Abstract
Calprotectin (S100A8/S100A9, MRP8/MRP14) is a major leukocyte protein found to be more sensitive than C-Reactive Protein (CRP) and Erythrocyte Sedimentation Rate (ESR) as a marker of inflammation in patients with rheumatoid arthritis (RA). The present objective was to explore the robustness of calprotectin assessments by comparing two different laboratory methods assessing calprotectin in plasma samples from patients with early or established RA. A total of 212 patients with early RA (mean (SD) age 52(13.3) years, disease duration 0.6(0.5) years) and 177 patients with established RA (mean (SD) age 52.9(13.0) years, disease duration 10.0(8.8) years) were assessed by clinical, laboratory, and ultrasound examinations. Frozen plasma samples (−80 °C) were analysed for calprotectin levels at baseline, 1, 2, 3, 6 and 12 months by use of either enzyme-linked immunosorbent assay (ELISA) or fluoroenzyme immunoassay (FEIA). The ELISA technique used kits from Calpro AS and the FEIA technology was assessed on an automated Thermo Fisher Scientific instrument. The results showed high correlations between the two methods at baseline and during follow-up, with Spearman correlation at baseline 0.93 (p < 0.001) in the early and 0.96 (p < 0.001) in the established RA cohorts. The correlations between each of the two calprotectin assessments and clinical examinations had similar range. Calprotectin correlated well with clinical examinations, with at least as high correlations as CRP and ESR. The present study showed similar results for the two analytical methods, supporting the robustness of calprotectin analyses, and suggest calprotectin in plasma to be included in the assessments offered by clinical routine laboratories.
Introduction
Calprotectin is a heterocomplex of the S100 proteins S100A8 and S100A9 (also called myeloid-related protein 8/14 (MRP8/MRP14)) which has a calcium-binding protein with EF hand calcium domains [Citation1], constituting the physiologically active conformation of this protein. Calprotectin is predominantly expressed by myelomonocytic cells, namely monocytes and neutrophils but also in early differentiation stages of macrophages [Citation2]. The protein constitutes 40% and 5%, respectively, of the polymorphonuclear neutrophil cytosolic and monocyte protein content [Citation3]. Calprotectin is an alarmin with important proinflammatory properties mainly secreted by activated neutrophils in a calcium-dependant manner.
In patients with rheumatoid arthritis (RA), calprotectin has been found to reflect clinical and ultrasound inflammation more sensitively than C-reactive protein (CRP) or Erythrocyte Sedimentation Rate (ESR) [Citation4–10]. In patients with RA, plasma calprotectin levels have been found to predict relapse [Citation11,Citation12] and response to biologic disease-modifying anti-rheumatic drugs (bDMARDs) [Citation13–16]. In addition, calprotectin has been shown to be associated with subclinical RA inflammatory activity [Citation17] and radiographic progression [Citation18–20]. Even if many studies use serum samples for assessing calprotectin concentration, plasma samples are shown to have higher associations to clinical and ultrasound assessments of inflammation [Citation21]. Calprotectin levels are about doubled in serum compared to plasma [Citation22], which is thought to be caused by ethylenediaminetetraacetic acid (EDTA) in plasma inhibiting the neutrophil activation by its binding to calcium, and thus inhibiting the release of calprotectin.
Ultrasound assessments are increasingly used in rheumatologic practice to assess arthritis and tenosynovitis in patients with RA. Ultrasound has been shown to have high reliability [Citation23], and use of comprehensive scores detects most of the ongoing inflammation [Citation24].
Prior to including plasma calprotectin in laboratory routine for disease management in RA, the concordance of calprotectin levels assessed by different methods should be investigated to explore the robustness of the assessments. The present study assessed the plasma calprotectin levels examined by two different established laboratory methods to explore their concordance in patients with RA having either early or established disease. In addition, we explored whether calprotectin assessed by the two methods had similar level of associations with clinical and ultrasound assessments of disease activity as found for CRP and ESR.
Methods
Patient and plasma samples
The present study is a sub-study of NORA (a Nordic multi-centre study exploring personalized medicine in RA) including plasma samples from patients with early or established RA. The patients with early RA were from the step-up, treat-to-target randomized control trial (ARCTIC) [Citation25], in which patients were treated with methotrexate and prednisolone from baseline, with additional medication as needed during follow-up according to a pre-defined step-up protocol. The established RA patients were part of the observational clinical ULRABIT study [Citation26] and included patients initiating biologic disease-modifying anti-rheumatic drugs.
Calprotectin assessments
In the early RA cohort, plasma for calprotectin analysis was collected from baseline, 1, 3 and 12 months for ELISA assessments, and from baseline, 1, 2, 3, 6 and 12 months for FEIA assessments. In patients with established RA, plasma was from baseline, 1, 2, 3, 6 and 12 months for both ELISA and FEIA examinations. Blood was collected in EDTA tubes and centrifugated for 15 min at a relative centrifugal force of 1400, performed within 30 min from collection of the EDTA blood. The upper third of the plasma samples (avoiding buffy coat) was used for calprotectin assessments. After centrifugation, the plasma was aliquoted into NUNC tubes with 0.5 mL plasma in each, and then stored frozen (−80 °C).
Calprotectin was assessed by use of two different methods (each method using one aliquot); Enzyme-Linked ImmunoSorbent Assay (ELISA) and FluoroEnzyme ImmunoAssay (FEIA). The ELISA method was developed by MK Fagerhol about 40 years ago [Citation27] with further refinements. In the early RA cohort, calprotectin had previously been analysed [Citation20] by a calprotectin ELISA alkaline phosphatase (ALP) kit from Calpro AS (Oslo, Norway) at the Broegelmann Research Laboratory. The assay is using plates coated with a mouse monoclonal antibody directed against S100A9. The detection antibody is a polyclonal rabbit antibody conjugated to ALP. All plasma samples from the individual patients were analysed on the same plate. Colour intensity was read at 405 nanometre wavelengths by an eMax reader from Molecular Devices (Sunnyvale, CA) using Soft Max pro software and a Synergy H1 Hybrid Reader from BioTek (Winooski, VT). Plasma levels of calprotectin in the established RA cohort was assessed by an ELISA technique also using kits from Calpro AS (Oslo, Norway) and the samples were assessed in a semi-automatic analysis machine Dynex DS2 (Dynex Technologies, VA) at Diakonhjemmet hospital. Plasma samples from each patient were examined on the same plate. The Calpro AS plates were coated by monoclonal antibodies against calprotectin, and the kits included all necessary solutions, substrates, standards, and controls (high and low calprotectin levels), and the Calpro AS protocol was used for the calprotectin assessments. The standards and controls were used as the mean of two measures, while all the patient samples were analysed as single measures. Normal median value (25, 75 percentile) of calprotectin was provided by the manufacturer by assessment of plasma from 100 healthy blood donors and was 560 (412, 788) µg/L.
The FEIA technology used wells coated with monoclonal antibodies to calprotectin in a research setting on a Thermo Fisher Scientific instrument with a 1:50 dilution of the plasma sample. The response to patient samples was compared directly with the response to calibrators and defined as Arbitrary Units per ml (AU/mL) [Citation22]. A total of 300 plasma samples from blood donors were measured to define a normal range for the research setting of the FEIA technology. The normal range was defined as below 20 AU/mL, with a mean (SD) of 5.4 (4.0) AU/mL and 25, 75 percentiles of 2.6, 6.7 AU/mL for the measured blood donor samples.
Routine laboratory assessments
CRP (immunoturbidimetric assay) and ESR (Westergren) were assessed in the routine at Diakonhjemmet hospital laboratory (normal levels; CRP ≤4 mg/L and ESR ≤12 men/≤17 women mm/h).
Ultrasound assessments
The ultrasound assessments used the Outcome Measures in Rheumatology (OMERACT) definitions of pathology [Citation28]. In the ARCTIC trial of early RA, ultrasound was performed at baseline, 1, 2, 3, 6 and 12 months in half of the cohort and at baseline and 12 months in the other half [Citation25]. Ultrasound was performed after extensive training of the sonographers as well as using a reference ultrasound atlas which has been shown to give high intra- and inter-rater reliability [Citation23] and included 32 joints scored semi-quantitatively for grey scale (GS) and power Doppler (PD) as described [Citation25].
The established RA cohort was assessed similar to the ARCTIC patients (as previously described [Citation26]; ultrasound of 36 joints performed by one experienced sonographer using the Norwegian ultrasound atlas as reference [Citation23]).
Clinical examinations
We only included the most objective clinical RA measures in the present study, i.e. number of swollen joints and examiner’s global score (EGA) (visual analogue scale (VAS, score 0–100)) to explore the associations between calprotectin/CRP/ESR and clinical assessments of inflammation. In the early RA cohort, the clinical assessments were performed by the treating physician, scoring number of swollen joints (the standard 28 joints [Citation29]) as well as EGA [Citation20]. In the cohort of established RA, clinical assessments were performed by one of two trained study nurses blinded to the ultrasound results; they assessed number of swollen joints (the standard 28 joints [Citation29] with the addition of ankles and the metatarsophalangeal joints 1–5 scored as one joint bilaterally (i.e. in total 32 joints)) as well as EGA.
Statistics
Sum scores of both GS synovitis and PD activity were calculated for each of the two cohorts and were used in the present calculations. Spearman’s rank correlations were used for intra-individual comparisons between calprotectin concentrations examined by the two methods as well as correlations between each of the laboratory assessments (calprotectin (two methods), CRP, and ESR) and each of the clinically most objective measures of inflammation (GS and PD ultrasound, swollen joint count, and EGA). Correlation coefficients were defined as; no <0.2, low 0.2 − 0.3, moderate >0.3–<0.5, substantial 0.5–0.7 and >0.7 high.
Results
In the early RA cohort, a total of 212 patients (mean (SD) age 52.0 (13.3) years, disease duration 0.6 (0.5) years, 61% women, 81% anti-CCP IgG positive and 71% RF IgM positive) were included. The established RA cohort included 177 patients (mean (SD) age 52.8 (13.0) years, disease duration 10.0 (8.8) years, 81% women, 82% anti-CCP IgG positive and 69% RF IgM positive).
Correlations between the two calprotectin assessments
shows the correlations between the two calprotectin assays at baseline for early RA, r = 0.93 (p < 0.001) and established RA, r = 0.96 (p < 0.001). The figures illustrate the range of baseline calprotectin concentrations in the two groups.
Figure 1. Spearman’s correlation at baseline between calprotectin levels examined by use of ELISA or FEIA in the ARCTIC cohort of early RA (A) as well the ULRABIT cohort of established RA (B).
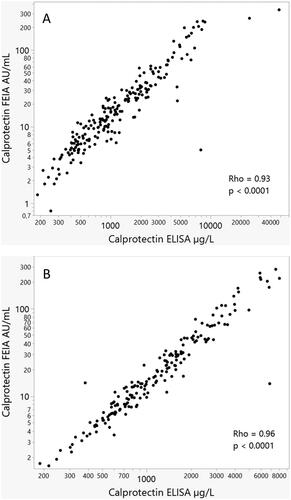
During follow-up (1, 2, 3, 6, and 12 months visits), the median (range) correlations between the two calprotectin measurements were 0.88 (0.87 − 0.89, all with p < 0.001) in the early RA cohort. The corresponding correlations were 0.96 (0.95 − 0.97) in the cohort of established RA.
Correlations between calprotectin/CRP/ESR levels and clinical assessments
shows the baseline correlations between the laboratory results and the more objective assessments of disease activity in the early and established RA patients (p < 0.001 for all the correlations). Correlation coefficients between calprotectin (assessed by use of the ELISA method) and CRP/ESR has previously been published [Citation5,Citation20]. Calprotectin, assessed by both methods, was found to have correlations with the clinical assessments in the same range as the established laboratory markers CRP and ESR. During follow-up, the correlations were low to moderate between calprotectin (assessed by both methods), CRP, ESR and clinical as well as ultrasound assessments (data not shown). The two calprotectin methods showed only marginally different correlation coefficients, and there were no statistically significant differences in the correlations between the two calprotectin methods regarding their correlations with CRP/ESR or clinical/ultrasound examinations during follow-up (data not shown).
Table 1. Spearman’s rank correlations at baseline between calprotectin (assessed by ELISA or FEIA)/CRP/ESR and clinical as well as ultrasound assessments of disease activity in the cohorts of early or established RA (p < 0.001 for all correlations).
Discussion
The present study is the first to explore the associations between ELISA and FEIA methods for assessments of calprotectin in patients with RA. In addition, both early and established RA were included to explore whether disease duration had any influence on the association. We found very high correlations between the two methods in both patient cohorts, and the two methods had at least as good levels of correlations with clinical and ultrasound assessments of disease activity as the two established inflammatory markers CRP and ESR.
Ultrasound is a sensitive imaging technique for detection of inflammation, and our calprotectin assessments had similar levels of association with ultrasound sum scores as previously described [Citation8]. We found both calprotectin assessments to have moderate to substantial correlations with clinical examinations of joint inflammation (EGA and SJC) at baseline, and similar levels of correlations have been shown previously [Citation7,Citation9,Citation20]. Previous studies have shown low or no associations between subjective evaluations (patient reported outcomes) and objective assessments (EGA and SJC) of disease activity [Citation26,Citation30,Citation31]. Thus, we did not include any subjective variables in our present calculations.
The calprotectin ELISA method has been developed during the last two decades [Citation27] and the laboratory examinations have been increasingly automized. Even if the patients with early RA had ELISA calprotectin assessed more manually, the calprotectin levels had as high correlations with the FEIA method as was found for the established RA cohort.
The FEIA method was presently found to have similar associations to clinical assessments as the ELISA method. Thus, even if FEIA is a method that has not been fully developed, and therefore not having an accreditation at this point of time, the method seems to be promising as a routine assessment for inflammatory joint diseases and may be used in an automated workflow.
A limitation of the present study is that only two laboratory methods have been compared. However, these methods are frequently used in clinical laboratories, and thus relevant to compare. Another limitation is that the ELISA method was somewhat differently performed for the early compared to the established cohort. However, both methods used the same Calpro AS kits, and the only difference was the manually compared to semi-automatic filling of syringes. Calprotectin levels has recently been shown to be increased with addition of haemoglobin [Citation32], where significant elevation was found in samples with low calprotectin levels. In the present study we included patients with high calprotectin levels, and even if plasma was collected independent of haemolysis, a potential haemolysis may not have influenced our results. A strength of our study is the large number of patients included, as well as having follow-up results. In addition, the inclusion of both early and established RA cohorts makes the present results covering the whole spectrum of RA patients.
To conclude, we found very high correlations between the calprotectin levels assessed by ELISA and FEIA, and we found each of the two methods to be associated with objective measures of inflammation, with no significant differences between the two methods. The present study indicates that both the ELISA and the FEIA assessments can be recommended for calprotectin assessments in clinical practice.
Acknowledgements
We acknowledge Inge Dale at Calpro, Marianne Eidsheim at the Broegelmann Research Laboratory, Ellen Moholt and Camilla Fongen at Diakonhjemmet Hospital, and the ARCTIC study group of early RA: Hallvard Fremstad, Tor Magne Madland, Åse Stavland Lexberg, Hilde Haukeland, Erik Rødevand, Christian Høili, Hilde Stray, Anne Noraas, Inger Johanne Widding Hansen, and Gunnstein Bakland. We also acknowledge our study nurses Anne Katrine Kongtorp and Britt Birketvedt who were important in the organization and assessments of the established RA cohort. We acknowledge Patricia Fischer for measurements on the Thermo Fisher Scientific instrument. Thanks for ongoing support with the calprotectin assay from the development team at Thermo Fisher Scientific, especially Laura Steller and Bernhard Hoermann.
Disclosure statement
Hilde Berner Hammer has honorary for teaching from AbbVie, UCB, Lilly and Novartis. Sigve Lans Pedersen has no competing interests to declare. Maria Jonsson has no competing interests to declare. Linda Mathsson-Alm is employee at Thermo Fisher Scientific. Isabel Gehring is employee at Thermo Fisher Scientific. Joe Sexton has no competing interests to declare. Espen A. Haavardsholm: have received grants from Norwegian Regional Health Authorities (interregional KLINBEFORSK grants), grants from The South-Eastern Norway Regional Health Authority and personal fees from Pfizer, AbbVie, Celgene, Novartis, Janssen, Gilead, Eli-Lilly and UCB. Johan Askling has received grant/research support from AbbVie, AstraZeneca, Bristol Myers Squibb, Eli Lilly, Janssen, Merck, Pfizer, Roche, Samsung Bioepis, Sanofi, and UCB.
Data availability statement
The data will be shared if there is a reasonable request for it.
Additional information
Funding
References
- Korndörfer IP, Brueckner F, Skerra A. The crystal structure of the human (S100A8/S100A9)2 heterotetramer, calprotectin, illustrates how conformational changes of interacting alpha-helices can determine specific association of two EF-hand proteins. J Mol Biol. 2007;370(5):887–898. doi: 10.1016/j.jmb.2007.04.065.
- Edgeworth J, Gorman M, Bennett R, et al. Identification of p8,14 as a highly abundant heterodimeric calcium binding protein complex of myeloid cells. J Biol Chem. 1991;266(12):7706–7713.
- Romand X, Bernardy C, Nguyen MVC, et al. Systemic calprotectin and chronic inflammatory rheumatic diseases. Joint Bone Spine. 2019;86(6):691–698. doi: 10.1016/j.jbspin.2019.01.003.
- Bach M, Moon J, Moore R, et al. A neutrophil activation biomarker panel in prognosis and monitoring of patients with rheumatoid arthritis. Arthritis Rheumatol. 2020;72(1):47–56. doi: 10.1002/art.41062.
- Nordal HH, Brokstad KA, Solheim M, et al. Calprotectin (S100A8/A9) has the strongest association with ultrasound-detected synovitis and predicts response to biologic treatment: results from a longitudinal study of patients with established rheumatoid arthritis. Arthritis Res Ther. 2017;19(1):3. doi: 10.1186/s13075-016-1201-0.
- Hammer HB, Odegard S, Fagerhol MK, et al. Calprotectin (a major leucocyte protein) is strongly and independently correlated with joint inflammation and damage in rheumatoid arthritis. Ann Rheum Dis. 2007;66(8):1093–1097. doi: 10.1136/ard.2006.064741.
- Hammer HB, Fagerhol MK, Wien TN, et al. The soluble biomarker calprotectin (an S100 protein) is associated to ultrasonographic synovitis scores and is sensitive to change in patients with rheumatoid arthritis treated with adalimumab. Arthritis Res Ther. 2011;13(5):R178. doi: 10.1186/ar3503.
- Hurnakova J, Hulejova H, Zavada J, et al. Relationship between serum calprotectin (S100A8/9) and clinical, laboratory and ultrasound parameters of disease activity in rheumatoid arthritis: a large cohort study. PLoS One. 2017;12(8):e0183420. doi: 10.1371/journal.pone.0183420.
- Hurnakova J, Zavada J, Hanova P, et al. Serum calprotectin (S100A8/9): an independent predictor of ultrasound synovitis in patients with rheumatoid arthritis. Arthritis Res Ther. 2015;17(1):252. doi: 10.1186/s13075-015-0764-5.
- Wang Y, Liang Y. Clinical significance of serum calprotectin level for the disease activity in active rheumatoid arthritis with normal C-reactive protein. Int J Clin Exp Pathol. 2019;12:1009–1014.
- de Moel EC, Rech J, Mahler M, et al. Circulating calprotectin (S100A8/A9) is higher in rheumatoid arthritis patients that relapse within 12 months of tapering anti-rheumatic drugs. Arthritis Res Ther. 2019;21(1):268. doi: 10.1186/s13075-019-2064-y.
- Inciarte-Mundo J, Ramirez J, Hernández MV, et al. Calprotectin strongly and independently predicts relapse in rheumatoid arthritis and polyarticular psoriatic arthritis patients treated with tumor necrosis factor inhibitors: a 1-year prospective cohort study. Arthritis Res Ther. 2018;20(1):275. doi: 10.1186/s13075-018-1764-z.
- Choi IY, Gerlag DM, Herenius MJ, et al. MRP8/14 serum levels as a strong predictor of response to biological treatments in patients with rheumatoid arthritis. Ann Rheum Dis. 2015;74(3):499–505. doi: 10.1136/annrheumdis-2013-203923.
- Inciarte-Mundo J, Ruiz-Esquide V, Hernández MV, et al. Calprotectin more accurately discriminates the disease status of rheumatoid arthritis patients receiving tocilizumab than acute phase reactants. Rheumatology (Oxford). 2015;54(12):2239–2243. doi: 10.1093/rheumatology/kev251.
- Inciarte-Mundo J, Victoria Hernández M, Ruiz-Esquide V, et al. Serum calprotectin versus Acute-Phase reactants in the discrimination of inflammatory disease activity in rheumatoid arthritis patients receiving tumor necrosis factor inhibitors. Arthritis Care Res (Hoboken). 2016;68(7):899–906. doi: 10.1002/acr.22795.
- Nair SC, Welsing PM, Choi IY, et al. A personalized approach to biological therapy using prediction of clinical response based on MRP8/14 serum complex levels in rheumatoid arthritis patients. PLoS One. 2016;11(3):e0152362. doi: 10.1371/journal.pone.0152362.
- Inciarte-Mundo J, Ramirez J, Hernández MV, et al. Calprotectin and TNF trough serum levels identify power doppler ultrasound synovitis in rheumatoid arthritis and psoriatic arthritis patients in remission or with low disease activity. Arthritis Res Ther. 2016;18(1):160. doi: 10.1186/s13075-016-1032-z.
- Hammer HB, Ødegård S, Syversen SW, et al. Calprotectin (a major S100 leucocyte protein) predicts 10-year radiographic progression in patients with rheumatoid arthritis. Ann Rheum Dis. 2010;69(1):150–154. doi: 10.1136/ard.2008.103739.
- Di Ceglie I, Kruisbergen NNL, van den Bosch MHJ, et al. Fc-gamma receptors and S100A8/A9 cause bone erosion during rheumatoid arthritis. Do they act as partners in crime? Rheumatology (Oxford). 2019;58(8):1331–1343. doi: 10.1093/rheumatology/kez218.
- Jonsson MK, Sundlisæter NP, Nordal HH, et al. Calprotectin as a marker of inflammation in patients with early rheumatoid arthritis. Ann Rheum Dis. 2017;76(12):2031–2037. doi: 10.1136/annrheumdis-2017-211695.
- Nordal HH, Fagerhol MK, Halse AK, et al. Calprotectin (S100A8/A9) should preferably be measured in EDTA-plasma; results from a longitudinal study of patients with rheumatoid arthritis. Scand J Clin Lab Invest. 2018;78(1-2):102–108. doi: 10.1080/00365513.2017.1419371.
- Van Hoovels L, Vander Cruyssen B, Bogaert L, et al. Pre-analytical and analytical confounders of serum calprotectin as a biomarker in rheumatoid arthritis. Clin Chem Lab Med. 2019;58(1):40–49. doi: 10.1515/cclm-2019-0508.
- Hammer HB, Bolton-King P, Bakkeheim V, et al. Examination of intra and interrater reliability with a new ultrasonographic reference atlas for scoring of synovitis in patients with rheumatoid arthritis. Ann Rheum Dis. 2011;70(11):1995–1998. doi: 10.1136/ard.2011.152926.
- Hammer HB, Kvien TK. Comparisons of 7- to 78-joint ultrasonography scores: all different joint combinations show equal response to adalimumab treatment in patients with rheumatoid arthritis. Arthritis Res Ther. 2011;13(3):R78. doi: 10.1186/ar3341.
- Haavardsholm EA, Aga A-B, Olsen IC, et al. Ultrasound in management of rheumatoid arthritis: ARCTIC randomised controlled strategy trial. BMJ. 2016;354:i4205. doi: 10.1136/bmj.i4205.
- Hammer HB, Uhlig T, Kvien TK, et al. Pain catastrophizing, subjective outcomes, and inflammatory assessments including ultrasound: results from a longitudinal study of rheumatoid arthritis patients. Arthritis Care Res (Hoboken). 2018;70(5):703–712. doi: 10.1002/acr.23339.
- Hetland G, Berntzen HB, Fagerhol MK. Levels of calprotectin (leukocyte L1 protein) during apheresis. Scand J Clin Lab Invest. 1992;52(6):479–482. doi: 10.3109/00365519209090124.
- Wakefield RJ, Balint PV, Szkudlarek M, et al. Musculoskeletal ultrasound including definitions for ultrasonographic pathology. J Rheumatol. 2005;32:2485–2487.
- Prevoo ML, van ‘t Hof MA, Kuper HH, et al. Modified disease activity scores that include twenty-eight-joint counts. Development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum. 1995;38(1):44–48. doi: 10.1002/art.1780380107.
- Hammer HB, Jensen Hansen IM, Järvinen P, et al. Rheumatoid arthritis patients with predominantly tender joints rarely achieve clinical remission despite being in ultrasound remission. Rheumatol Adv Pract. 2021;5:rkab030.
- Hammer HB, Hansen I, Järvinen P, et al. Major reduction of ultrasound-detected synovitis during subcutaneous tocilizumab treatment: results from a multicentre 24 week study of patients with rheumatoid arthritis. Scand J Rheumatol. 2021;50(4):262–270. doi: 10.1080/03009742.2020.1845394.
- Blavnsfeldt AG, Parkner T, Knudsen CS. Plasma calprotectin - preanalytical stability and interference from hemolysis. Scand J Clin Lab Invest. 2022;82(5):349–355. doi: 10.1080/00365513.2022.2092901.
