Abstract
Persisting inflammation has been discovered in lungs and other parenchymatous organs of some COVID-19 convalescents. Calprotectin, neutrophil extracellular traps (NETs), syndecan-1 and neopterin are general key inflammatory markers, and systemically enhanced levels of them may remain after the COVID-19 infection. These inflammatory markers were therefore measured in serum samples of 129 COVID-19 convalescent and 27 healthy blood donors or employees at Oslo Blood bank, Norway. Also antibodies against SARS-CoV-2 nucleocapsid antigen were measured, and timing of sampling and severity of infection noted. Whereas neopterin and NETs values remained low and those for syndecan-1 were not raised to statistically significant level, concentrations for calprotectin, as measured by a novel mixed monoclonal assay, were significantly increased in the convalescents. Antibodies against SARS-CoV-2 nucleocapsid antigen were elevated, but did not correlate with levels of inflammatory markers. Difference between the groups in only one biomarker makes evaluation of ongoing or residual inflammation in the convalescents difficult. If there is a low-grade inflammation, it would in that case involve neutrophils.
Introduction
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)/Coronavirus disease 2019 (COVID-19) pandemic originating in Wuhan, China, in December 2019, is still on-going over two years later but under control in countries with high vaccination rate, despite recent occurrence of far more contagious Omicron variants. The clinical state of the disease has ranged from very mild flu-like symptoms to death from lung ingestion and immunothrombosis, where neutrophil extracellular traps (NETs) have been considered as driver of endothelial damage [Citation1]. Moreover, it has been discovered that inflammation can persist in lungs, mediastinal lymph nodes, spleen and liver in convalescing COVID-19 patients with repeated negative corona RT-PCR test [Citation2]. Although most convalescents initially seem to have a clinically mild post-COVID course [Citation3], the inflammation is persistent over time and gives different signs and symptoms for a varying number of subjects [Citation4].
Calprotectin is a major component in NETs [Citation5], which are generated in various diseases to trap and destroy invading pathogens in the extracellular space. Calprotectin, NETs, syndecan and neopterin are key inflammatory markers with patho-physiological effects:
L1 protein, the main cytosolic protein in neutrophil granulocytes and monocytes [Citation6], was later called calprotectin when its calcium- and zinc-binding and antimicrobial properties were discovered [Citation7]. The metal-binding mechanism limits essential nutrients and hence growth of invading pathogens [Citation8]. Extracellular calprotectin is increased in patients with rheumatoid arthritis and inflammatory bowel disease [Citation9,Citation10]. Furthermore, the protein has strong apoptosis-inducing activity against cells in culture [Citation11]. Elevated calprotectin levels are also found in severe COVID-19 disease, which suggests neutrophil involvement in inflammation and respiratory compromise in COVID-19 [Citation12].
Elevated NETs levels have also been found in hospitalized COVID-19 patients [Citation13]. NETs can block capillary circulation [Citation14], and have been associated with thrombosis formation in COVID-19 [Citation15]. However, DNase may disrupt NETs by degrading their content of DNA [Citation16].
Syndecans represent a family of cell surface proteoglycans carrying covalently attached glycosaminoglycan chains of the heparan sulphate or chondroitin sulphate type in the extracellular domain of the protein. The protein part is transmembrane and the cytosolic part is actively engaged in signal transduction [Citation17]. There are five different syndecans, syndecan 1–5, with differences in cell and tissue distribution. The different domains of syndecans contribute to their functions as links between the extracellular and intracellular environment and their dynamic involvement in normal and pathological conditions. Shedding of syndecans has been linked to several types of diseases [Citation18,Citation19]. Recently, shedding of syndecan-1 has also been studied in relation to the severity of COVID-19 complications [Citation20].
Neopterin is an antioxidant produced by INFγ-stimulated mononuclear phagocytes [Citation21]. Its function is to protect the cells against toxic oxidants. Increased neopterin levels are seen in a wide range of conditions including cancer, autoimmune diseases and viral infections [Citation21], including COVID-19 [Citation22]. Neopterin has been used in Austrian blood banks as an unspecific marker for emerging novel viral infections [Citation23,Citation24].
The immune response to COVID-19 infection can be monitored by serum levels of antibodies to the nucleocapsid antigen (N) of SARS-CoV-2 [Citation25]. The concentration of these antibodies has been shown to positively correlate with concentration of neutralizing antibodies in neutralization assays [Citation26]. Besides the COVID-19 antigen or PCR assays, increased levels of such N antibodies are also proof of previous COVID-19 infection.
Thus, in COVID-19 disease there are reports of elevated levels of both calprotectin, NETs, syndecan-1 and neopterin. Since persisting inflammation has been discovered in COVID-19 convalescents, the purpose of this paper was to use key inflammatory markers to examine whether a systemic inflammatory response normally still remains in the aftermath of COVID-19 infection, similar to the antibody response, in previously healthy blood donors.
Materials and methods
Blood samples from COVID-19 convalescent blood donors (n = 129), as documented for most* participants by positive SARS-CoV-2 PCR test, and healthy employees with a negative test (n = 20) were collected in April and May 2020, from subjects who had registered in Koronastudien.no, and from current SARS-CoV-2 negative blood donors (n = 7) [Citation27]. All participants were asked to participate and give consent to research through Koronastudien.no [Citation28]. Convalescent blood donors were sampled minimum 2 weeks after (increased to 4 weeks in mid-May), but mostly 3 weeks (median value) after complete resolution of COVID-related symptoms or positive PCR test, with the intention to identify donors for convalescent plasma production. They, therefore, only donated blood for research at this particular occasion. *Not all participants included had been tested with PCR because the test was not available to all at the beginning of the epidemic when the current material was collected. Population characteristics among the 61 convalescents who we were able to reach, is given in . Controls were similar relative to gender (most women) but younger, median age about 35 years. Symptoms volunteered by convalescents (n = 12): loss of smell or taste, nose congestion, cough, shortness of breath, fever, throat ache, stomach ache, muscle ache, head ache, chest pain, nausea and diarrhea.
Table 1. Characteristics of COVID-19 convalescent blood donors, median and (range).
Serum was analyzed at the Department of Microbiology, Oslo University Hospital, where a commercial anti-SARS-CoV-2 assay is performed (Roche). Remnants of serum were used in the current study and number of samples analyzed varied somewhat between assays due to availability.
Calprotectin mixed monoclonal assay
A novel calprotectin ELISA based on a mixture of monoclonal antibodies was established [Citation29]. Since a considerable proportion of calprotectin in biological materials contain both histone and DNA fragments, a combination of several, different monoclonals will be necessary to obtain a reliable assay. The monoclonals here were selected so that the mixture reacted with all fractions obtained from chromatography of stool extracts from patients with inflammatory bowel syndrome. The mixed monoclonal (MiMo) antibodies were used both for coating of microwells and preparation of a horse radish peroxidase (HRP)-conjugate. The assay range of the ELISA was from 5 to 1000 ng/mL with a CV of about 10% for the standards.
NETs dual hybrid ELISAs
In brief, separate wells in Maxisorp (NUNC) 96-well microplates were coated with rabbit anti-Histone 3 (H3) (produced by Agrisera AB, Sweden, by immunization with synthetic histone 3 typical peptides) and anti-S100A12 (produced by Genovac GmbH, by immunization of mice with recombinant S100A12 produced by the same company). The monoclonal anti-calprotectin antibodies were all conjugated with HRP. The wells were coated either with anti-H3, anti-S100A12 or anti-calprotectin, the latter to establish a standard curve for bound calprotectin in complexes with histone 3 or S100A12 [Citation30].
Syndecan-1
Syndecan-1 was measured using commercial ELISA kits detecting human syndecan-1 (Diaclone, 950.640.192).
Neopterin
Neopterin ELISA kit (DEIA 1640) used was from Creative Diagnostics®.
CRP
C-reactive protein (CRP) in serum was analyzed by immune-turbidimetric method by use of Cobas 8000 instrument and standards from Roche Diagnostics.
Antibodies against SARS-CoV-2 nucleocapsid (N) antigen
The Elecsys® Anti-SARS-CoV-2 - Roche (Basel, Switzerland) Diagnostics CE-marked commercial assay was used to test serum samples from all participants. The assay uses a recombinant protein representing the nucleocapsid (N) antigen for the determination of antibodies against SARS-CoV-2. The result of a sample is given either as reactive, ≥100 s/co or non-reactive < 100 s/co according to the manufacturer’s recommendations.
Statistics
t-test was used when data was normally distributed, and Wilcoxon when not, as determined by statistical programs Jandel SigmaStat/Plot or GraphPad Prism 8.4.3. Pearsson correlation was used for comparison of normally distributed data, and otherwise Spearman correlation was applied. Differences with p < .05 were considered statistically significant.
Results
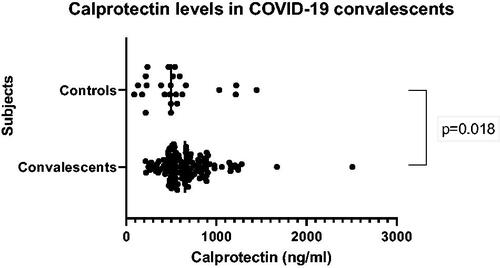
Inflammatory markers were examined in serum of blood donors who had undergone COVID-19 infection and compared with those who had not been affected by the infection; healthy blood donors and employees at the blood bank. Calprotectin levels were found to be significantly higher in the convalescents than in controls (p = .018) (), but the difference was modest (24%) and within normal values. H3-NETs was not discovered and A12-NETs was only detected (63 ng/mL) in one of the convalescents who also had high values of calprotectin (10,500 ng/mL) and syndecan-1 (291 ng/mL).
Figure 1. Levels of calprotectin measured by novel mixed monoclonal assay in COVID-19 convalescent blood donors and non-COVID-19 controls.
Levels for syndecan-1 were not statistically increased compared with controls (). There was a positive correlation between calprotectin and syndecan-1 values in the convalescents (rr = 0.227, p = .009), but it was too low to be of interest (). There were, as expected, highly elevated levels (mean 46.9 ± 32.2, range 1.3–130.0 S/Co) of antibodies to SARS-CoV-2 nucleocapsid in convalescents compared to controls (0.7 ± 0.0 S/Co) but no correlation with levels of inflammatory markers ().
Table 2. Levels of Syndecan-1, neopterin and CRP in COVID-19 convalescent blood donors and non-COVID-19 controls; healthy employees at the Blood bank and healthy blood donors who were unvaccinated and without COVID-19 antibodies.
Table 3. Correlations between values for inflammatory markers, and between these and antibody levels in COVID-19 convalescents.
When measuring neopterin there was no difference in levels between convalescent and normal blood donors (). Although CRP levels in the remaining samples tended to be higher (median value by 35% and upper range fivefold, p = .08) in the convalescents than controls, both levels were normal.
When interviewing the mostly middle aged convalescents about their past COVID-19 infection, median duration of illness was 2 weeks and median number of symptoms was 3 (), usually fever, head ache and loss of smell and/or taste, in that order.
Discussion
Our results show that only oneof several inflammatory markers, calprotectin, was moderately elevated in the blood of the COVID-19 infection convalescents. However, although calprotectin mainly is a neutrophil product [Citation6], activated neutrophils proposedly are linked to the damage of the lung microcirculation in COVID-19 and increased neutrophil count represents a risk factor for intensive care need and respiratory failure [Citation31], our finding does not support a neutrophil-driven inflammation in convalescents. The calprotectin levels, albeit being increased relatively to controls, were within normal range (0.8–20 ug/mL) [Citation32]. However, since half-life of calprotectin in plasma is only 5 h [Citation33], some calprotectin-related process may be on-going. Also, it is possible that a few individuals in the convalescent group lacking positive COVID-19 PCR test due to test scarcity, had experienced symptoms similar to those of COVID-19 without actually having had the disease. If such possible true COVID negatives could have been excluded from the group, the difference in convalescents’ inflammatory markers could have been greater.
Since calprotectin is contained in NETs [Citation5], one could anticipate that also NET remnants were increased in the convalescents. However, this was only observed in one of those subjects with very high calprotectin values, who also had a high syndecan-1 value. Although also median CRP levels were within normal range, the upper range value in the convalescents was highly increased compared with that of controls, which may suggest a tendency to inflammation in the former.
The recently described persisting inflammation in convalescing COVID-19 patients was located in well vascularized parenchymatous organs; lungs, liver, spleen, in addition to mediastinal lymph nodes [Citation2], where the glycocalyx may have been damaged and syndecan-1 shedded [Citation34]. However, except for cough, shortness of breath and breast pain in a few, none of our convalescents complained about symptoms from other organs besides lungs. The convalescents usually had suffered from head cold symptoms. Our finding is in contrast to what is reported on a cohort of home-isolated patients with long, also called post-COVID-19 syndrome [Citation35], which may be driven by long-term tissue damage and pathological inflammation [Citation4].
Not surprisingly, increased serum neopterin levels have been found in COVID-19 patients and higher in severe than mild disease [Citation22]. Neopterin has, therefore, been suggested as predictive marker for disease severity in hospitalized COVID-19 patients [Citation36,Citation37]. Similar to findings with calprotectin [Citation12], fecal neopterin levels were also raised in COVID-19 patients, especially those with GI symptoms [Citation38]. However, the lack of increase in neopterin levels in the convalescents, in contrast to what is reported in COVID-19 patients [Citation22], indicates that the viral infection as such is overcome and that the oxidative state in convalescents does not require any longer macrophage production and release of the neopterin antioxidant for protection [Citation21]. Likewise, levels of NETs remnants have also normalized, suggesting that the tendency for netosis-immune-thrombosis generation has subsided [Citation15].
Levels of antibodies against SARS-CoV-2 nucleocapsid antigen were as expected significantly raised in the COVID-19 convalescent blood donors. One motive for the collection of samples from COVID-19 convalescents in the blood bank and determination of anti-SARS-CoV-2 antibody concentrations, was to identify suitable donors for convalescent plasma [Citation27]. Plasma from convalescents containing anti-COVID-19 antibodies has been widely used for therapeutic measures in patients with COVID-19 infections and is considered safe [Citation39], but it seems justified only for particular patients [Citation40].
In conclusion, there is some evidence for neutrophil activity in the convalescents. However, since difference between groups was found in only one biomarker, i.e., calprotectin, it is difficult to evaluate whether there is an on-going or residual low-grade inflammation. Similar but larger and more quality controllable studies may give a better answer.
Ethical approval and patient consent statement
The study was approved by the independent ethics committee of Region South-East, Norway (REK number 124170), and all participants gave consent to research through Koronastsudien.no. The study was registered at www.clinicaltrials.gov (Identifier: NCT04320732).
Author contributions
GH interpreted the data and wrote the paper. MKF generated the novel Calprotectin Mimo, NETs and DNase ELISAs and MRM applied them on the blood samples. VPDS introduced the anti-SARS-CoV-2 assay into routine test repertoire at Microbiology Department and together with AL applied it on the samples. NTN did the neopterin test and interviewed participants, and SOK was responsible for the Syndecan-1 test. LSHNM collected the samples and was responsible for the biobanking and AVLS for regulatory issues regarding them. All authors read and commented on the paper and approved the final version of the manuscript.
| Abbreviations | ||
| COVID-19 | = | Coronavirus disease 2019 |
| ELISA | = | enzyme linked immunosorbent assay |
| NETs | = | neutrophil extracellular traps |
Acknowledgement
The authors thank Department of Medical Biochemistry, Oslo University Hospital for the CRP measurements.
Disclosure statement
No potential conflict of interest was reported by the authors.
Data availability statement
The data supporting the findings of this study are available from the corresponding author upon request. The complete data are not publicly available due to ethical restrictions.
Additional information
Funding
References
- Yang J, Wu Z, Long Q, et al. Insights Into immunothrombosis: the interplay Among neutrophil extracellular trap, von willebrand factor, and ADAMTS13. Front Immunol. 2020;11:610696.
- Bai Y, Xu J, Chen L, et al. Inflammatory response in lungs and extrapulmonary sites detected by [18F] fluorodeoxyglucose PET/CT in convalescing COVID-19 patients tested negative for coronavirus. Eur J Nucl Med Mol Imaging. 2021;48(8):2531–2542.
- Cabrera Martimbianco AL, Pacheco RL, Bagattini ÂM, et al. Frequency, signs and symptoms, and criteria adopted for long COVID-19: a systematic review. Int J Clin Pract. 2021;75(10):e14357.
- Yong SJ. Long COVID or post-COVID-19 syndrome: putative pathophysiology, risk factors, and treatments. Infect Dis (Lond). 2021;53(10):737–754.
- Urban CF, Ermert D, Schmid M, et al. Neutrophil extracellular traps contain calprotectin, a cytosolic protein complex involved in host defense against Candida albicans. PLoS Pathog. 2009;5(10):e1000639.
- Fagerhol MK, Dale I, Andersson T. A radioimmunoassay for a granulocyte protein as a marker in studies on the turnover of such cells. Bull Eur Physiopathol Respir. 1980(16 Suppl):273–282.
- Steinbakk M, Naess-Andresen CF, Lingaas E, et al. Antimicrobial actions of calcium binding leucocyte L1 protein, calprotectin. Lancet. 1990;336(8718):763–765.
- Rosen T, Nolan EM. Metal sequestration and antimicrobial activity of human calprotectin are pH-Dependent. Biochem. 2020;59(26):2468–2478.
- Berntzen HB, Olmez U, Fagerhol MK, et al. The leukocyte protein L1 in plasma and synovial fluid from patients with rheumatoid arthritis and osteoarthritis. Scand J Rheumatol. 1991;20(2):74–82.
- Røseth AG, Fagerhol MK, Aadland E, et al. Assessment of the neutrophil dominating protein calprotectin in feces. A methodologic study. Scand J Gastroenterol. 1992;27(9):793–798.
- Yui S, Nakatani Y, Mikami M. Calprotectin (S100A8/S100A9), an inflammatory protein complex from neutrophils with a broad apoptosis-inducing activity. Biol Pharm Bull. 2003;26(6):753–760.
- Shi H, Zuo Y, Yalavarthi S, et al. Neutrophil calprotectin identifies severe pulmonary disease in COVID-19. J Leukoc Biol. 2021;109(1):67–72.
- Zuo Y, Yalavarthi S, Shi H, et al. Neutrophil extracellular traps in COVID-19. JCI Insight. 2020;5(11):e138999.
- Boneschansker L, Inoue Y, Oklu R, et al. Capillary plexuses are vulnerable to neutrophil extracellular traps. Integr Biol (Camb). 2016;8(2):149–155.
- Zuo Y, Zuo M, Yalavarthi S, et al. Neutrophil extracellular traps and thrombosis in COVID-19. J Thromb Thrombolysis. 2021;51:446–453.
- Jiménez-Alcázar M, Rangaswamy C, Panda R, et al. Host DNases prevent vascular occlusion by neutrophil extracellular traps. Science. 2017;358(6367):1202–1206.
- Alexopoulou AN, Multhaupt HA, Couchman JR. Syndecans in wound healing, inflammation and vascular biology. Int J Biochem Cell Biol. 2007;39(3):505–528.
- Manon-Jensen T, Itoh Y, Couchman JR. Proteoglycans in health and disease: the multiple roles of syndecan shedding. FEBS J. 2010;277(19):3876–3889.
- Svennevig K, Hoel TN, Thiara AS, et al. Syndecan-1 plasma levels during coronary artery bypass surgery with and without cardiopulmonary bypass. Perfusion. 2008;23(3):165–171.
- Ogawa F, Oi Y, Nakajima K, et al. Temporal change in syndecan-1 as a therapeutic target and a biomarker for the severity classification of COVID-19. Thromb J. 2021;19(1):55.
- Gieseg SP, Baxter-Parker G, Lindsay A. Neopterin, inflammation, and oxidative stress: what could we be missing? Antioxidants (Basel). 2018;7(7):80.
- Özger HS, Dizbay M, Corbacioglu SK, et al. The prognostic role of neopterin in COVID-19 patients. J Med Virol. 2021;93(3):1520–1525.
- Nübling CM, Chudy M, Volkers P, et al. Neopterin levels during the early phase of human immunodeficiency virus, hepatitis C virus, or hepatitis B virus infection. Transfusion. 2006;46(11):1886–1891.
- Mayersbach P, Fuchs D, Schennach H. Performance of a fully automated quantitative neopterin measurement assay in a routine voluntary blood donation setting. Clin Chem Lab Med. 2010;48(3):373–377.
- Okba N, Müller MA, Li W, et al. Severe acute respiratory syndrome coronavirus 2 − specific antibody responses in coronavirus disease patients. Emerg Infect Dis. 2020;26(7):1478–1488.
- Zhou P, Yang XL, Wang XG, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270–273.
- Nissen-Meyer LSH, Brantsaeter AB. The story of an extraordinary year: challenges and opportunities in responding to covid-19. Transfus Apher Sci. 2021;60(2):103092.
- Søraas AVL. Risk factors for community- and workplace transmission of COVID-19. ClinicalTrials.gov. Identifier: NCT04320732.
- Fagerhol MK, Rugtveit J. Heterogeneity of fecal calprotectin reflecting generation of neutrophil extracellular traps (NETs) in the gut: new immunoassays are available. JMP. 2022;3(1):38–51.
- Fagerhol MK, Johnson E, Tangen JM, et al. NETs analysed by novel calprotectin-based assays in blood donors and patients with multiple myeloma or rheumatoid arthritis: a pilot study. Scand J Immunol. 2020;91(5):e12870.
- Kåsine T, Dyrhol-Riise AM, Barratt-Due A, et al. Nor-solidarity study group. Neutrophil count predicts clinical outcome in hospitalized COVID-19 patients: results from the NOR-Solidarity trial. J Intern Med. 2022;291(2):241–243.
- Jarlborg M, Courvoisier DS, Lamacchia C, et al. Serum calprotectin: a promising biomarker in rheumatoid arthritis and axial spondyloarthritis. Arthritis Res Ther. 2020;22(1):105.
- Fagerhol MK, Nielsen HG, Vetlesen A, et al. Increase in plasma calprotectin during long-distance running. Scand J Clin Lab Invest. 2005;65(3):211–220.
- Dogné S, Flamion B. Endothelial glycocalyx impairment in disease: focus on hyaluronan shedding. Am J Pathol. 2020;190(4):768–780. Apr
- Blomberg B, Mohn KG, Brokstad KA, et al. Long COVID in a prospective cohort of home-isolated patients. Nat Med. 2021;27(9):1607–1613.
- Bellmann-Weiler R, Lanser L, Burkert F, et al. Neopterin predicts disease severity in hospitalized patients With COVID-19. Open Forum Infect Dis. 2021;8(1):ofaa521.
- Robertson J, Gostner JM, Nilsson S, et al. Serum neopterin levels in relation to mild and severe COVID-19. BMC Infect Dis. 2020;20(1):942.
- Grabherr F, Effenberger M, Pedrini A, et al. Increased fecal neopterin parallels gastrointestinal symptoms in COVID-19. Clin Transl Gastroenterol. 2021;12(1):e00293.
- Joyner MJ, Bruno KA, Klassen SA, et al. Safety update: COVID-19 convalescent plasma in 20,000 hospitalized patients. Mayo Clin Proc. 2020;95(9):1888–1897.
- Hueso T, Pouderoux C, Péré H, et al. Convalescent plasma therapy for B-cell–depleted patients with protracted COVID-19. Blood. 2020;136(20):2290–2295.
