Dear Editor
We developed a method to measure calprotectin in whole blood (b-calprotectin) [Citation1] and showed that it could be used to quantitate blood neutrophils (b-neutrophils) [Citation2]. The method works because neutrophils contain practically all calprotectin in blood [Citation2]. This principle could be used in a bedside test for b-neutrophils if someone wanted to develop such a test. A problem with the method was that we had to use heparin blood, while EDTA blood is mostly used for hematological samples. Now we have modified the extraction part of the method, so that the yield from EDTA blood is as good as from heparin blood. We have used the modified extraction method to establish a relationship between b-neutrophils and b-calprotectin in EDTA blood.
It is well known that the secondary and tertiary structures of calprotectin are influenced by the availability of divalent ions, such as calcium and zinc ions [Citation3]. One would expect, therefore, that concentrations of free calcium and zinc ions would influence the binding of calprotectin to antibodies in an immunoassay. In EDTA blood calcium and zinc ions preferentially bind to EDTA, as the dissociation constants of calcium and zinc ions from EDTA are several orders of magnitude lower than those from calprotectin [Citation4–6]. Besides, EDTA binds zinc ions much stronger than calcium ions [Citation5]. According to Nyborg & Peersen, ‘a few minutes of exposure to EDTA can result in significant stripping of Zn2+ from many proteins’ [Citation5].
Previously, we had limited success with adding calcium ions to the extraction mixture with EDTA blood, as the recovery increased from 56% to just 78% compared to heparin blood [Citation1]. The goal was to neutralize the effect of EDTA in blood samples obtained using 4 mL Vacuette EDTA tubes, which are spray-dried with 1.2–2.0 mg EDTA per mL blood, corresponding to 0.342 µmol EDTA in the 50 µL used for extraction [Citation1]. Now we studied the recovery after adding both zinc and calcium ions to the extraction mixture. Compared to heparin blood, adding 0.2, 0.4 and 0.8 µmol calcium ions (from a solution of 40 mmol/L calcium chloride) increased the recovery from 58% (no calcium added) to 63%, 68%, and 71%, respectively. The corresponding figures for zinc ions (from a solution of 40 mmol/L zinc acetate) was 99%, 110%, and 112%, respectively. Surprisingly, adding calcium in addition to zinc did not increase the recovery any further. Presumably EDTA binds zinc ions so much stronger than calcium ions that the calcium ions from blood were already released from EDTA and available to the calprotectin molecules, so adding extra calcium ions had no effect.
Thus, as a standard procedure, we decided to add 50 µL of a 10 mmol/L solution of zinc acetate dihydrate (Sigma-Aldrich, Merck, KGaA, Darmstadt, Germany, product number 96459), i.e. 0.5 µmol zinc ions to the mixture of 50 µL EDTA-blood and 450 µL M-PER Mammalian Protein Extraction Reagent (ThermoFisher, Pittsburg, PA, USA, product number 78501). The mixture was vortexed vigorously for 30 s, and left to stand for 10 min at room temperature. Then we added B-CAL-EX (Bühlmann Laboratories AG, Schönenbuch, Switzerland) to a final volume of 5.05 mL, vortexed for 15 s and centrifuged at 2000g for 10 min before analysis on a Siemens Advia XPT instrument (Siemens, Erlangen, Germany) with the fCAL turbo test from Bühlmann.
Using this modified extraction method we analysed b-calprotectin in 130 anonymous left-over EDTA blood samples from our routine hematology laboratory. B-neutrophils were measured with Sysmex XN analysers (Sysmex Corporation, Kobe, Japan), except for two samples in which b-neutrophils were measured with a Siemens Advia 2120i instrument (Siemens, Erlangen, Germany). All results were validated for clinical use. The samples were kept frozen at −40 °C until analysis. The Regional Committees for Medical and Health Research Ethics decided that the project did not require a formal ethics approval.
Of the 130 specimens, 6 had to be excluded due to missing data. In 4 cases b-neutrophils were registered as < 0.07 × 109/L, whereof 2 had unmeasurable b-calprotectin and the two others had b-calprotectin of 1.2 and 0.04 mg/L. The lower limit of the measuring range is 4 mg/L [Citation1], but in these cases we registered all numeric results from the instrument. In 2 other specimens b-neutrophil was missing for one and b-calprotectin for the other. That left 124 specimens with data on both b-neutrophils and b-calprotectin, with b-neutrophils in the range from 0.097 to 83 × 109/L (median 2.6 × 109/L) and b-calprotectin in the range from 1.0 to 2190 mg/L (median 78 mg/L). Three b-calprotectin values were below 4 mg/L. Median b-calprotectin per neutrophil, 30 pg, was the same as in heparin blood samples from 75 patients with possible neutropenia [Citation2].
The association between b-neutrophils and b-calprotectin is shown in (all 124 data pairs) and (36 data pairs with b-neutrophil <1.5 × 109/L). The overall Spearman rank correlation coefficient between b-neutrophils and b-calprotectin was 0.986, the same as previously found in heparin blood from patients with possible neutropenia [Citation2]. For 33 data pairs with b-calprotectin >4 mg/L and b-neutrophil <1.5 × 109/L the association between b-neutrophils and b-calprotectin was modeled by ordinary least squares linear regression. The regression line along with the 99% prediction interval (for new observations) are indicated in , from where it can be deduced that most patients with b-neutrophils less than 1 × 109/L will have b-calprotectin below 40 mg/L. The corresponding figure in heparin blood from patients with possible neutropenia was 50 mg/L [Citation2], estimated from fewer observations and with another statistical procedure.
Figure 1. B-neutrophils (y) and b-calprotectin (x) in 124 routine EDTA blood samples. Both axes are logarithmic. The lower limit of the measuring range of b-calprotectin is indicated by a black vertical line.
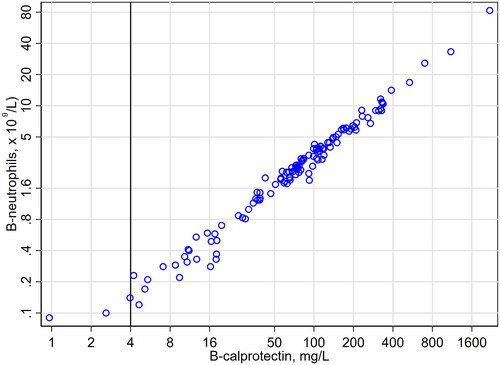
Figure 2. B-neutrophils (y) and b-calprotectin (x) in 36 routine EDTA blood samples with b-neutrophil <1.5 × 109/L. The regression line and the 99% prediction interval is calculated from the 33 data pairs with b-calprotectin > 4 mg/L (lower limit of the measuring range [black vertical line]). The limit of b-neutrophil equal to 1.0 × 109/L is indicated by a red horizontal line.
![Figure 2. B-neutrophils (y) and b-calprotectin (x) in 36 routine EDTA blood samples with b-neutrophil <1.5 × 109/L. The regression line and the 99% prediction interval is calculated from the 33 data pairs with b-calprotectin > 4 mg/L (lower limit of the measuring range [black vertical line]). The limit of b-neutrophil equal to 1.0 × 109/L is indicated by a red horizontal line.](/cms/asset/9a835288-23ae-4aa0-af9d-8564f4cb2e46/iclb_a_2137690_f0002_c.jpg)
In conclusion, we have shown that b-calprotectin extracted from EDTA blood can be used to detect neutropenia below 1 × 109/L.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Åsberg A, Løfblad L, Felic A, et al. Measuring calprotectin in plasma and blood with a fully automated turbidimetric assay. Scand J Clin Lab Invest. 2019;79(1–2):50–57.
- Åsberg A, Løfblad L, Felic A, et al. Using blood calprotectin as a measure of blood neutrophils. Scand J Clin Lab Invest. 2021;81(4):303–306.
- Imani M, Bahrami Y, Jaliani HZ, et al. In solution cation-induced secondary and tertiary structure alterations of human calprotectin. Protein J. 2014;33(5):465–473.
- Naess-Andresen CF, Egelandsdal B, Fagerhol MK. Calcium binding and concomitant changes in the structure and heat stability of calprotectin (L1 protein). Clin Mol Pathol. 1995;48(5):M278–284.
- Nyborg JK, Peersen OB. That zincing feeling: the effects of EDTA on the behaviour of zinc-binding transcriptional regulators. Biochem J. 2004;381(Pt 3):e3–e4.
- Thompson MW. Regulation of zinc-dependent enzymes by metal carrier proteins. Biometals. 2022;35(2):187–213.
