiol. 2017;240:25–29.
- Gonzalez-Pacheco H, Amezcua-Guerra LM, Sandoval J, et al. Prognostic implications of serum albumin levels in patients with acute coronary syndromes. Am J Cardiol. 2017;119(7):951–958.
- Cakal S, Cakal B, Güven Z, et al. Switching ticagrelor to 600 mg or 300 mg clopidogrel loading bridge in patients with unstable angina. JCM. 2021;10(11):2463.
- Cui H, Zhang X, Ding X, et al. Urinary Alpha1-Microglobulin: a new predictor for in-Hospital mortality in patients with ST-Segment elevation myocardial infarction. Med Sci Monit. 2021;27:e927958.
- Wang Z, Wang J, Cao D, et al. Correlation of neutrophil-to-lymphocyte ratio with the prognosis of non-ST-segment elevation in patients with acute coronary syndrome undergoing selective percutaneous coronary intervention. J Int Med Res. 2020;48(10):300060520959510. Oct
- Glaser R, Selzer F, Jacobs AK, et al. Effect of gender on prognosis following percutaneous coronary intervention for stable angina pectoris and acute coronary syndromes. Am J Cardiol. 2006;98(11):1446–1450.
- Vicent L, Ariza-Solé A, Díez-Villanueva P, et al. Statin treatment and prognosis of elderly patients discharged after Non-ST segment elevation acute coronary syndrome. Cardiology. 2019;143(1-2):14–21.
- Vogrin S, Harper R, Paratz E, et al. Comparative effectiveness of routine invasive coronary angiography for managing unstable angina. Ann Intern Med. 2017;166(11):783–791.
- Braunwald E, Morrow DA. Unstable angina: is it time for a requiem? Circulation. 2013;127(24):2452–2457.
- Saito Y, Tanaka A, Node K, et al. Uric acid and cardiovascular disease: a clinical review. J Cardiol. 2021;78(1):51–57.
- Han M, Kim H, Kim HJ, et al. Serum uric acid is associated with coronary artery calcification in early chronic kidney disease: a cross-sectional study. BMC Nephrol. 2021;22(1):247.
- Kumbhalkar S, Deotale R. Association between serum uric acid level with presence and severity of coronary artery disease. J Assoc Physicians India. 2019;67(4):29–32.
- Maloberti A, Bossi I, Tassistro E, et al. Uric acid in chronic coronary syndromes: Relationship with coronary artery disease severity and left ventricular diastolic parameter. Nutr Metab Cardiovasc Dis. 2021;31(5):1501–1508.
- Mozzini C, Girelli D, Setti A, et al. Serum uric acid levels, but not rs7442295 polymorphism of SCL2A9 gene, predict mortality in clinically stable coronary artery disease. Curr Probl Cardiol. 2021;46(5):100798.
- Wei H, Liu Y, Wen H. Association between uric acid and N-Terminal Pro-B-Type natriuretic peptide in patients with unstable angina pectoris. Am J Med Sci. 2020;360(1):64–71.
- Yang LJ, Feng YX, Li T, et al. Serum albumin levels might be an adverse predictor of long term mortality in patients with acute myocardial infarction. Int J Cardiol. 2016;223:647–648.
- Polat N, Oylumlu M, Işik MA, et al. Prognostic significance of serum albumin in patients with acute coronary syndrome. Angiology. 2020;71(10):903–908.
- Wada H, Dohi T, Miyauchi K, et al. Independent and combined effects of serum albumin and C-reactive protein on long-term outcomes of patients undergoing percutaneous coronary intervention. Circ J. 2017;81(9):1293–1300.
- Cinar T, Cagdas M, Rencuzogullari I, et al. Prognostic efficacy of C-reactive protein/albumin ratio in ST elevation myocardial infarction. Scand Cardiovasc J. 2019;53(2):83–90.
- Karabağ Y, Çağdaş M, Rencuzogullari I, et al. Usefulness of the C-Reactive protein/albumin ratio for predicting No-Reflow in ST-elevation myocardial infarction treated with primary percutaneous coronary intervention. Eur J Clin Invest. 2018; 48(6):e12928.
- Özgür Y, Akın S, Yılmaz NG, et al. Uric acid albumin ratio as a predictive marker of short-term mortality in patients with acute kidney injury. Clin Exp Emerg Med. 2021;8(2):82–88.
- Çakmak E, Bayam E, Çelik M, et al. Uric acid-to-Albumin ratio: a novel marker for the extent of coronary artery disease in patients with Non-ST-Elevated myocardial infarction. Pulse. 2020;8(3-4):99–107. )
- Xu M, Li HW, Chen H, et al. Sex and age differences in patients with unstable angina pectoris: a single-center retrospective study. Am J Med Sci. 2020;360(3):268–278.