Abstract
Individuals with familial hypercholesterolemia (FH) have increased cardiovascular risk despite lipid-lowering therapy, and additional therapy is warranted. Omega-3 polyunsaturated fatty acid (n-3 PUFA) supplements have demonstrated an effect on cardiovascular endpoints in some clinical trials. Platelet-modifying and anti-inflammatory properties are among the proposed beneficial effects of n-3 PUFA. We investigated the effect of a high-dose n-3 PUFA supplement on platelet function and inflammatory markers in FH subjects. We performed a randomized, double-blind trial with a crossover design. Inclusion criteria were genetically verified heterozygous FH, stable disease, statin treatment >12 months, and age 18-75 years. Trial participants were allocated to two treatment periods in random order. The treatment periods (three months each) were separated by a three-month washout period. N-3 PUFA (1840 mg eicosapentaenoic acid and 1520 mg docosahexaenoic acid) and placebo (olive oil) were administered in four capsules daily. Endpoints were platelet function and inflammatory markers, assessed by platelet function analyzer, soluble markers P-selectin, vascular cell adhesion molecule (VCAM) and intercellular adhesion molecule (ICAM), 27 cytokines, and hematological parameters. Thirty-four heterozygous FH individuals completed the trial. No treatment effect (p = 0.93) from n-3 PUFA on the platelet function analyzer was found (2 s, 95% CI [-13, 6]). In our FH population, n-3 PUFA did not influence the levels of P-selectin (-2.0, 95% CI [-5.0, 2.0], p = 0.41), VCAM (0, 95% CI [-14.2, 14.2], p > 0.99), ICAM (-27.0, 95% CI [-70.1, 16.5]; p = 0.21), cytokine levels, or hematological parameters. In statin-treated FH individuals, high dose n-3 PUFA supplement did not affect platelet function and inflammatory markers.
Trial registration number: EUDRACTNR 2012-000505-68; ClinicalTrials.gov NCT01813006
Trial studying the effect of omega-3 fatty acids supplements in familial hypercholesterolemia.
High-dose omega-3 fatty acids supplements had no impact on platelet function.
Cytokine levels were unchanged after three months of omega-3 fatty acid supplementation.
No effect of omega-3 fatty acids on C-reactive protein was observed.
Highlights
Introduction
Individuals with familial hypercholesterolemia (FH) are at increased risk of cardiovascular events despite statin treatment [Citation1,Citation2]. In FH, low-density lipoprotein cholesterol (LDL-C) exposure since early childhood is considered the primary driver for this increased risk, with proinflammation and altered platelet function as possible accomplices [Citation3–9]. The link between cardiovascular disease (CVD) and inflammation is widely accepted [Citation10,Citation11]. Inflammation is involved in all stages of atherosclerosis, and therapy targeting pro-inflammatory cytokines has proven beneficial in reducing biomarkers of inflammation and recurrent cardiovascular events after myocardial infarction [Citation12,Citation13]. Still, other trials investigating anti-inflammatory treatment in CVD lack an effect on cardiovascular events [Citation14,Citation15].
After the introduction of statins, the evidence of positive cardiovascular effects from omega-3 polyunsaturated fatty acids (n-3 PUFA) has been limited [Citation16]. Nonetheless, the Reduction of Cardiovascular Events with Icosapent Ethyl-Intervention Trial (REDUCE-IT) results have nuanced the role of n-3 PUFA in cardiovascular disease prevention [Citation17]. Icosapent ethyl supplement is currently included in the European guidelines for the management of hypertriglyceridemia in statin-treated patients [Citation18]. For FH subjects, n-3 PUFA supplement is not part of the recommended medication [Citation18].
The pathway of the potential effect of n-3 PUFA is to be elucidated, yet an anti-inflammatory and antithrombotic effect of n-3 PUFA are among the proposed mechanisms of action [Citation17,Citation19]. Meta-analyses assessing the impact of n-3 PUFA on inflammatory markers have shown a reduction in C-reactive protein (CRP) after n-3 PUFA supplementation [Citation20,Citation21]. In addition, reductions in interleukin (IL) 6 and tumor necrosis factor (TNF) after n-3 supplement was found in healthy individuals and individuals with chronic disease [Citation21]. In patients with poor health, n-3 PUFA supplement was associated with reduced platelet aggregability, but the same effect from n-3 PUFA was not found in healthy individuals [Citation22]. One report showed reduced platelet aggregability after n-3 PUFA in trial participants with FH and familial combined hyperlipidemia. Still, the knowledge concerning the effects of n-3 PUFA on platelet function and inflammation in FH is scarce [Citation23].
We hypothesized that a high-dose n-3 PUFA supplement would reduce inflammatory markers in FH individuals. We specifically studied the effects of n-3 PUFA on platelet function, soluble platelet function markers, cytokines, and hematological parameters in mutation-positive heterozygous FH patients.
Patients and methods
The design, study population, and eligibility criteria have been described previously [Citation24,Citation25]. In short, this was a randomized trial with a crossover design (two treatment periods of three months, separated by a washout period). The study population was individuals with mutation-positive heterozygous FH from the Lipid Clinic at Nordland Hospital (Bodø, Norway). The trial had a three-month washout period to preclude a possible carry-over effect between the two treatments. The participants did not have dietary restrictions during the trial, but fish oil supplements were discontinued three months before trial enrollment. We followed a per-protocol analysis. The inclusion criteria for age were changed at the beginning to enhance the inclusion rate, from 18-60 years to 18-75 years. The trial participants were randomized to treatment sequence at inclusion, with an allocation ratio of 1:1. BASF (Lysaker, Norway) provided the study medication, and Apotekproduksjon AS (Oslo, Norway) provided the randomization and the labeling of the study medication. Marine n-3 PUFA (daily dose of 1840 mg eicosapentaenoic acid and 1520 mg docosahexaenoic acid) and placebo (olive oil) was administered as two capsules twice daily.
Before each treatment period, the participants received the study medication in sequentially concealed containers. Unused medicine was returned after treatment to ensure compliance requirements (use at least 50% of the drug). The sequence randomization was kept secret for the trial participants and care providers, and the randomization key was opened after trial completion. The outcome assessors knew the randomization of the participants. Primary outcomes and secondary lipoprotein outcomes are previously published [Citation24,Citation25]. Here we report on the secondary endpoints of changes in platelet function and inflammatory markers. Sample size determination was based on a power calculation for changes in the primary endpoint, in vivo endothelial function.
Blood samples
Fasting blood samples were taken at each hospital visit. Hematological parameters were analyzed consecutively on a Siemens ADVIA 2120i Hematology System (Siemens Healthcare Diagnostics Ltd., Camberly, UK). Ethylenediaminetetraacetic acid blood obtained for soluble platelet and inflammatory markers was centrifuged at 3220 g for 15 min at 4 °C, and plasma was frozen in aliquots at −70° C and analyzed in batch after trial completion.
Platelet function analyzer
The platelet function analyzer Innovance PFA-200 System (Siemens Healthineers, Erlangen, Germany) and Dade collagen/ADP cartridges (Siemens Healthineers) was used. The analysis was performed following the instructions from the manufacturer. In brief, citrated whole blood is passed by a capillary, and through an aperture in a coated membrane, the system measures the time required to obstruct this aperture. Closure time (CT) in seconds is the outcome measure, and the analysis was performed at every hospital visit (baseline, after treatment one, after washout, and after treatment two). We used the cartridge with collagen and adenosine diphosphate-coated membrane, with reference values for CT from 62 to 100 s.
Multiplex and singleplex immunoassays
The concentration of 27 cytokines in ethylenediaminetetraacetic acid plasma was assessed by a humane cytokine 27-plex assay (Bio-Plex Human Cytokine Grp I Panel 27-Plex; Bio-Rad Laboratories Inc, Hercules, CA) containing the following analytes; interleukin-1 beta (IL-β), IL-1 receptor antagonist (IL-1ra), IL-2, IL-4, IL-5, IL-6, IL-7, IL-8, IL-9, IL-10, IL-12, IL-13, IL-15, IL-17, eotaxin-1 (C-C motif chemokine ligand 11, CCL11), basic fibroblast growth factor (FGF basic), granulocyte colony-stimulating factor (G-CSF), granulocyte-macrophage colony-stimulating factor (GM-CSF), interferon-gamma (IFN-γ), interferon-gamma-inducing protein 10 or IP-10 (CXCL10), monocyte chemoattractant protein 1 (MCP-1 or CCL2), macrophage inflammatory protein-1-alpha (MIP-1α or CCL3), MIP-1β (or CCL4), platelet-derived growth factor-BB (PDGF-BB), RANTES (CCL5), tumor necrosis factor (TNF) and vascular endothelial growth factor (VEGF). The multiplex immunoassay was performed following the instruction of the manufacturer.
The analysis of vascular cell adhesion molecule (VCAM), intercellular adhesion molecule (ICAM), and P-selectin was done as a single plex from eBioscience (Thermo Fisher Scientific, San Diego, CA).
Statistics
The statistical analyses were performed with Prism version 9.0 (GraphPad Software, San Diego, CA). The period effect was assessed by a two-sample t-test or Mann–Whitney test of the differences between the treatment periods in the two sequence groups. The average of the treatment periods in each sequence group was compared by a two-sample t-test or Mann–Whitney test, giving the treatment period interaction. A paired samples t-test estimated the treatment effect of the two treatments for normally distributed differences and Wilcoxon matched-pairs signed rank for not normally distributed differences. The Shapiro–Wilk test was applied for the assessment of normality distribution. Mean (standard deviation) and median (interquartile range) are presented by the distribution of differences. A two-tailed p-level <0.05 was considered statistically significant.
Cytokine values below the lower detection limit were accepted as valid numbers. Immeasurable cytokine levels were replaced by a random number between 0.001 and the lower detection limit using the random function in Excel version 15.56 (Microsoft Corporation, Redmond, Washington). Cytokines missing ≥70 percent of their 136 values were not analyzed for baseline values or treatment effect. The Holm-Sídák method for multiple comparisons was applied for period effect and treatment period interaction for all outcome variables and treatment effect for cytokines.
Ethics
We obtained informed written consent from all trial participants at trial inclusion. The trial was conducted in compliance with the Declaration of Helsinki and was approved by the regional ethics committee of northern Norway, P REK 2011/899, and by the Norwegian Medicines Agency (EUDRACTNR 2012-000505-68; ClinicalTrials.gov NCT01813006).
Results
Sixty-five patients were assessed for eligibility, and 38 individuals with FH were randomized to sequence, 19 in each sequence group. Three individuals left the trial (one due to fertility treatment, one withdrew because of abdominal pain, and one left the trial for an unknown reason), and one participant was excluded from the analysis because of pregnancy. Baseline characteristics in total and a participant flow diagram are previously published [Citation24,Citation25]. The trial was conducted from September 2012 to July 2016. Outcomes from 34 individuals (16 and 18 in each sequence) were analyzed. Apart from one participant reporting abdominal pain during n-3 PUFA treatment, we did not register any harm or unintended effects during the trial.
The mean age and body mass index were slightly higher, and more women were in the group starting with placebo (). Thirty-five per cent of the trial participants were treated with antiplatelet or anticoagulant therapy, which was stable during the trial. More individuals were on antiplatelet therapy or anticoagulants in the group starting with placebo (20%) than in the group beginning with n-3 PUFA (15%) ().
Table 1. Baseline population characteristics presented by sequence and by total.
The trial was conducted at the research outpatient clinic at Nordland Hospital (Bodø, Norway). In agreement with the prespecified sample size required, the trial inclusion was terminated after including 38 individuals. No period effect or treatment period interaction was found for the secondary endpoints.
Platelet function analyzer
The first trial participant was included before the platelet function analyzer was operative. Therefore, the median of 17 participants in the group starting with a placebo is presented as the baseline for treatment one. The median CT increased from baseline treatment one to post-washout, but the increase was equal in the two treatment sequence groups (). One sample after n-3 PUFA treatment was coagulated. Subsequently, 33 pairs of CT were analyzed (). No difference in CT was detected between treatment with n-3 PUFA (median: 92 s; interquartile range (IQR): 83–106) and placebo (median: 95.5 s; IQR: 86–106) (p = 0.93, median of differences 2 s, with 95% CI [-13, 6], ).
Figure 1. Closure time (A), intercellular adhesion molecule (ICAM) (B), vascular cell adhesion molecule (VCAM) (C), and P-selectin (D) after omega-3 polyunsaturated fatty acids (n-3 PUFA) and after placebo. SD: standard deviation. IQR: interquartile range.
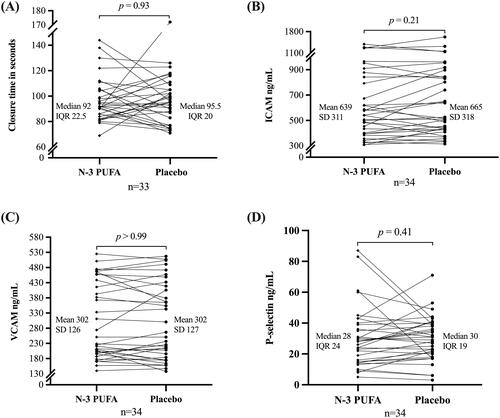
Table 2. Outcome variables at baseline are presented by sequence, treatment period, and total.
Soluble platelet function markers
The treatment sequence group starting with placebo had a larger variation in baseline ICAM than the group starting with n-3 PUFA (). On average, the ICAM level was lower after n-3 PUFA treatment (mean: 639 ng/mL; standard deviation (SD) 311 ng/mL) than after placebo (mean: 665 ng/mL; SD 318 ng/mL), but not statistically significant (p = 0.21, mean of differences: −27 ng/mL, 95%CI [–70, 16], t (33) = 1.27). No difference was found in VCAM levels after n-3 PUFA (mean: 302 ng/mL; SD = 126 ng/mL) and placebo (mean: 302 ng/mL, SD 127 mg/mL) (p > 0.99, mean of differences 0, 95%CI [–5, 2], t(33) = 0). P-selectin was lower after n-3 PUFA (median 28 ng/mL, IQR: 1539) than after placebo (median: 30 ng/mL; IQR: 20–39), but not statistically significant (p = 0.91, the median of differences −2 ng/mL, 95% CI [–5, 2], ).
Hematological parameters and C-reactive protein
There were no differences at baseline between the two sequence groups regarding hematological parameters and C-reactive protein (). Furthermore, hemoglobin, leukocytes, thrombocytes, neutrophil granulocytes, lymphocytes, monocytes, eosinophils, and C-reactive protein were unaffected by n-3 PUFA compared to placebo (). One individual had thrombocytes slightly below the reference level after n-3 PUFA, thrombocytes 127 x10E9/L (reference level 130-400 x10E9/L).
Table 3. Hematological parameters and C-reactive protein by treatment.
Cytokines
Several cytokines had values below lower detection limits or levels out of range. Complete values were obtained for six cytokines, and 16 cytokines had 1- 41% values below the lower detection limit or out of range (Supplementary Table 1). Five cytokines had ≥70% values below the lower detection limit and/or out of range (IL-5, IL-15, basic FGF, VEGF, and IFN-γ) and were excluded from the statistical analyses (Supplementary Table 1). The group starting with placebo had a higher median and variability in cytokine values at baseline than the group beginning with n-3 PUFA (Supplementary Table 2). There were no treatment effects of n-3 PUFA compared to placebo on cytokine levels (). IL-1β (p = 0.04) difference was not significant after correction for multiple comparisons ().
Table 4. Cytokine results presented by treatment.
Discussion
In this study, we found that platelet function, inflammatory markers, and hematological parameters were unaffected by the n-3 PUFA supplement in heterozygous FH patients. A low level of inflammation in our FH population is a possible explanation for this finding, as the trial participants were on lipid-lowering therapy plus acetylsalicylic acid when indicated.
None of the trial participants had anemia, thrombocytopenia, or known primary hemostasis defects at baseline, variables known to influence the CT result [Citation26]. The platelet function analyzer assesses the primary hemostasis in citrated whole blood under high shear stress [Citation27]. A low CT value corresponds to higher platelet reactivity. The CT values in our trial were comparable to prior trials investigating closure time in FH patients [Citation6,Citation28]. Our neutral results align with a study on healthy individuals where no change in CT (collagen ADP cartridge) was found after 28 days of n-3 PUFA supplement [Citation29].
One explanation for the lack of effect from n-3 PUFA in our trial is that the FH population was well-treated with statins. One trial described increased platelet survival in FH individuals after low-dose n-3 PUFA treatment [Citation23]. Statin use among the trial participants was not stated, but the elevated cholesterol levels reported indicate that the trial participants were not statin-treated [Citation23]. In a meta-analysis investigating the effect of n-3 PUFA on platelet aggregability, a reduction in platelet aggregability was found in subjects with CVD on antiplatelet treatment using lower doses of n-3 PUFA (<1.83 g/day) over a short period (< 8 weeks) [Citation22]. No effect from n-3 PUFA was found in healthy individuals on platelet aggregability [Citation22].
The platelet alpha granules release soluble P-selectin, IL-8, PDGF, and RANTES. All these variables were unaffected by the n-3 PUFA supplement in the present trial, proposing that the level of platelet activation was stable during the current trial ( and ). Other studies have communicated the same lack of effect from n-3 PUFA on soluble P-selectin in healthy individuals and individuals with high CVD risk [Citation30–32]. The pleiotropic effects of statins can be one reason for this lack of n-3 PUFA effect on platelet activation. Still, platelet activation has been reported in mutation-positive FH patients on lipid-lowering therapy [Citation33]. We found no effect of n-3 PUFA on the adhesion molecules ICAM and VCAM in our FH population. The effect of n-3 PUFA supplementation on adhesion molecules shows divergent results in meta-analyses [Citation20,Citation32]. Both neutral results and reductions in ICAM were found in healthy individuals and subjects with dyslipidemia; the effect of n-3 PUFA on VCAM seems to be limited [Citation20,Citation32]. The use of statins in our trial participants could explain the lack of effect on adhesion molecules. In observational studies on FH children, ICAM levels were higher in untreated FH children than in healthy controls. In contrast, ICAM and VCAM levels were equivalent in statin-treated FH children and healthy peers [Citation8,Citation9].
The overall neutral results in our trial suggest that n-3 PUFA supplements do not influence cytokine levels in FH individuals. Prior reports have described increased pro-inflammatory cytokines in FH individuals compared to healthy controls. Children with FH have higher pro-inflammatory cytokines TNF, IL-1β, and IL-6 than healthy control children [Citation7,Citation8]. In addition, FH children had lower levels of the anti-inflammatory cytokine IL-10 than healthy peers [Citation7]. Increased expression of TNF-related genes in peripheral blood mononuclear cells and higher levels of RANTES have been described in statin-treated FH adults compared to healthy controls [Citation4,Citation5]. As the crossover design in our study entails, a direct comparison to a healthy population is unavailable. However, except for IL-7, the cytokine results by treatment in our study are within the reference ranges of cytokines (in ethylenediaminetetraacetic acid plasma) proposed by Henno et al. [Citation34]. Our study population consisted of individuals with and without established coronary heart disease, but most did not have CVD. In CVD patients, possible effects of n-3 PUFA supplement on IL-6 levels are reported [Citation21,Citation35]. In line with our neutral findings, n-3 PUFA supplement in healthy individuals seems to have a limited effect on inflammatory markers. However, a possible, reducing the impact on TNF has been reported [Citation20,Citation21,Citation35].
In our study, the trial participants were treated with a high dose of a mixture of EPA and DHA. The diversity in n-3 PUFA preparations and dosages can explain the diverging results in n-3 PUFA trials. In one clinical trial with individuals at risk of CVD, DHA was more effective than EPA on the inflammatory markers IL-18 and adiponectin, and EPA was more effective than DHA in reducing IL-6 [Citation36]. In contrast, a meta-analysis showed an equal effect of DHA and EPA on the inflammatory markers IL-6, TNF, and adiponectin [Citation37]. Another explanation for varying results in previous n-3 PUFA trials has been the diversity in placebo choice. In our trial, the placebo was olive oil which has been used in several other studies. There are some indications that olive oil can reduce inflammatory markers [Citation38].
We found no treatment effect from n-3 PUFA supplements on our trial subjects’ hematological parameters. Earlier reports have demonstrated monocytosis in statin-treated FH children, with an increased subset of pro-inflammatory monocytes than healthy controls [Citation9]. The monocyte levels in the present trial were within the reference range and were unchanged throughout the trial. In a previous trial with healthy individuals, n-3 PUFA reduced the hemoglobin and thrombocyte levels after 28 days of supplementation [Citation29]. Despite a more extended intervention period in our trial, we did not find similar effects on hemoglobin and thrombocyte levels from the n-3 PUFA supplement.
Meta-analyses have shown a reduction in CRP by n-3 PUFA supplementation in healthy individuals and subjects with chronic non-autoimmune diseases [Citation20,Citation21]. The CRP level in the present trial was unaffected by the n-3 PUFA supplement; one reason can be that our trial participants had low levels of inflammation in general. It was previously shown that statin-treated FH individuals do not have higher CRP than healthy controls [Citation4,Citation5,Citation39].
The strengths of this trial were that this was a single-centre study, and the trial subjects were well characterized. We used a high dosage of n-3 PUFA supplement, and the length of the treatment periods was comparable to previous n-3 PUFA trials.
This trial has some limitations. First, the outcome presented was secondary endpoints and not power tested; thus, the result must be interpreted carefully. Still, the overall neutral results are convincing. Second, the trial population consisted of primary and secondary CVD prevention. A future trial investigating the effect of n-3 PUFA supplements in FH individuals with established CVD would be interesting. Third, the study population was relatively small, but the crossover design is strengthening. The results from this trial can be generalized to statin-treated FH subjects but not to a non-FH population with increased CVD risk or the general population.
In conclusion, high dose n-3 PUFA supplement in statin-treated FH subjects did not affect platelet function, inflammatory markers, or selected hematological parameters. This finding does not support n-3 PUFA supplementation in FH individuals, but further research is needed.
Supplemental Material
Download PDF (151.7 KB)Acknowledgments
BASF supplied study medication (n-3 PUFA and placebo) free of charge. The company received the manuscript 14 days before submission but did not influence the writing process.
Additional information
Funding
References
- Svendsen K, Krogh HW, Igland J, et al. 2.5-fold increased risk of recurrent acute myocardial infarction with familial hypercholesterolemia. Atherosclerosis. 2021;319:28–34.
- Besseling J, Hovingh GK, Huijgen R, et al. Statins in familial hypercholesterolemia: consequences for coronary artery disease and All-Cause mortality. J Am Coll Cardiol. 2016;68(3):252–260.
- Chiva-Blanch G, Padro T, Alonso R, et al. Liquid biopsy of extracellular microvesicles maps coronary calcification and atherosclerotic plaque in asymptomatic patients With familial hypercholesterolemia. Arterioscler Thromb Vasc Biol. 2019;39(5):945–955.
- Holven KB, Narverud I, Lindvig HW, et al. Subjects with familial hypercholesterolemia are characterized by an inflammatory phenotype despite long-term intensive cholesterol lowering treatment. Atherosclerosis. 2014;233(2):561–567.
- Hovland A, Narverud I, Lie Oyri LK, et al. Subjects with familial hypercholesterolemia have lower aortic valve area and higher levels of inflammatory biomarkers. J Clin Lipidol. 2021;15(1):134–141.
- Barale C, Bonomo K, Frascaroli C, et al. Platelet function and activation markers in primary hypercholesterolemia treated with anti-PCSK9 monoclonal antibody: a 12-month follow-up. Nutr Metab Cardiovasc Dis. 2020;30(2):282–291.
- Narverud I, Ueland T, Nenseter MS, et al. Children with familial hypercholesterolemia are characterized by an inflammatory imbalance between the tumor necrosis factor alpha system and interleukin-10. Atherosclerosis. 2011;214(1):163–168.
- Charakida M, Tousoulis D, Skoumas I, et al. Inflammatory and thrombotic processes are associated with vascular dysfunction in children with familial hypercholesterolemia. Atherosclerosis. 2009;204(2):532–537.
- Christensen JJ, Osnes LT, Halvorsen B, et al. Altered leukocyte distribution under hypercholesterolemia: a cross-sectional study in children with familial hypercholesterolemia. Atherosclerosis. 2017;256:67–74.
- Libby P. Inflammation in atherosclerosis. Arterioscler Thromb Vasc Biol. 2012;32(9):2045–2051.
- Hansson GK, Hermansson A. The immune system in atherosclerosis. Nat Immunol. 2011;12(3):204–212.
- Ridker PM, Devalaraja M, Baeres FMM, et al. IL-6 inhibition with ziltivekimab in patients at high atherosclerotic risk (RESCUE): a double-blind, randomised, placebo-controlled, phase 2 trial. The Lancet. 2021;397(10289):2060–2069.
- Ridker PM, Everett BM, Thuren T, et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med. 2017;377(12):1119–1131.
- Ridker PM, Everett BM, Pradhan A, et al. Low-Dose methotrexate for the prevention of atherosclerotic events. N Engl J Med. 2019;380(8):752–762.
- O'Donoghue ML, Glaser R, Cavender MA, et al. Effect of losmapimod on cardiovascular outcomes in patients hospitalized With acute myocardial infarction: a randomized clinical trial. JAMA. 2016;315(15):1591–1599.
- Abdelhamid AS, Brown TJ, Brainard JS, et al. Omega-3 fatty acids for the primary and secondary prevention of cardiovascular disease. The Cochrane Database of Systematic Reviews. 2020;2020(3):CD003177.
- Bhatt DL, Steg PG, Miller M, et al. Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. N Engl J Med. 2019;380(1):11–22.
- Mach F, Baigent C, Catapano AL, et al. 2019 ESC/EAS guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J. 2020;41(1):111–188.
- Adili R, Voigt EM, Bormann JL, et al. In vivo modeling of docosahexaenoic acid and eicosapentaenoic acid-mediated inhibition of both platelet function and accumulation in arterial thrombi. Platelets. 2019;30(2):271–279.
- AbuMweis S, Jew S, Tayyem R, et al. Eicosapentaenoic acid and docosahexaenoic acid containing supplements modulate risk factors for cardiovascular disease: a meta-analysis of randomised placebo-control human clinical trials. J Hum Nutr Diet. 2018;31(1):67–84.
- Li K, Huang T, Zheng J, et al. Effect of marine-derived n-3 polyunsaturated fatty acids on C-reactive protein, interleukin 6 and tumor necrosis factor alpha: a meta-analysis. PloS One. 2014;9(2):e88103.
- Gao LG, Cao J, Mao QX, et al. Influence of omega-3 polyunsaturated fatty acid-supplementation on platelet aggregation in humans: a meta-analysis of randomized controlled trials. Atherosclerosis. 2013;226(2):328–334.
- Pirich C, Gaszo A, Granegger S, et al. Effects of fish oil supplementation on platelet survival and ex vivo platelet function in hypercholesterolemic patients. Thromb Res. 1999;96(3):219–227.
- Hande LN, Thunhaug H, Enebakk T, et al. Addition of marine omega-3 fatty acids to statins in familial hypercholesterolemia does not affect in vivo or in vitro endothelial function. J Clin Lipidol. 2019;13(5):762–770.
- Hande LN, Kjellmo C, Pettersen K, et al. Effect of N-3 polyunsaturated fatty acids on lipid composition in familial hypercholesterolemia: a randomized crossover trial. Biomedicines. 2022;10(8):1809.
- Hayward CP, Harrison P, Cattaneo M, et al. Platelet function analyzer (PFA)-100 closure time in the evaluation of platelet disorders and platelet function. J Thromb Haemost. 2006;4(2):312–319.
- Paniccia R, Priora R, Liotta AA, et al. Platelet function tests: a comparative review. Vasc Health Risk Manag. 2015;11:133–148.
- Blazek M, Blaha M, Pecka M, et al. Primary hemostasis in patients treated with LDL-apheresis for severe familiar hypercholesterolemia: a prospective pilot trial using PFA-100 analysis to rationalize therapeutic LDL-apheresis procedure. Hematology. 2007;12(6):571–576.
- Larson MK, Shearer GC, Ashmore JH, et al. Omega-3 fatty acids modulate collagen signaling in human platelets. Prostaglandins Leukot Essent Fatty Acids. 2011;84(3-4):93–98.
- Yusof HM, Miles EA, Calder P. Influence of very long-chain n-3 fatty acids on plasma markers of inflammation in Middle-aged men. Prostaglandins Leukot Essent Fatty Acids. 2008;78(3):219–228.
- Lee KW, Blann AD, Lip GY. Effects of omega-3 polyunsaturated fatty acids on plasma indices of thrombogenesis and inflammation in patients post-myocardial infarction. Thromb Res. 2006;118(3):305–312.
- Yang Y, Lu N, Chen D, et al. Effects of n-3 PUFA supplementation on plasma soluble adhesion molecules: a meta-analysis of randomized controlled trials. Am J Clin Nutr. 2012;95(4):972–980.
- Suades R, Padro T, Crespo J, et al. Liquid biopsy of extracellular microvesicles predicts future major ischemic events in genetically characterized familial hypercholesterolemia patients. Arterioscler Thromb Vasc Biol. 2019;39(6):1172–1181.
- Henno LT, Storjord E, Christiansen D, et al. Effect of the anticoagulant, storage time and temperature of blood samples on the concentrations of 27 multiplex assayed cytokines - Consequences for defining reference values in healthy humans. Cytokine. 2017;97:86–95.
- Rangel-Huerta OD, Aguilera CM, Mesa MD, et al. Omega-3 long-chain polyunsaturated fatty acids supplementation on inflammatory biomakers: a systematic review of randomised clinical trials. Br J Nutr. 2012;107 Suppl 2(Suppl 2):S159–S170.
- Allaire J, Couture P, Leclerc M, et al. A randomized, crossover, head-to-head comparison of eicosapentaenoic acid and docosahexaenoic acid supplementation to reduce inflammation markers in men and women: the comparing EPA to DHA (ComparED) study. Am J Clin Nutr. 2016;104(2):280–287.
- Vors C, Allaire J, Mejia SB, et al. Comparing the effects of docosahexaenoic and eicosapentaenoic acids on inflammation markers using pairwise and network Meta-Analyses of randomized controlled trials. Adv Nutr. 2021;12(1):128–140.
- Schwingshackl L, Christoph M, Hoffmann G. Effects of olive oil on markers of inflammation and endothelial function– a systematic review and meta-analysis. Nutrients. 2015;7(9):7651–7675.
- Hovland A, Aagnes I, Brekke OL, et al. No evidence of impaired endothelial function or altered inflammatory state in patients with familial hypercholesterolemia treated with statins. J Clin Lipidol. 2010;4(4):288–292.
