Abstract
Chronic kidney disease (CKD) and low-grade inflammation are associated with increased risk of cardiovascular disease (CVD). Calprotectin, a protein mainly secreted by activated neutrophils during inflammatory conditions, has been linked to CVD risk in general populations. The aim of this study was to evaluate the association of calprotectin with CVD risk in CKD patients, relative to C-reactive protein (CRP). One hundred and fifty-three patients with moderate CKD were prospectively followed up at 5 and 10 years. We used Cox regression modelling with stepwise adjustments for other relevant covariates (age, sex, cystatin C, previous CVD, systolic blood pressure, HDL cholesterol and HbA1c) to assess the association of baseline calprotectin and CRP with the risk of fatal or non-fatal CVD events. Twenty-nine and 44 patients experienced a CVD event during median follow-up of 4.8 and 10.9 years, respectively. Higher calprotectin was associated with increased CVD risk at both time points, which remained statistically significant after multivariable adjustments, including adjustment for CRP. For CRP, the associations did not remain statistically significant after final multivariable adjustments. In conclusion, we have shown that in patients with CKD, calprotectin was independently associated with the risk of future CVD events, suggesting that calprotectin may provide prognostic information of CVD risk.
Introduction
Individuals with chronic kidney disease (CKD) represent a high-risk group for developing cardiovascular disease (CVD) [Citation1]. The increased CVD risk associated with CKD is only partly due the high prevalence of traditional CVD risk factors [Citation1,Citation2] and thus non-traditional kidney-specific risk factors, in particular, oxidative stress and low-grade inflammation, may be important links between CKD and CVD [Citation3].
Several inflammatory biomarkers, such as C-reactive protein (CRP), fibrinogen, albumin, white blood cells (WBCs), tumour necrosis factor (TNF) and interleukin (IL)-6 are associated with CVD risk in individuals with CKD [Citation4,Citation5]. Polymorphonuclear leukocytes are suggested to play a key role in accelerating atherosclerosis by releasing reactive oxygen species (ROS), granule content and inflammatory mediators [Citation3,Citation6,Citation7]. Neutrophils contain relatively large amounts of the cytoplasmatic proteins S100A8 and S100A9. Intracellularly, they preferentially form heterodimeric complexes, S100A8/A9, and once discharged into the extracellular space they also form tetramer complexes, (S100A8/A9)2 [Citation8]. These aggregates are known by the name ‘calprotectin’. Calprotectin is released to the circulation during inflammatory processes; either passively by cellular damage [Citation9] or by active secretion [Citation10]. Among the numerous extracellular biological functions ascribed to calprotectin, it is found to exhibit proinflammatory effects on human microvascular endothelial cells [Citation11] and leukocytes [Citation12]. Besides being a biomarker of disease activity in a variety of inflammatory conditions [Citation13,Citation14], increased levels of calprotectin predict cardiovascular events in general populations [Citation15–17] and is also suggested to be a mediator of CVD [Citation18,Citation19].
In this study, we evaluated calprotectin as a biomarker of CVD risk in a CKD population, comparing it to the well-established inflammatory marker CRP. Our hypothesis was that calprotectin concentrations would reflect the negative effects of low-grade chronic inflammation on future CVD risk.
Methods
Study design and population
The study population has previously been described in detail [Citation20]. Briefly, 160 participants were recruited from an outpatient clinic at a University Hospital in 2007–2009 and followed up at 5 years and 10 years. The inclusion criteria were: (1) having a diagnosis of CKD and (2) seeing a nephrologist on a regular basis. Of the 160 study participants, we excluded five individuals with high CRP values likely to be caused by infection, and two individuals with missing baseline calprotectin or CRP values. In total, 153 patients were included in the analysis. In the absence of data from other prospective studies using calprotectin as a CVD biomarker in a CKD population, this study was conducted as an exploratory study using the sample size available.
Data collection and baseline variables
Medical history, clinical measurements and blood samples were collected at inclusion. Among various laboratory analyses performed at inclusion were: serum and urine creatinine, blood HbA1c and haemoglobin, serum or plasma albumin, cystatin C, triglycerides, total cholesterol and HDL-cholesterol. Diabetes was defined as having a diagnosis of either diabetes type 1 or 2 according to the medical records. Smoking was recorded as non-smoking or current smoking. Hypertension was defined as systolic blood pressure (BP) ≥140 mmHg and/or diastolic BP ≥90 mmHg or use of antihypertensive medication. Previous CVD was defined as a history of coronary disease, cerebrovascular disease or peripheral artery disease. Estimated glomerular filtration rate (eGFR) was calculated using the Modification of Diet in Renal Disease (MDRD) study equation [Citation21]. Body mass index (BMI) was calculated as weight (kg) divided by the square of the height (m). Urine albumin/creatinine ratio (u-ACR) was also recorded.
Laboratory analyses
Blood sample handling, most laboratory analyses and analytical methods have previously been described [Citation20]. Blood samples drawn at baseline were routinely centrifuged (serum tubes with separation gel after 30 min of coagulation) before they were stored at 4 °C. Except for a few samples, which were stored for a maximum of 72 h, most samples were aliquoted and frozen within the same day as collection. Then they were stored at –80 °C until analysis. Serum calprotectin was measured using the Bühlmann MRP8/MRP14™ ELISA (enzyme-linked immunoassay) kit (Bühlmann Laboratories AG, Schönenbuch, Switzerland), which has a measuring range of 0.4–24 mg/L. Studies performed by the manufacturer using human samples ranging from 0.21 to 21.64 mg/L and 0.9 to 12.1 mg/L, showed within-run and between-run coefficients of variation (CVs) of 2.4–7.4% and 4.7–7.4%, respectively. Plasma CRP was measured using a high sensitivity immunoturbidimetric assay with the measuring range 0.1–20.0 mg/L (Tina-quant CRP (Latex), Roche Diagnostics, Rotkreuz, Switzerland). Using human and control samples ranging from 0.55 to 12.36 mg/L, the assay provider found within-run and between-run CVs of 0.43–1.34% and 2.51–5.7%, respectively.
Follow-up and end-point assessment
The study outcome was non-fatal or fatal CVD events found by review of the medical records at each of the two follow-up assessments. For participants with multiple events, only the first event was recorded. Non-fatal CVD events were defined as hospitalisation due to acute myocardial infarction (AMI), unstable angina (UAP), ischemic stroke, transient ischemic attack (TIA), percutaneous coronary intervention (PCI), percutaneous transluminal coronary angioplasty (PTCA) or coronary artery bypass grafting (CABG). Fatal CVD events were defined as death by CVD according to the International Classification of Disease code version 9 or 10 (ICD-9: 401-459.9; ICD-10: I10-99) using data from the Norwegian Cause of Death Registry and medical records. Participants lost to follow-up by clinical visits were censored on the relevant date (n = 5 during the first follow-up period, n = 4 during the second).
Statistical analyses
Data are summarised as medians with interquartile range (IQR) or as total numbers with corresponding percentages. The Mann–Whitney U-test (continuous variables) or χ2 test (categorical variables) was used to compare characteristics of patients with and without positive outcome. Spearman’s correlation coefficient was used to explore correlation between calprotectin, CRP and the adjustment variables. Data distributions were assessed by graphical inspection of normal Q–Q plots and performance of the skewness and kurtosis test for normality.
Endpoint analyses were performed for 5 years and 10 years follow-up. We used Cox proportional hazards (PHs) modelling with age as the time variable, thereby adjusting for age in all the models and also ensuring that individuals of the same age were compared. Calprotectin and CRP were non-normally distributed and therefore log-transformed in the analyses to achieve better model fit. The effects of calprotectin and CRP were compared by evaluating the 95% confidence intervals (CIs) of hazard ratios (HRs) computed per standard deviation (SD) increment of log-transformed values. The SD for baseline loge calprotectin concentration was 0.53, which corresponds to a 1.7-fold difference (i.e. e0.53) in mg/L on the original scale of calprotectin measurement. Likewise, 1 SD loge CRP was 1.25, corresponding to a 3.5-fold higher CRP-concentration.
Separate models for calprotectin and CRP were adjusted for covariates in a stepwise manner, and finally calprotectin and CRP were also tested in the same model. Few positive outcomes limited the number of covariates we could adjust for, more so after 5 years, than after 10 years. Analyses were adjusted for covariates known to be important CVD risk factors in CKD. These include age, sex, kidney function (cystatin C), previous CVD, systolic BP, HDL cholesterol and diabetes (HbA1c). Smoking was omitted as a potential covariate because of very few (n = 5) smokers among the participants with a CVD event, and we also had no information of previous smoking history. BMI was omitted as a covariate because the literature indicated a very complex relationship between BMI and cardiovascular mortality in CKD, spanning from no significant associations in individuals with CKD stages 3–5, to an inverse relationship in certain subgroups (e.g. haemodialysis or end-stage kidney failure) [Citation22].
On step 1, the crude (adjusted by age as time variable) associations of calprotectin and CRP with risk of CVD events were examined. On step 2, we further adjusted for sex and cystatin C. On step 3, the analyses were additionally adjusted for previous CVD. There were more outcome events at 10 years follow-up, allowing for additional adjustments for systolic BP, HDL cholesterol and HbA1c (step 4). Finally, we included both calprotectin and CRP in the same fully adjusted model (step 5). Model fit was compared using the Akaike (AIC) and Bayesian information criteria (BIC), where lower values indicate better fit. In a sensitivity analysis at 10 years follow-up, we excluded patients with previous CVD (step 2).
The PHs assumption was assessed with Schoenfeld’s residuals, and to assess the linearity and functional form of the continuous variables, we plotted the Martingale residuals. In order to meet the PH assumption, the models were stratified based on previous CVD. Data were analysed using the software package STATA (version 17.0, StataCorp, College Station, TX). p Values <.05 were considered statistically significant.
Ethical declaration
All patients provided written informed consent at inclusion. This study was approved by the Norwegian Data Inspectorate and by the Regional Committee for Medical and Health Research Ethics (2018/646).
Results
Median age at study entry was 60 years (45–70) and 148 (97%) were of Caucasian origin. Forty-three patients (28%) had a history of previous CVD and 28 (18.3%) had a diagnosis of diabetes at inclusion. Baseline characteristics of the total study population and by outcome at 10 years follow-up are shown in .
Table 1. Baseline characteristics.
In total, 29 and 44 patients experienced a CVD event during median follow-up times of 4.8 and 9.7 years, respectively. Patients who experienced a CVD event were older, had higher incidence of previous CVD, higher systolic BP and higher levels of creatinine, HbA1c, cystatin C, calprotectin and CRP. Further, they had lower eGFR, HDL cholesterol and haemoglobin. Calprotectin and CRP were weakly correlated (Spearman’s rho = 0.31, p = .001), and even less to the adjustment variables age, previous CVD, cystatin C, systolic BP, HDL cholesterol and HbA1c.
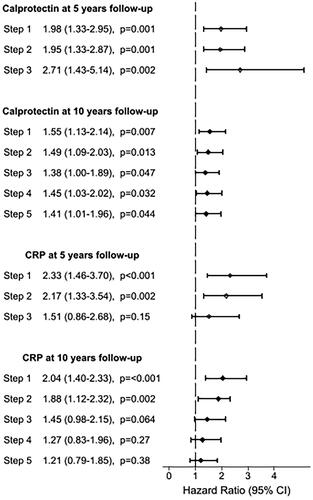
In Cox regression analyses, higher levels of calprotectin were statistically significantly associated with increased CVD risk at 5 and 10 years follow-up. These associations remained significant after adjustment for the other covariates through steps 1–5 (). We found similar associations for CRP (steps 1 and 2), but the associations did not remain statistically significant after final adjustments at 5 years (step 3) and 10 years (steps 3–5). When including calprotectin and CRP in the same model (step 5), the associations of calprotectin remained statistically significant. The associations were similar in the sensitivity analysis, excluding patients with previous CVD (n = 43).
Figure 1. Hazard ratios for biomarkers associated with cardiovascular events. HR is expressed per 1 SD increase of loge calprotectin and loge CRP at 5 and 10 years follow-up. Age was used as time axis and thereby adjusted for in all models. Step 1: adjusted for age. Step 2: adjusted for age, sex and cystatin C. Step 3: adjusted for age, sex, cystatin C and previous CVD. Step 4: adjusted for age, sex, cystatin C, previous CVD, systolic blood pressure, high-density lipoprotein (HDL) cholesterol and HbA1c. Step 5: adjusted for age, sex, cystatin C, previous CVD, HDL cholesterol, HbA1c, CRP and calprotectin. HR: hazard ratio; CI: confidence interval; CVD: cardiovascular disease; CRP: C-reactive protein.
Discussion
In the present cohort study of 153 patients with CKD, calprotectin was independently associated with the risk of CVD events after adjustments for other relevant risk factors. Additionally, we demonstrated that this finding was consistent during 5 and 10 years of follow-up. The association of CRP with risk of CVD after multivariable adjustments was not statistically significant.
Comparison with previous work
As far as we know, prospective studies investigating calprotectin in relation to CVD risk among CKD populations are lacking, but calprotectin is a biomarker of CVD risk, predicting incident CVD in studies of general populations [Citation15–17] and in survivors of acute coronary syndromes (ACSs) [Citation23]. Healy et al. found that calprotectin predicted CVD events in a prospective nested case-control study of 255 healthy postmenopausal women [Citation15]. Cotoi et al. reported an independent association of calprotectin with coronary events and CVD mortality in a cohort of 644 healthy middle-aged individuals [Citation16]. In a population-based study of 5290 individuals without CVD at study entry, Kunutsor et al. found an independent association of calprotectin with CVD events, and that adding calprotectin to the Framingham CVD Risk Score improved risk assessment [Citation17]. In a case control study of patients with ACS, elevated levels of calprotectin 30 days following the event were associated with increased risk of CVD death or recurrent myocardial infarction [Citation23].
Calprotectin and other CVD risk factors
Prior studies indicate that the increased CVD risk seen in CKD patients can only partly be explained by traditional CVD risk factors [Citation1], perhaps implicating that other non-traditional risk factors might be equally or more important in this population. Smoking and measures of impaired glucose metabolism (e.g. HbA1c, insulin resistance), obesity (BMI, body fat percentage), hyperlipidaemia (LDL cholesterol, HDL cholesterol) and CKD (albuminuria) are all CVD risk factors that have been associated with increased levels of calprotectin [Citation16,Citation24,Citation25]. In our study, calprotectin may therefore represent a composite effect of several of the measured CVD risk factors.
As suggested by Schiffrin et al., endothelial dysfunction, low-grade inflammation, dyslipidaemia and oxidative stress associated with CKD may accelerate atherosclerosis, thereby explaining the higher CVD risk in this population [Citation26]. Besides being a biomarker of inflammatory conditions and perhaps CVD risk, some even suggest that calprotectin may be an important mediator of CVD in itself [Citation18,Citation19,Citation27]. Among the proatherogenic mechanisms proposed for calprotectin is its ability to promote immune cell recruitment in the arterial wall by triggering secretion of inflammatory cytokines by the vascular endothelium and by impairing endothelial integrity [Citation11,Citation28].
In summary, calprotectin seems to play some role in the complex interactions between the immune system, traditional risk factors and CVD, but to what extent it directly contributes to the pathogenesis of CVD remains unknown.
Study limitations
The main limitation of this study is the relatively small sample size and thereby few outcome events per adjustment variable. The rule of thumb of ten or more events per variable in Cox models is not well defined and may be relaxed in analysis of causal influences in observational data, where adequate control of confounding is important [Citation29]. In the context of a low number of events, Vittinghoff and McCulloch state that there is a higher risk of making type 2 errors, than of type 1 errors. In our case, this would mean that there is a greater risk of misleading negative conclusions such as regarding CRP as a non-significant marker, than falsely reporting a statistical significant association between calprotectin and CVD risk [Citation29].
Another limitation of our study is that a significant proportion of the study participants had a history of previous CVD (established atherosclerosis) at inclusion, and/or were using statins. Known to affect inflammation in opposite directions [Citation30], this may have attenuated the associations between the baseline inflammatory biomarkers and the outcome.
Although we sought to handle all samples as standardised as possible, a few of the samples were stored at 4 °C for up to 72 h before freezing; thus, we cannot eliminate a possible impact on the measurements. However, we have previously shown that serum calprotectin is stable for up to 48 h [Citation31] when stored at 4 °C and 20 °C, and a recent study found that serum calprotectin is stable for up to seven days at 2–8 °C [Citation32]. Therefore, we expect the influence from this pre-analytical factor of delayed freezing to be minimal.
Lastly, when considering the generalisability of our results, it should be noted that the patients were mostly Caucasians and recruited from a single centre, but our population is probably representative of the majority of patients attending a Norwegian nephrology outpatient clinic.
Conclusions
In conclusion, we found that increased calprotectin concentrations at baseline were associated with higher risk of future CVD events in CKD patients. Circulating levels of calprotectin may provide prognostic information on CVD risk in addition to some traditional risk factors, but this needs to be verified in larger cohorts of patients with CKD.
Author contributions
L.L., V.V., G.G.H. and A.Å. contributed to the study concept and design. G.G.H. and L.L. contributed to the acquisition of data. L.L. and V.V. analysed and interpreted the data. L.L. drafted the manuscript. V.V. substantially revised the manuscript. G.G.H. and A.Å. revised the manuscript for important intellectual content. All authors approved the final version of the manuscript.
Acknowledgements
The authors are grateful for the assistance from the Department of Nephrology and the Department of Clinical Chemistry at St. Olav’s University Hospital in recruiting patients for this study and managing blood test analysis.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Gansevoort RT, Correa-Rotter R, Hemmelgarn BR, et al. Chronic kidney disease and cardiovascular risk: epidemiology, mechanisms, and prevention. Lancet. 2013;382(9889):339–352.
- Matsushita K, van der Velde M, Astor BC, et al. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet. 2010;375(9731):2073–2081.
- Cachofeiro V, Goicochea M, de Vinuesa SG, et al. Oxidative stress and inflammation, a link between chronic kidney disease and cardiovascular disease. Kidney Int Suppl. 2008;74:S4–S9.
- Weiner DE, Tighiouart H, Elsayed EF, et al. Inflammation and cardiovascular events in individuals with and without chronic kidney disease. Kidney Int. 2008;73(12):1406–1412.
- Amdur RL, Feldman HI, Dominic EA, et al. Use of measures of inflammation and kidney function for prediction of atherosclerotic vascular disease events and death in patients with CKD: findings from the CRIC study. Am J Kidney Dis. 2019;73(3):344–353.
- Sela S, Shurtz-Swirski R, Cohen-Mazor M, et al. Primed peripheral polymorphonuclear leukocyte: a culprit underlying chronic low-grade inflammation and systemic oxidative stress in chronic kidney disease. J Am Soc Nephrol. 2005;16(8):2431–2438.
- Silvestre-Roig C, Braster Q, Ortega-Gomez A, et al. Neutrophils as regulators of cardiovascular inflammation. Nat Rev Cardiol. 2020;17(6):327–340.
- Vogl T, Gharibyan AL, Morozova-Roche LA. Pro-inflammatory S100A8 and S100A9 proteins: self-assembly into multifunctional native and amyloid complexes. Int J Mol Sci. 2012;13(3):2893–2917.
- Voganatsi A, Panyutich A, Miyasaki KT, et al. Mechanism of extracellular release of human neutrophil calprotectin complex. J Leukoc Biol. 2001;70(1):130–134.
- Tardif MR, Chapeton-Montes JA, Posvandzic A, et al. Secretion of S100A8, S100A9, and S100A12 by neutrophils involves reactive oxygen species and potassium efflux. J Immunol Res. 2015;2015:296149.
- Viemann D, Strey A, Janning A, et al. Myeloid-related proteins 8 and 14 induce a specific inflammatory response in human microvascular endothelial cells. Blood. 2005;105(7):2955–2962.
- Pruenster M, Kurz AR, Chung KJ, et al. Extracellular MRP8/14 is a regulator of beta2 integrin-dependent neutrophil slow rolling and adhesion. Nat Commun. 2015;6:6915.
- Ehrchen JM, Sunderkotter C, Foell D, et al. The endogenous toll-like receptor 4 agonist S100A8/S100A9 (calprotectin) as innate amplifier of infection, autoimmunity, and cancer. J Leukoc Biol. 2009;86(3):557–566.
- Pruenster M, Vogl T, Roth J, et al. S100A8/A9: from basic science to clinical application. Pharmacol Ther. 2016;167:120–131.
- Healy AM, Pickard MD, Pradhan AD, et al. Platelet expression profiling and clinical validation of myeloid-related protein-14 as a novel determinant of cardiovascular events. Circulation. 2006;113(19):2278–2284.
- Cotoi OS, Duner P, Ko N, et al. Plasma S100A8/A9 correlates with blood neutrophil counts, traditional risk factors, and cardiovascular disease in middle-aged healthy individuals. Arterioscler Thromb Vasc Biol. 2014;34(1):202–210.
- Kunutsor SK, Flores-Guerrero JL, Kieneker LM, et al. Plasma calprotectin and risk of cardiovascular disease: findings from the PREVEND prospective cohort study. Atherosclerosis. 2018;275:205–213.
- Averill MM, Kerkhoff C, Bornfeldt KE. S100A8 and S100A9 in cardiovascular biology and disease. Arterioscler Thromb Vasc Biol. 2012;32(2):223–229.
- Croce K. S100A8/A9 complex: more than just a biomarker of cardiovascular risk? Circ J. 2010;74(4):626–627.
- Hov GG, Sagen E, Hatlen G, et al. Arginine/asymmetric dimethylarginine ratio and cardiovascular risk factors in patients with predialytic chronic kidney disease. Clin Biochem. 2011;44(8–9):642–646.
- Levey AS, Coresh J, Greene T, et al. Using standardized serum creatinine values in the Modification of Diet in Renal Disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145(4):247–254.
- Ladhani M, Craig JC, Irving M, et al. Obesity and the risk of cardiovascular and all-cause mortality in chronic kidney disease: a systematic review and meta-analysis. Nephrol Dial Transplant. 2017;32:439–449.
- Morrow DA, Wang Y, Croce K, et al. Myeloid-related protein 8/14 and the risk of cardiovascular death or myocardial infarction after an acute coronary syndrome in the pravastatin or atorvastatin evaluation and infection therapy: thrombolysis in myocardial infarction (PROVE IT-TIMI 22) trial. Am Heart J. 2008;155(1):49–55.
- Ortega FJ, Sabater M, Moreno-Navarrete JM, et al. Serum and urinary concentrations of calprotectin as markers of insulin resistance and type 2 diabetes. Eur J Endocrinol. 2012;167(4):569–578.
- Pedersen L, Nybo M, Poulsen MK, et al. Plasma calprotectin and its association with cardiovascular disease manifestations, obesity and the metabolic syndrome in type 2 diabetes mellitus patients. BMC Cardiovasc Disord. 2014;14:196.
- Schiffrin EL, Lipman ML, Mann JF. Chronic kidney disease: effects on the cardiovascular system. Circulation. 2007;116(1):85–97.
- Schiopu A, Cotoi OS. S100A8 and S100A9: DAMPs at the crossroads between innate immunity, traditional risk factors, and cardiovascular disease. Mediators Inflamm. 2013;2013:828354.
- Viemann D, Barczyk K, Vogl T, et al. MRP8/MRP14 impairs endothelial integrity and induces a caspase-dependent and -independent cell death program. Blood. 2007;109(6):2453–2460.
- Vittinghoff E, McCulloch CE. Relaxing the rule of ten events per variable in logistic and Cox regression. Am J Epidemiol. 2007;165(6):710–718.
- Diamantis E, Kyriakos G, Quiles-Sanchez LV, et al. The anti-inflammatory effects of statins on coronary artery disease: an updated review of the literature. Curr Cardiol Rev. 2017;13:209–216.
- Åsberg A, Løfblad L, Felic A, et al. Measuring calprotectin in plasma and blood with a fully automated turbidimetric assay. Scand J Clin Lab Invest. 2019;79(1–2):50–57.
- Mylemans M, Nevejan L, Van Den Bremt S, et al. Circulating calprotectin as biomarker in neutrophil-related inflammation: pre-analytical recommendations and reference values according to sample type. Clin Chim Acta. 2021;517:149–155.
