Abstract
Acute kidney injury (AKI) is common in COVID-19 and is diagnosed using relative serum creatinine increase. Estimated GFR (eGFR) is a more accurate measure of glomerular filtration due to compensation for age and sex. Serum Cystatin-C, less affected by non-renal factors than creatinine, may further improve renal function estimation and add prognostic information. Our aim is to investigate the importance of a calculated eGFR in relation to creatinine as well as the value of Cystatin-C in patients with severe COVID-19. This study is a retrospective cohort study investigating levels and trends of routine laboratory parameters combined with clinical data from 286 consecutive patients with severe COVID-19 from Karolinska University Hospital. AKI developed in 38% of the patients and 15% were treated with hemodialysis. Mortality in the AKI group was 42% compared to 5% in the non-AKI group. At admission, eGFR, but not creatinine, was significantly associated with AKI development, need of intubation and mortality. Moreover, discrepant results between eGFR creatinine (eGFRCR) and eGFR Cystatin-C (eGFRCYS) was common in the ICU patients compared to non-ICU patients and related to outcome. In addition, we found that daily median Cystatin-C levels during the hospital stay were correlated to neutrophil count. eGFRCR was found to be an overall better prognostic marker than creatinine regarding AKI development and prognosis in severe COVID-19. Fulfillment of Shrunken pore syndrome criteria indicated a higher mortality risk. Cystatin-C may be related to neutrophil count, which could be a clue to the discrepant eGFR results.
Introduction
Kidney involvement in coronavirus disease 2019 (COVID-19) have been of interest since the first phase of the pandemic. Development of acute kidney injury (AKI) is seen in 0–46% symptomatic COVID-19 patients and in 68–80% of the patients in need of intensive care [Citation1–5]. Patients hospitalized with COVID-19 has been found to have a higher incidence of severe AKI compared with controls [Citation6] and the development of AKI is an independent prognostic factor of morbidity and mortality [Citation7].
The pathophysiology explaining the kidney damage is not fully understood but could be due to changes in blood pressure or a direct viral effect causing cellular damage as the virus, SARS-Cov2, have been found in kidney tissue of patients with severe COVID-19 [Citation8]. Other complicating factors discussed are impairment of the renin-angiotensin-aldosterone system, hypovolemia and stress from a highly catabolic state or damage via COVID-19 coagulopathy [Citation9–11].
Biomarkers estimating kidney function have been investigated for their prognostic value of morbidity and mortality. Creatinine has been reported to be higher at admission in patients with developing severe disease and a prognostic factor of death [Citation12–14].
Cystatin-C, another protein biomarker of kidney function, is not frequently measured in the intensive care unit (ICU). An advantage of Cystatin-C is that it, contrary to creatinine, is independent of muscle mass, and it is suggested that Cystatin-C is more reliable than creatinine in identifying early disturbances in glomerular filtration [Citation15,Citation16]. A few studies of COVID-19 have included Cystatin-C as a potential prognostic biomarker, and it has been used in models of detecting severe COVID-19 [Citation17–19].
Estimated glomerular infiltration rate (eGFR) calculated by modern equations (developed using standardized analytical methods and exogenous clearance determinations as reference methods) from creatinine or Cystatin-C gives an estimate of kidney function without the need for laborious and time-consuming invasive clearance measurements. Since muscle mass is declining in patients with severe illness in ICU, plasma creatinine and eGFR formulas using creatinine are considered unreliable. However, combining creatinine based estimated glomerular filtration rate (eGFRCR) and Cystatin-C-based estimated glomerular filtration rate (eGFRCYS), mean eGFR has been shown to be a better filtration estimate than either biomarker alone, also in severe ill patients in ICU [Citation20]. Decreased eGFR is seen in the acute phase of COVID-19 but emerging evidence also show that eGFR is continuously decreased among patients with a history of COVID-19-associated AKI [Citation21,Citation22].
A discrepancy between eGFRCR and eGFRCYS, in different patient groups, have been observed and studied prior to the pandemic [Citation23–25]. This could perhaps be explained by increased synthesis or reduced elimination of Cystatin-C, especially in combination with a reduction in creatinine due to muscle mass reduction. Corticosteroids are known to increase synthesis of Cystatin C and other endogenous factors may also affect Cystatin C synthesis, but to a lesser extent. Another explanation to the discrepancy could be presence of shrunken pore syndrome (SPS) a condition where the glomerular pores of the kidney decrease in size and molecules with a size of 5- to 40-kDa molecules have a reduced ability to pass through the pores. This means that Cystatin-C (13 kDa) accumulate in plasma while creatinine (113 Da) passes thru the pores. The original definition of SPS is eGFRCYS being less than 60% of eGFRCR, using modern eGFR equations [Citation26]. Fulfilling SPS-criteria is common in severe illness, and a greater discrepancy between eGFRCR and eGFRCYS is related to increased mortality [Citation27]. A recent study showed divergent levels of eGFRCR and eGFRCYS in critically ill COVID-19 patients at admission to the ICU [Citation28], however in that study only admission levels were presented.
We performed a study of hospitalized patients with severe COVID-19 with the aim to investigate which of the common routine kidney parameters, calculated eGFR, creatinine and Cystatin-C, is the best estimate to predict severe disease and mortality. Evaluation of the discrepancy between eGFRCR and eGFRCYS was also included.
Material and methods
The study is a retrospective observational cohort study of patients with severe, RT-PCR confirmed, COVID-19 disease. Severe COVID-19 was defined as having <94% SaO2 without oxygen treatment [Citation29,Citation30]. The project has been given ethical approval (D-nr 2020-01752) by the Swedish Ethical Review Authority.
From March to April 2020, 305 consecutive patients admitted to a hospital in the Stockholm region were included. The cohort comprised patients admitted to Karolinska’s two sites: Huddinge and Solna. They were followed from admission until discharge or death, last day of data collection being 30 June 2020. Patients could have been transferred from another hospital in the Stockholm Region, but all were treated at some time point at Karolinska. The referring hospitals and outpatient clinics in the region are connected to the Karolinska University Laboratory enabling collection of laboratory information from admittance and through follow-up.
Nineteen patients were excluded due to lost to follow up due to being transferred to a hospital outside of the Stockholm region before full recovery, and the final study cohort consisted of 286 patients.
For statistical analysis of discrepancy between eGFRCR and eGFRCYS patients deceased, due to co-morbidities not directly related to COVID-19, in the first week of hospitalization (N = 8) were excluded. Since the present study was conducted before dexamethasone was introduced in the treatment of severe COVID-19 (the RECOVERY study) [Citation31], none of our patient’s received dexamethasone.
Data collection
Descriptive data about the patients were extracted retrospectively from medical records including age, sex, hypertension, diabetes mellitus type 2, chronic obstructive pulmonary disease (COPD) and preexisting kidney disease. During the study period data were collected regarding onset of symptoms, date of admission to hospital, duration at the ICU (including need of intubation), thrombotic events and in hospital death.
From the Karolinska university laboratory database, available results from inflammation and kidney function parameters; C-reactive protein, procalcitonin, interleukin-6, lymphocyte and neutrophil count as well as creatinine and Cystatin-C were collected continuously and daily during the study period or a maximum of 21 days (after three weeks the biomarker pattern was considered unreliable due to secondary insults and not primary COVID-19).
Estimated glomerular filtration rate was calculated by CAPA (Caucasian, Asian, pediatric and adult) [Citation32] and the revised Lund-Malmö [Citation33] GFR estimating equations for Cystatin-C and creatinine respectively.
For more information on laboratory assays, analytical methods and reference intervals, see Supplemental Table 1.
Outcomes
‘Acute kidney injury’ (AKI) was defined as per Kidney Disease Improving Global Outcomes (KDIGO) criteria: an increase in serum creatinine of >26.6 µmol/L (>0.3 mg/dl) over a 48-h period or 50% increase in baseline creatinine [Citation34]. Fulfilling ‘shrunken pore syndrome’ criteria was determined by eGFRCYS being less than 60% of eGFRCR. Thrombotic events were confirmed by routine radiology assessment. Presence of ‘ICU care’ was defined as the patient admitted to a unit that provides intensive and specialized medical and nursing care, with a high capacity for monitoring and organ support, to patients with life-threatening organ system insufficiencies [Citation35]. The need of either ‘dialysis’ or ‘mechanical ventilation’ was only present in the ICU cohort and implicated treatment with continuous renal replacement therapy (CRRT), respectively intubation and connection to ventilatory support. ‘Discharge’ was characterized as discharged from hospital to the patient’s home or to an inpatient rehabilitation facility. ‘Deceased’ were patients that died during hospitalization, as noted in the medical records.
Statistical analysis
Statistical analysis was performed using SPSS (version 26.0, IBM Corp.). Normality of data was assessed using Shapiro–Wilk test. Pearson’s χ2 test was used to evaluate possible differences in distributions between groups. Binary logistic regression was used to calculate the odds ratio (OR) and investigate the effect of clinical variables on the risk of death and the risk of acute kidney injury. Medians at each time point were calculated from existing laboratory results, and no interpolation of data was performed. The Mann–Whitney U-test was used for pairwise comparisons. A p value <.05 was considered significant.
Results
Descriptive statistics of the total cohort and subgroups
The cohort was divided in two groups, one where the patients needed critical care (ICU), and where the patients did not need critical care (non-ICU) (see ). There were a larger proportion of women in the non-ICU group compared to in the ICU group but otherwise no significant differences regarding other descriptive parameters at admission. In the cohort, 10% (19 ICU and 9 non-ICU patients) had the diagnosis of asthma in their patient medical record but only 6 had low dose maintenance treatment with inhaled steroids.
Table 1. Clinical characteristics on admittance of 286 COVID-19 patients.
Comparing admission laboratory characteristics to levels after a week of hospitalization shows an increasing inflammatory response and declining kidney function (see ).
Table 2. Clinical routine laboratory characteristics of 286 patients with severe COVID-19.
Outcomes of the total cohort and subgroups
During the hospital stay a large proportion (38%) of the cohort developed AKI according to the KDIGO criteria. In the ICU, 48% of the patients developed AKI stage 3 (see ).
Table 3. Clinical outcome characteristics of 286 patients with severe COVID-19.
Analysis and associations of the kidney function parameters
At admission a low eGFRCR was associated with higher odds of acute kidney injury (OR: 0.976; 95% CI: 0.964–0.990, p < .001), intubation (OR: 0.986; 95% CI: 0.974–0.998, p = .021) and death (OR: 0.983; 95% CI: 0.969–0.998, p = .023) using binary logistic regression adjusted for presence of diabetes mellitus type 2, hypertension and COPD. In other words, a decrease of eGFR by 10 mL/min/1,73m2 would increase the odds of death by 18,5%. Importantly, there were no statistically significant association between AKI, intubation and death and admission levels of creatinine (p = .063, p = .255, p = .191, respectively). The associations between creatinine and AKI, intubation or death occurred at day 1, 2 and 4, respectively.
Increased levels of Cystatin-C at admission were associated with higher odds of death (p = .044) and lower eGFRCYS with higher odds of AKI (p = .048) using the same model as for analysis of creatinine stated above.
Dynamics of kidney function parameters over time
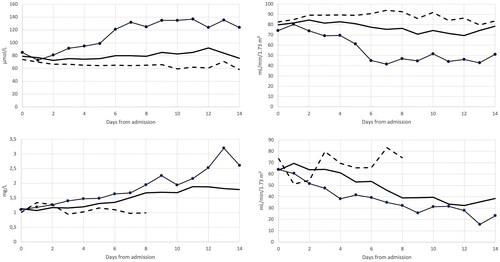
Median creatinine increased and creatinine based eGFR decreased over time in the patients who did not survive () and both parameters reached peak levels around hospital day 7. As creatinine dynamics indicated, an association between increased levels of creatinine and higher odds of death began by day 4 of hospitalization and remained for the rest of the study period. Similar dynamics was also seen with Cystatin-C and Cystatin-C based eGFR (Supplemental Figure 1), but whereas creatinine reached a peak level around day 7, Cystatin-C continued to increase during the study period.
Figure 1. Median plasma creatinine (left upper plot) and creatinine based eGFR (right upper lot), median plasma Cystatin-C (left lower plot) and Cystatin-C based eGFR (right lower plot) divided in groups of ICU deceased (black line with dots), ICU survivors (black whole line) and non-ICU patients (broken black line).
Discrepant eGFR; descriptive statistics and association to death
Paired results of eGFRCR and eGFRCYS was available in 201 patients. Of these 201 patients, 58% developed an eGFRCYS/eGFRCR ratio <0.6 during their hospital stays (see and ). In routine samples from an outpatient material, the frequency of an eGFRCYS/eGFRCR ratio <0.6 was less than 1%, n = 3584 (data not published).
Figure 2. Left panel: median creatinine based eGFR (broken black line) and Cystatin-C based eGFR (black line) over time. Right panel: eGFRCYS/eGFRCR ratio (grey line) over time.
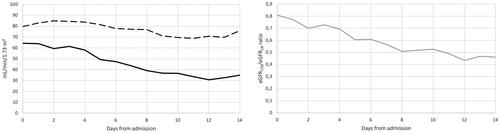
Table 4. Discrepant eGFR in patients with severe COVID-19.
Mortality was significantly higher in the group with eGFRCYS/eGFRCR ratio <0.6 (fulfilling SPS criteria) compared to the group with eGFRCYS/eGFRCR ratio >0.6 (p = .030 calculated with chi-square test). Over the study period, lower eGFRCYS/eGFRCR ratio was associated with increased odds of death in the total cohort, also when adjusted for hypertension, Diabetes mellitus type 2 and COPD, (OR: 0.977; 95% CI: 0.961–0.993). For every decrease of 0.1 in eGFRCYS/eGFRCR ratio, the odds of death increase by 27%. Early development of an eGFR discrepancy >40%, during the first seven days of hospitalization, resulted in a mortality rate of 39%.
Correlation of kidney function and inflammation parameters
Correlation between daily medians over the first 15 days of hospitalization were found between eGFRCYS/eGFRCR ratio and neutrophil count (r2 = 0.94). In addition, very strong correlations could be found between median eGFRCYS and neutrophil count as well as Cystatin-C and neutrophil count (r2 = 0.89 and r2 = 0.88, respectively). We also found a correlation, however less strong, between PCT and eGFRCYS/eGFRCR ratio (r2 = 0.59). Regarding CRP, IL-6, creatinine and eGFRCR no notable correlations were seen in our cohort.
Discussion
In this study of patients with severe COVID-19, we found a high frequency of patients fulfilling the SPS criteria, that a lower eGFRCYS/eGFRCR ratio was associated with higher mortality and that a decrease in eGFRCR is an earlier predictor of poor outcome than an increase in creatinine. We also found a very high frequency of AKI, in particular, in patients in the ICU.
Already at admission, low eGFRCR, was associated with development of AKI, dialysis and death. This is likely due to the fact that calculation of eGFR, adjusting for sex and age, provides a better estimate of kidney function, on an individual basis, using the patients’ own characteristics.
Measurement of both Cystatin-C and creatinine allows for calculation of an eGFRCYS/eGFRCR ratio with prognostic value, but it also allows for calculation of mean eGFR ((eGFRCR + eGFRCYS)/2), which has been shown to be the best agreement between estimated and measured GFR [Citation20].
We found that about 60% of the patients fulfilled the criteria for SPS in our cohort, and in this subgroup more patients died. In addition, a lower eGFRCYS/eGFRCR ratio was associated with increased odds of death. This suggests that a low eGFRCYS/eGFRCR ratio, and fulfillment of SPS criteria, can be used as a prognostic marker. Our findings of very strong correlations between Cystatin-C, eGFRCYS and mean neutrophils in the ICU group, could indicate that neutrophils are important for the development of a discrepancy between eGFRCR and eGFRCYS and other inflammatory factors, like CRP and IL-6 are not, which is in agreement with Mårtensson et al. [Citation24]. In an anonymized cohort of patients from the routine laboratory, there was no correlation to be found, supporting the assumption that activation of neutrophils is related to the condition, (data not presented). There was only a poor correlation between creatinine and neutrophil count found in our cohort, and this may suggest that activation of neutrophils affect the levels of Cystatin-C. We cannot however exclude the reverse situation, that Cystatin C affects neutrophil numbers.
Increasing discrepancy between eGFRCR and eGFRCYS seems to develop over the cause of the disease and is found in higher frequency in the later stages of the disease. This might also be influenced by the catabolic state of the critically ill patients in our cohort. Creatinine decreases with about 1% per day in patients admitted to the ICU [Citation36], which may correspond to reduced muscle mass. This could be part of the explanation of the discrepant eGFR levels, but it does not change the fact that the eGFR discrepancy is an indicator of poor prognosis.
The frequency of AKI and dialysis in our cohort was 38% and 15% respectively. Of the patients developing AKI 36% had stage 1, 20% stage 2 and 44% stage 3 according to the KDIGO criteria. Compared to Chan et al. the frequency of AKI and proportion of the different KDIGO stages are similar but the proportion with dialysis was higher, 40% compared to 19% [Citation5]. This might be explained by the number of ICU patients in our cohort being considerably larger, 57% vs 24%, and therefore more patients could be treated with CRRT, and this may account for the observed difference.
The mortality rate of patients with AKI was 42%, compared to 5% of the patients without AKI which is slightly lower than in Chan et.al. (50% and 8%, respectively) despite including more ICU patients.
Sampathkumar et al. found a smaller proportion of AKI in their cohort (7%), but similar mortality (44%) in their group of patients with AKI [Citation37]. This finding of fewer patients with AKI, may be caused by different diagnostic criteria or different composition of the cohort.
This a single-center cohort study and this may confer limitations compared to multicenter research, including risk of smaller sample sizes and homogenous study population leading to less generalizable findings. However, to increase the generalizability of the study, a relatively large study population has been recruited, which originates from the entire Stockholm region consisting of 2.4 million inhabitants (December 2021) and about a quarter of these were born abroad [Citation38]). Karolinska University Hospital is the primary tertiary hospital of the Stockholm region and severely ill patients were transferred here in the beginning of the pandemic.
This study is conducted partly on ICU patients with severe COVID-19 that are subjects to invasive treatments and monitoring that might affect the biomarker levels such that the observations not only represent the progress of the disease itself. However, an important strength with the present study is that all patients included in this study were hospitalized before the RECOVERY study [Citation31] and did not receive treatment with dexamethasone, a treatment well known to increase Cystatin-C production and falsely lowering eGFR based on Cystatin-C. Of the total cohort, only 6 patients had the diagnose of asthma with daily low dose inhaled steroids as maintenance treatment. Being so few patients, we assess that this does not affect the outcome of the study.
Conclusions
We found that it is common with discrepant eGFRCR and eGFRCYS and fulfillment of SPS criteria in severe COVID-19, and the discrepancy is aggravated during the course of the disease. A pronounced difference, as seen in SPS, is associated with severe disease and mortality. An early decrease in eGFRCR has a significantly higher odds of AKI, dialysis requirement and death compared to increased creatinine. We also found that Cystatin-C level and eGFRCYS/eGFRCR ratio is correlated to neutrophil count, a fact that may help to explain the occurrence of the discrepancy between eGFRCR and eGFRCYS.
Ethical approval
The project has been given ethical approval (D-nr 2020-01752) by the Swedish Ethical Review Authority
Author contributions
AS conceived the study, gained ethical approval, collected data and wrote the first draft of the manuscript. All authors were involved in data analysis, reviewed and edited the manuscript and approved the final version of the manuscript.
Supplemental Material
Download PDF (54.9 KB)Acknowledgements
The authors are grateful to all the nurses and doctors who worked day and night, caring for these severely ill patients, making it possible for us to conduct this study.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Na KR, Kim HR, Ham Y, et al. Acute kidney injury and kidney damage in COVID-19 patients. J Korean Med Sci. 2020;35(28):e257.
- Rubin S, Orieux A, Prevel R, et al. Characterization of acute kidney injury in critically ill patients with severe coronavirus disease 2019. Clin Kidney J. 2020;13:354–361.
- Wang L, Li X, Chen H, et al. Coronavirus disease 19 infection does not result in acute kidney injury: an analysis of 116 hospitalized patients from Wuhan, China. Am J Nephrol. 2020;51(5):343–348.
- Hirsch JS, Ng JH, Ross DW, et al. Acute kidney injury in patients hospitalized with COVID-19. Kidney Int. 2020;98(1):209–218.
- Chan L, Chaudhary K, Saha A, et al. AKI in hospitalized patients with COVID-19. J Am Soc Nephrol. 2021;32(1):151–160.
- Fisher M, Neugarten J, Bellin E, et al. AKI in hospitalized patients with and without COVID-19: a comparison study. J Am Soc Nephrol. 2020;31(9):2145–2157.
- Cheng Y, Luo R, Wang K, et al. Kidney disease is associated with in-hospital death of patients with COVID-19. Kidney Int. 2020;97(5):829–838.
- Su H, Yang M, Wan C, et al. Renal histopathological analysis of 26 postmortem findings of patients with COVID-19 in China. Kidney Int. 2020;98(1):219–227.
- Gabarre P, Dumas G, Dupont T, et al. Acute kidney injury in critically ill patients with COVID-19. Intensive Care Med. 2020;46(7):1339–1348.
- Soleimani M. Acute kidney injury in SARS-CoV-2 infection: direct effect of virus on kidney proximal tubule cells. Int J Mol Sci. 2020;21(9):3275.
- Glowacka M, Lipka S, Mlynarska E, et al. Acute kidney injury in COVID-19. Int J Mol Sci. 2021;22(15):8081.
- Chen L, Yu J, He W, et al. Risk factors for death in 1859 subjects with COVID-19. Leukemia. 2020;34(8):2173–2183.
- Deng X, Liu B, Li J, et al. Blood biochemical characteristics of patients with coronavirus disease 2019 (COVID-19): a systemic review and meta-analysis. Clin Chem Lab Med. 2020;58(8):1172–1181.
- Shao M, Li X, Liu F, et al. Acute kidney injury is associated with severe infection and fatality in patients with COVID-19: a systematic review and meta-analysis of 40 studies and 24,527 patients. Pharmacol Res. 2020;161:105107.
- Shlipak MG, Sarnak MJ, Katz R, et al. Cystatin C and the risk of death and cardiovascular events among elderly persons. N Engl J Med. 2005;352(20):2049–2060.
- Inker LA, Schmid CH, Tighiouart H, et al. Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med. 2012;367(1):20–29.
- Wei XY, Jing D, Jia B, et al. Characteristics of in peripheral blood of 70 hospitalized patients and 8 diarrhea patients with COVID-19. Int J Med Sci. 2020;17(9):1142–1146.
- Yang Z, Shi J, He Z, et al. Predictors for imaging progression on chest CT from coronavirus disease 2019 (COVID-19) patients. Aging. 2020;12(7):6037–6048.
- Zinellu A, Mangoni AA, Cystatin C. COVID-19 severity and mortality: a systematic review and meta-analysis. J Nephrol. 2022;35(1):59–68.
- Ravn B, Rimes-Stigare C, Bell M, et al. Creatinine versus cystatin C based glomerular filtration rate in critically ill patients. J Crit Care. 2019;52:136–140.
- Nugent J, Aklilu A, Yamamoto Y, et al. Assessment of acute kidney injury and longitudinal kidney function after hospital discharge among patients with and without COVID-19. JAMA Netw Open. 2021;4(3):e211095.
- Bowe B, Xie Y, Xu E, et al. Kidney outcomes in long COVID. J Am Soc Nephrol. 2021;32(11):2851–2862.
- Ito R, Yamakage H, Kotani K, et al. Comparison of cystatin C- and creatinine-based estimated glomerular filtration rate to predict coronary heart disease risk in Japanese patients with obesity and diabetes. Endocr J. 2015;62(2):201–207.
- Martensson J, Martling CR, Oldner A, et al. Impact of sepsis on levels of plasma cystatin C in AKI and non-AKI patients. Nephrol Dial Transplant. 2012;27(2):576–581.
- Nakashima A, Horita S, Matsunaga T, et al. Factors contributing to discrepant estimated glomerular filtration values measured by creatinine and cystatin C in patients with rheumatoid arthritis. Sci Rep. 2021;11(1):9884.
- Akesson A, Lindstrom V, Nyman U, et al. Shrunken pore syndrome and mortality: a cohort study of patients with measured GFR and known comorbidities. Scand J Clin Lab Invest. 2020;80(5):412–422.
- Grubb A. Shrunken pore syndrome - a common kidney disorder with high mortality. Diagnosis, prevalence, pathophysiology and treatment options. Clin Biochem. 2020;83:12–20.
- Liu Y, Xia P, Cao W, et al. Divergence between serum creatine and cystatin C in estimating glomerular filtration rate of critically ill COVID-19 patients. Ren Fail. 2021;43(1):1104–1114.
- Infectious Diseases Society of America. IDSA guidelines on the treatment and management of patients with COVID-19; 2020 [cited 2021 Mar 14]. Available from: https://www.idsociety.org/practice-guideline/covid-19-guideline-treatment-and-management/
- National Institutes of Health. Clinical spectrum of SARS-CoV-2 infection; 2020 Dec 17 [cited 2021 Mar 14]. Available from: https://www.covid19treatmentguidelines.nih.gov/overview/clinical-spectrum/
- Horby P, Lim WS, Emberson JR, et al. Dexamethasone in hospitalized patients with Covid-19. N Engl J Med. 2021;384(8):693–704.
- Grubb A, Horio M, Hansson LO, et al. Generation of a new cystatin C-based estimating equation for glomerular filtration rate by use of 7 assays standardized to the international calibrator. Clin Chem. 2014;60(7):974–986.
- Nyman U, Grubb A, Larsson A, et al. The revised Lund-Malmo GFR estimating equation outperforms MDRD and CKD-EPI across GFR, age and BMI intervals in a large Swedish population. Clin Chem Lab Med. 2014;52:815–824.
- (KDIGO) KDIGO. Acute Kidney Injury (AKI). 2021. [cited 2021-09-10]; Available from https://kdigo.org/guidelines/acute-kidney-injury/.
- Marshall JC, Bosco L, Adhikari NK, et al. What is an intensive care unit? A report of the task force of the World Federation of Societies of Intensive and Critical Care Medicine. J Crit Care. 2017;37:270–276.
- Volbeda M, Hessels L, Posma RA, et al. Time courses of urinary creatinine excretion, measured creatinine clearance and estimated glomerular filtration rate over 30 days of ICU admission. J Crit Care. 2021;63:161–166.
- Sampathkumar Hanumaiah H, Rajiv A. Incidence, risk factors and outcome of COVID-19 associated AKI – a study from South India. J Assoc Phys India. 2021;69:11–12.
- Region_Stockholm. Modellutveckling av demografisk prognos 2018–2060/2040 efter personers bakgrund. [cited; DEMOGRAFIRAPPORT 2019 02]. Available from: https://www.regionstockholm.se/globalassets/4.-regional-utveckling/alla-projekt-inom-regional-utveckling/demografidagen2019/2-modellutveckling-bakgrund-2018-2060_2040.pdf
