Abstract
Previous studies have identified occasional cases of heterozygous Hb Tacoma in areas that have attracted Finnish immigrants, especially in Sweden and North America, but large studies of this slightly unstable beta variant in vitro have not been carried out. Here we determined the prevalence of hemoglobin variants across Finland. A total of 5059 samples from 11 different hospital districts were analyzed using HbA1c capillary electrophoresis and reviewed for atypical profiles (HbA1c, Capillarys 3 Tera, Sebia). 38 heterozygous Hb Tacoma cases were found (0.75%). The prevalence was highest in South Ostrobothnia (2.0%), located in western Finland, and second highest in the neighboring provinces (1.0–1.4%), but only two districts were devoid of variants. Heterozygous Hb Tacoma was confirmed by genetic testing (NM_000518.5(HBB):c.93G > T (p.Arg31Ser)). In addition, five other variants were found, suggestive of HbE, Hb Helsinki (two cases) and an alpha variant, as interpreted from the electropherograms. The fifth variant, belonging to the South Ostrobothnian cohort, remained unknown at the time of the initial analyses, but was later interpreted as homozygous Hb Tacoma and confirmed by hemoglobin fraction analysis (Hemoglobin(E), Capillarys 3 Tera). In a subsequent retrospective study of the electropherograms of routine HbA1c diagnostics, altogether nine homozygous Hb Tacoma cases were identified in South Ostrobothnia. While heterozygous Hb Tacoma is usually an incidental finding, it interferes with several HbA1c assays. The present study is the first demonstration of homozygous Hb Tacoma. The clinical presentations of homozygous Hb Tacoma are not known and need to be addressed in future studies.
Introduction
Hemoglobin is a universal name for a wide variety of molecules. Hemoglobin A (HbA) is the main form of hemoglobin, comprising 95–98% of total adult hemoglobin. It consists of two alpha and two beta subunits. Two other forms are HbA2 and fetal hemoglobin (HbF), which comprise 2–3% and 0–2%, and are formed of two alpha and two delta subunits, and two alpha and two gamma subunits, respectively. In medicine, glycated hemoglobin (HbA1c) is utilized both in the monitoring and diagnosis of diabetes [Citation1]. HbA1c is formed when glucose attaches to the N-terminus of the beta chain of hemoglobin A, and the more glucose in the surrounding fluid, the more HbA1c is formed [Citation2]. The reference range for HbA1c is 20–42 mmol/mol, which is about 4–6% of hemoglobin A. The N-terminus is also glycated by other sugar moieties, but to a lesser extent [Citation3].
More than 1400 genetic hemoglobin variants have so far been reported [Citation4]. Most variants have one single nucleotide point mutation but also double mutations, insertions, deletions and hybrids occur. The majority of heterozygote variants do not cause clinical symptoms, whereas homozygous and compound heterozygote forms of some variants cause high morbidity and mortality [Citation5]. In addition to direct health effects, variants may also cause analytical interference on HbA1c methods [Citation6,Citation7], and interference on the diagnostical principle, which assumes a mean red blood cell life span of 120 days [Citation8–11]. Thalassemias are defects in the quantity of globins, and may also co-occur with variants.
Hemoglobin Tacoma is a single nucleotide point mutation of the beta subunit at position 93, where guanine is replaced with thymine causing arginine to serine change at the amino acid level (NM_000518.5(HBB):c.93G > T (p.Arg31Ser), HGVS nomenclature) [Citation4]. The same amino acid change may also be due to guanine to cytosine mutation, namely Hb Tacoma II (NM_000518.5(HBB):c.93G > C (p.Arg31Ser)) [Citation4]. These variants can be identified based on a characteristic electropherogram in a routine HbA1c analysis on capillary electrophoresis. The method separates molecules based on their electrophoretic mobility (size and charge), which also depends on the pH and ionic strength of the buffer. HbA1c is separated due to a change in net charge caused by the N-terminal glycation and, simultaneously, the method allows for the detection of some of the variant hemoglobins. Hemoglobin(E) is a capillary electrophoretic method designed more specifically for the detection of hemoglobin variants, without the separation of glycated hemoglobins.
Previous studies have identified occasional cases of heterozygous Hb Tacoma in areas that have attracted Finnish immigrants, especially in Sweden and North America [Citation12–16]. As yet, the prevalence of hemoglobin Tacoma in Finland has not been known, since the majority of laboratories use immunological and enzymatic HbA1c methods that cannot identify the presence of Hb variants. The aim of this study was to determine the prevalence of heterozygous Hb Tacoma and other possible variants across Finland using HbA1c capillary electrophoresis. Regarding homozygous Hb Tacoma, this is also the first report on the occurrence of such cases.
Materials and methods
HbA1c from a total of 5059 samples was analyzed by capillary electrophoresis method and reviewed for atypical profiles (HbA1c method, Capillarys 3 Tera, Sebia, Evry, France). Samples referred for HbA1c analysis were collected in a consecutive manner from 11 different hospital districts, around 500 samples each, and stored for analysis under temperature control (2–8 °C). The majority of samples were analyzed in the next two days after the sample was drawn and all of them within a week. The analyses were performed in the FINAS accredited (SFS-EN ISO 15189) Clinical Chemistry Laboratory of Seinäjoki Central Hospital (South Ostrobothnia, Finland) in 2018–2019. The expected fractions for a non-variant sample were HbA1c, other HbA (other N-terminal glycations than HbA1c), HbA0 (the remaining HbA), HbF (not all patients) and HbA2, and a zero baseline between separate fractions. Confirmatory genetic testing was carried out for five heterozygous Hb Tacoma samples, each originating from a different district (HBB single gene test, Blueprint Genetics, Espoo, Finland) [Citation17]. Previously 20 samples from South Ostrobothnia with similar atypical electropherograms have been genetically identified by Lenters-Westra et al. [Citation6], all being heterozygous Hb Tacoma. The other variants were interpreted from the electropherograms based on the material provided by the assay manufacturer. The study utilized anonymized left-over samples of routine diagnostics, and therefore no informed consent from the patients was obtained. No data was collected about the patients. The study was approved by the Regional Ethics Committee of Tampere University Hospital, Tampere, Finland.
In 2021, a sample with HbA1c electropherogram suggestive of possible homozygous Hb Tacoma (the index case) was discovered among routine HbA1c diagnostics in South Ostrobothnia and sent for hemoglobin fraction analysis at Huslab, Helsinki, Finland (Hemoglobin(E) method, Capillarys 3 Tera, Sebia, Evry, France) along with one Hb Tacoma heterozygote sample. In general, the expected fractions for non-variants are HbA, HbF (not all patients) and HbA2, and a zero baseline between separate fractions. Both samples were at first analyzed as native and then mixed 1 + 1 with Hb AFSC control (Sebia, Evry, France). The control contains HbA, HbF, HbS and HbC and is used to achieve more precise migration positions of variant hemoglobins in the absence of HbA. The samples were stored frozen (–70 °C) until the analyses. A retrospective study on the analyzers database was performed in South Ostrobothnia to re-evaluate unidentified cases for possible Hb Tacoma homozygosity.
Results
The numbers and percentages of heterozygous Hb Tacoma cases found from the 11 different hospital districts are shown in (column A). In the total population, 38 cases (0.75%) were found. Regionally, the occurrence of variants was clustered in South Ostrobothnia (2.0%), but notable percentages of cases were also found in Ostrobothnia (1.4%), Pirkanmaa (1.0%), North Ostrobothnia (0.80%), North Savo (0.80%) and Southwest Finland (0.80%). Only two districts were devoid of variants. Genetic testing confirmed heterozygous Hb Tacoma.
Table 1. Numbers (n) and percentages of hemoglobin variants in Finland by region.
The findings on other variants are also summarized in (column B). A total of five other cases were found: one from Uusimaa, two from Southwest Finland, one from Kainuu and one from South Ostrobothnia. The South Ostrobothnian sample remained unknown at the time of the initial analyses but was later identified as homozygous Hb Tacoma. The other cases were interpreted from HbA1c electropherograms as heterozygote HbE (Uusimaa), heterozygote Hb Helsinki (two cases, Southwest Finland), and an alpha hemoglobin variant (Kainuu).
show the representative HbA1c electropherograms of non-variant and heterozygous Hb Tacoma samples. In non-variant cases, peaks of HbA1c, other HbA, HbA0 and HbA2, respectively, are clearly separated from the baseline and from each other. In heterozygous Hb Tacoma samples, two flat peaks below the non-variant peaks are observed, the first one extending from point 40 to 140, and the other one from point 180 to 280. At point 280, a peak characteristic of degraded hemoglobin is observed (marked 5), and the percentage of HbA2 (colored peak area) is above normal, in the example in 3.4% (HbA2 > 3% is considered elevated with Capillarys 3 HbA1c method).
Figure 1. HbA1c electropherograms of (A) non-variant and (B) heterozygous Hb Tacoma. In (B) two very flat peaks (points 40–140 and 180–280, marked by arrows) below the normal peaks are observed and a peak characteristic of degraded hemoglobin (marked 5). HbA2 percentage is 3.4% (colored area).
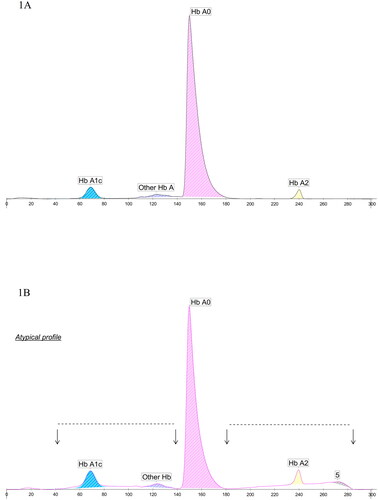
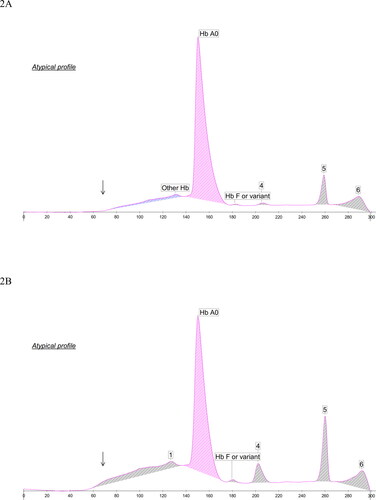
demonstrate HbA1c electropherograms of the index case of homozygous Hb Tacoma and the homozygote of the South Ostrobothnian cohort. In both cases, non-glycated Hb Tacoma is falsely interpreted as HbA0. The false recognition of Hb Tacoma to HbA shifts scale to left causing the unaffected fractions, HbF and HbA2, to be misplaced and located around points 205 and 260 (marked 4 and 5), respectively. also shows the absence of HbA1c, which may be considered unlikely if HbA0 were present. Instead, glycated Hb Tacoma elutes as a flat peak, similar to heterozygote cases. In , the HbA1c area is masked by the white area (unstable hemoglobin). In a retrospective study on the analyzers database in South Ostrobothnia altogether nine homozygous cases were identified.
Figure 2. HbA1c electropherograms of (A) the index case of homozygous Hb Tacoma, and (B) the homozygote Hb Tacoma observed among the South Ostrobothnian cohort. Non-glycated Hb Tacoma is falsely interpreted as HbA0, which shifts scale to left and misplaces HbF (at x-axis around 205, marked 4) and HbA2 (at x-axis around 260, marked 5). Peak 6 is characteristic of degraded hemoglobin. In (A) HbA1c is absent, and in (B) the corresponding area is masked by the white area (marked by arrows).
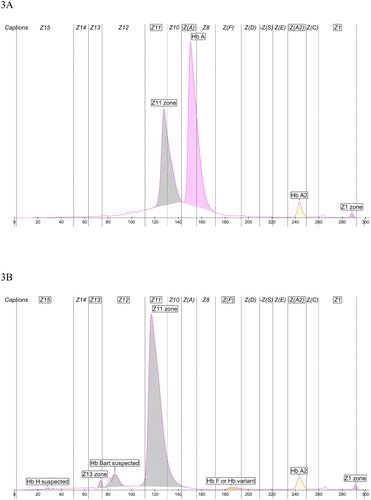
show Hemoglobin(E) electropherograms of heterozygote and homozygote Hb Tacoma. Hb Tacoma elutes at zone Z11 in the heterozygous case, and in the homozygous case, the major peak is seen at the same zone but to a little left. Migration in the latter is imprecise due to lack of any HbA. In analyses mixed with Hb AFSC control, the migration position of Hb Tacoma hemoglobin between heterozygote and homozygote cases was equivalent (figures not shown). The amount of Hb Tacoma hemoglobin in the heterozygote case is 37%. The amount of HbA2 in the heterozygous and homozygous cases are 3.1% and 3.0%, respectively (2.2–3.2% being considered as a normal range with Capillarys 3 Hemoglobin(E) method). No baseline elevation is observed, except for the heterozygote below Hb Tacoma and HbA due to their proximity. In the homozygous case, HbH and Hb Bart (which denote the hemoglobin of four beta and four gamma subunits, respectively) are falsely suspected and are considered as degradation products together with peaks Z13 and Z1.
Figure 3. Hemoglobin(E) electropherograms of (A) heterozygote Hb Tacoma, and (B) homozygote Hb Tacoma. Hb Tacoma elutes at zone Z11. The amount of Hb Tacoma hemoglobin in (A) is 37%, and of HbA2 in (A) and (B) is 3.1% and 3.0%, respectively. HbH and Hb Bart are falsely suspected and are degradation products together with peaks Z13 and Z1.
Discussion
The present study provides important insights into the prevalence of Hb Tacoma in Finland. The findings underscore notable regional differences in the prevalence of Hb Tacoma variant across the country, the highest prevalence being observed in the area of the South Ostrobothnia Hospital District, located in western Finland. The study also describes, for the first time, cases with homozygous Hb Tacoma.
So far, there are less than 20 case reports on Hb Tacoma variant [Citation12–16,Citation18–27]. It was described for the first time in 1965 [Citation12,Citation28]. The characterizations were carried out at the amino acid level until 1993 when the nucleotide substitution was identified as (HBB):c.93G > T [Citation14]. Interestingly, many of the cases have been reported with Finnish ancestry [Citation14–16,Citation19,Citation23], and it seems that Finnish emigration in the past has played role in the spreading of this variant. The emigration has been greatest from South Ostrobothnia and Ostrobothnia and with the highest attraction to Sweden and North America [Citation29–32]. Occasional cases of Hb Tacoma have been reported in these areas [Citation12–16], and thus the prevalence of Hb Tacoma may deserve large studies in their current populations as well. Nevertheless, it is not probable that all the Hb Tacoma patients share the same ancestor. The overall ethnic variety of cases suggests multicentric origin and it is also supported by the findings of the same amino acid substitution caused by a c.93G > C change [Citation4,Citation27,Citation33]. This variant has recently been named Hb Tacoma II [Citation4,Citation27].
Clinically, heterozygous Hb Tacoma is usually an incidental finding. Mild anemia has been observed in some of the patients [Citation14,Citation16,Citation18,Citation21,Citation25,Citation27], whereas some have shown a normal blood count [Citation12,Citation14,Citation16,Citation18,Citation19,Citation23]. History of slight hemolysis has been described only once [Citation14]. The percentage of Tacoma hemoglobin has been estimated between 27–43% of total hemoglobin [Citation12,Citation14,Citation15,Citation21,Citation34]. In capillary electrophoretic studies, including ours, the percentage has been 36–40% [Citation16,Citation25,Citation27]. To explain the relatively lower level, as compared to HbA, precipitation in vivo [Citation16,Citation19] and due storage [Citation15], continuous selective intracellular degradation [Citation20], and effects through impacts on splicing (codon 31 is divided by an intron) [Citation15,Citation27] has been suggested. On the other hand, if not ruled out, thalassemias may also co-occur and confound the percentages. Both normal [Citation14,Citation19,Citation21], and elevated HbA2 have been reported [Citation12,Citation14,Citation34]. With the capillary electrophoretic HbA1c method, elevated HbA2 is a constant finding, but may also be due to Hb Tacoma’s instability in the assay, which causes biased total hemoglobin as only colored area is quantitated. Hemoglobin fraction method here and previously has shown borderline to slightly elevated HbA2 proportions [Citation16,Citation27], but has white area and bias caused by peak proximity of Hb Tacoma to HbA.
Analytically, heterozygous Tacoma hemoglobin poses significant problems with HbA1c methods. In a study by Lenters-Westra et al. [Citation6] results with Tina-quant Gen.2 (immunoassay, Roche Diagnostics), Premier Hb9210 (affinity chromatography HPLC, Trinity Biotech), Tosoh G8 (cation exchange HPLC, Tosoh Bioscience) and Afinion (affinity chromatography point-of-care test, Abbott) were all at different level. In addition, the correlation between Tosoh G8 and others was weak. Conclusions can’t be made as to which method (Tina-quant Gen.2 or Premier Hb9210, which differed by 17%) is analytically correct. In the capillary electrophoretic HbA1c method, the flat peak interferes with HbA1c and the result is unreliable. Non-glycated Hb Tacoma may also partially elute with and interfere with HbA0. We have found that results from HbA1c on capillary electrophoresis and from DCA Vantage (immunological point-of-care test, Siemens Healthineers) usually differ and that the difference is not uniform but varies from sample to sample (personal experience). Many methods measure numerous variants correctly, in analytical means, but this appears not to apply Hb Tacoma.
Diagnostically, heterozygous Tacoma hemoglobin may cause some misinterpretation on HbA1c results through the assumption of RBC life span of 120 days. There is very little data about the variant, but almost all predicting tools suggest that Tacoma mutation makes hemoglobin more vulnerable to damage [Citation35]. Accordingly, Hb Tacoma has been characterized as an unstable variant in vitro [Citation12,Citation14,Citation19,Citation21,Citation34,Citation36,Citation37] and the baseline elevation observed here in the HbA1c method and degradation products in the fraction method are also in line with this. According to assay descriptions, degradation products in the HbA1c method migrate particularly to HbA2 position or more anodically than HbA0, i.e. in the Other HbA position. In the hemoglobin fraction method degradation products typically migrate one or two zones (depending on the zone width) more anodically than the original fraction.
The fact that Hb Tacoma shows more instability in the HbA1c method than in the hemoglobin fraction method is interesting. The reason for this may only be speculated at this point. The HbA1c method is not specifically designed for variant detection, and according to the manufacturer, especially the hemolysing solutions between methods differ. Both methods have assay buffer pH 9.4. It is known that the replacement of positive arginine by neutral serine results in a conformational change of a single amino acid side chain and the loss of several hydrogen bonds at the α1β1 contact surface, and the latter is thought to cause increased dissociation into free alpha and beta subunits by various agents [Citation37]. However, stability does not preclude effects on RBC lifespan either. Two of the most common beta variant hemoglobins worldwide, HbS and HbC, are stable variants and heterozygotes usually have normal hematological and clinical presentation, but their RBC life span is shortened [Citation8–10]. Until the characteristics of Hb Tacoma are better understood, every patient should only become his/her own reference for follow-up, and not to be compared against common reference limits.
Finally, it should be noted that the present work is the first report on homozygous Hb Tacoma cases. The recognition of an index patient in routine diagnostics in South Ostrobothnia prompted a re-evaluation of HbA1c electropherograms on analyzer’s database, and a total of nine cases were found. Based on the high prevalence of heterozygosity, this finding of homozygosity is not surprising. The white area, which refers to degenerated hemoglobin, was multiplied in HbA1c electropherograms of homozygous cases as compared to heterozygote cases. Previously, two compound heterozygote beta thalassemia/Hb Tacoma cases have been reported [Citation15,Citation33], the severity in the other exceeding what would be expected from patient’s thalassemia alone [Citation33]. It should however be noted, that this case was of type Tacoma II, which may have slightly lower expression levels than Hb Tacoma [Citation27].
Each study has its limitations. In this study, the samples were analyzed without any data about the age, sex, reason why the sample was taken and so on, i.e. about the representativeness of the Finnish general population, and therefore the prevalences reported should be considered indirect approximates. In Finland, HbA1c may be ordered based on monitoring or suspicion of diabetes, but also as part of a general health check-up. The study also included 11 but not all 19 special health care areas in Finland, although it extended to all five university hospital districts. Not all variants were genetically confirmed. Hb Tacoma has obvious interference on capillary electrophoretic HbA1c, but this was not studied in enough detail to draw conclusions. Studies on this and the clinical and laboratory findings of homozygous Hb Tacoma are needed, and are currently ongoing in our laboratory.
Acknowledgments
The authors would like to thank Professor Sohvi Hörkkö, University of Oulu, for the contribution of North Ostrobothnia, Lapland and Kainuu into the study.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Correction Statement
This article has not been published elsewhere and has not been submitted simultaneously for publication elsewhere.
Additional information
Funding
References
- American Diabetes Association. Standards of medical care in diabetes–2022. Diabetes Care. 2022;45(Suppl 1):S1–S264.
- Gebel E. The start of something good: the discovery of HbA(1c) and the American Diabetes Association Samuel Rahbar outstanding discovery award. Diabetes Care. 2012;35(12):2429–2431.
- Friedman S, Humbert JR. A simple microchromatographic column for determination of hemoglobins A1a + b and A1c. Hemoglobin. 1979;3(6):411–428.
- Giardine B, Borg J, Viennas E, et al. Updates of the HbVar database of human hemoglobin variants and thalassemia mutations. Nucleic Acids Res. 2014;42(Database issue):D1063–D1069.
- Mulumba LL, Wilson L. Sickle cell disease among children in Africa: an integrative literature review and global recommendations. Int J Afr Nurs Sci. 2015;3:56–64.
- Lenters-Westra E, Strunk A, Campbell P, et al. Can the Afinion HbA1c point-of-care instrument be an alternative method for the Tosoh G8 in the case of Hb-Tacoma? Scand J Clin Lab Invest. 2017;77(1):2–7.
- Little RR, La’ulu SL, Hanson SE, et al. Effects of 49 different rare Hb variants on HbA1c measurement in eight methods. J Diabetes Sci Technol. 2015;9(4):849–856.
- Gordon DK, Hussain M, Kumar P, et al. The sickle effect: the silent titan affecting glycated hemoglobin reliability. Cureus. 2020;12(8):e9685.
- Rhea JM, Roberts-Wilson TK, Molinaro RJ. Impact of hemoglobin variants on Hb A1c interpretation: do we assume too much? MLO Med Lab Obs. 2012;44(6):8, 10, 12 passim: quiz 20.
- Rhea JM, Molinaro R. Pathology consultation on HbA(1c) methods and interferences. Am J Clin Pathol. 2014;141(1):5–16.
- Urrechaga E. Incidental detection of Hb disorders during HbA1c analysis. J Diabetes Sci Technol. 2015;9(3):713.
- Baur EW, Motulsky AG. Hemoglobin tacoma – a beta-chain variant associated with increased hb A2. Humangenetik. 1965;1(7):621–634.
- Gray GR, Marion RB. Hemoglobinopathies in a hospital population in Vancouver. Can Med Assoc J. 1978;119(7):701–704.
- Landin B, Jeppsson JO. Rare beta chain hemoglobin variants found in Swedish patients during HBA1c analysis. Hemoglobin. 1993;17(4):303–318.
- Landin B, Alvelius G, Rai DK, et al. Compound heterozygosity for Hb Tacoma [beta30(B12)Arg–>Ser] and beta+-thalassemia. Hemoglobin. 2000;24(3):253–257.
- Merkeley H, Sandercock S, Halchuk L, et al. A novel means of identifying hemoglobin Tacoma utilizing capillary electrophoresis with a hemoglobin A1c software platform. Cogent Med. 2021;8(1):2002502.
- Blueprint Genetics. HBB single gene test; [cited 2022 Nov 10]. Available from: https://blueprintgenetics.com/tests/single-gene-tests/hbb-single-gene-test/.
- Idelson LI, Didkowsky NA, Casey R, et al. New unstable haemoglobin (Hb Moscva, beta24 (B4) Gly leads to Asp) found in the USSR. Nature. 1974;249(459):768–770.
- Deacon-Smith RA, Lee-Potter JP. An unstable haemoglobin, Hb Tacoma beta30 (B12) arg leads to ser, detected at birth by the demonstration of red cell inclusions. J Clin Pathol. 1978;31(9):883–887.
- Honig GR, Mason RG, Shamsuddin M, et al. Two new sickle cell syndromes: HbS, Hb Camden, and alpha-thalassemia; and HbS in combination with Hb Tacoma. Blood. 1980;55(4):655–660.
- Harano K, Harano T, Ueda S, et al. Hb Tacoma [beta 30(B12) Arg––Ser], a slightly unstable hemoglobin variant found in Japan. Hemoglobin. 1985;9(6):635–639.
- Idel’son LI, Molchanova TP, Aseeva EA, et al. [A second case of an Hb Takoma carrier in Moscow]. Gematol Transfuziol. 1987;32(9):45–46. Russian.
- Baudin-Chich V, Wajcman H, Gombaud-Saintonge G, et al. Hemoglobin Brest [beta 127 (H5)Gln––Lys] a new unstable human hemoglobin variant located at the alpha 1 beta 1 interface with specific electrophoretic behavior. Hemoglobin. 1988;12(2):179–188.
- Sangkitporn SK, Eksiri L, Sangnoi A, et al. Identification of beta-globin gene mutations in Thailand using an automated fluorescence-based DNA sequencer. Int J Lab Hematol. 2009;31(5):521–527.
- Keegan A, Curnow J. A rare haemoglobin-variant produces discrepant results between haemoglobinopathy screening techniques. Pathology. 2015;47(Suppl 1):S90.
- Nybo J, Hansen AT, Petersen JB, et al. Hemoglobin variants found in relation to HbA1c testing: high occurrence of Hb Athens–Georgia in the Northern Jutland, Denmark. Clin Chem Lab Med. 2019;57(6):e108–e110.
- Moore JA, Pullon BM, Wang D, et al. Hb Tacoma: G > T or G > C, and does it matter? Hemoglobin. 2021;45(3):203–206.
- Brimhall B, Jones RT, Baur EW, et al. Structural characterization of hemoglobin Tacoma. Biochemistry. 1969;8(5):2125–2129.
- Uudet tilastot, yhä enemmän suomalaistaustaisia Ruotsissa. Sveriges Radio Finska. 2019 Sep 17; [cited 2022 Nov 10]. Finnish. Available from: https://sverigesradio.se/artikel/7300130.
- Kero R. Suureen Länteen. Siirtolaisuus Suomesta Pohjois-Amerikkaan. Suomalaisen Siirtolaisuuden Historia I. Turku: Siirtolaisuusinstituutti; 1996. Finnish.
- United States Census Bureau. Table B04006 – People reporting ancestry – 2019 American community survey 1-year estimates; [cited 2022 Nov 10]. Available from: https://data.census.gov/cedsci/table?q=Ancestry&t=Ancestry&tid=ACSDT1Y2019.B04006.
- Statistics Canada. Immigration and ethnocultural diversity highlight tables 2016; [cited 2022 Nov 10]. Available from: https://www12.statcan.gc.ca/census-recensement/2016/dp-pd/hlt-fst/imm/Table.cfm?Lang=E&T=31&Geo=01.
- el-Kalla S, Mathews AR. A significant beta-thalassemia heterogeneity in the United Arab Emirates. Hemoglobin. 1997;21(3):237–247.
- Idelson LI, Didkowsky NA, Casey R, et al. Structure and function of haemoglobin Tacoma (beta 30 Arg yields Ser) found in a second family. Acta Haematol. 1974;52(5):303–311.
- Carlice-Dos-Reis T, Viana J, Moreira FC, et al. Investigation of mutations in the HBB gene using the 1,000 genomes database. PLOS One. 2017;12(4):e0174637.
- Hayashi A, Suzuki T, Stamatoyannopoulos G. Electrophoretic and functional abnormalities of haemoglobin Tacoma β30(B12) Arg→Ser. Biochim Biophys Acta. 1974;351(2):453–456.
- Tucker PW, Perutz MF. Mechanism of charge compensation and impairment of co-operative functions in haemoglobin Tacoma (Arg B12(30)β→Ser). J Mol Biol. 1977;114(3):415–420.
