Abstract
Physiological changes in hemostasis during pregnancy have been reported by several authors. This study aimed at establishing reference intervals for the hemostasis biomarkers thrombin-antithrombin complex (TAT), α2-plasmininhibitor-plasmin complex (PIC), thrombomodulin (TM) and tissue plasminogen activator-inhibitor complex (tPAI-C), in healthy pregnancies. After excluding outliers, a total of 496 healthy pregnant women (128 first-trimester, 142 second-trimester, 107 third-trimester and 119 pre-labor) and 103 healthy nonpregnant women were enrolled from Shenzhen Bao’an Women’s and Children’s Hospital. Hemostasis biomarkers, TAT, PIC, TM and tPAI-C, were measured by using a quantitative chemiluminescence enzyme immunoassay performed on HISCL automated analysers. The median and reference intervals (the 2.5th and 97.5th percentiles) were calculated to establish trimester-specific reference intervals for healthy pregnant women. The reference intervals for TAT, PIC, TM and tPAI-C in the first trimester were 0.7–7.6 1 µg/L, 0.2–0.9 mg/L, 2.8–11.0 TU/ml, and 1.2–6.5 1 µg/L, respectively. The reference intervals in the second trimester were 1.7–12.0 1 µg/L, 0.2–1.0 mg/L, 3.7–11.6 TU/ml, and 2.8–8.8 1 µg/L, respectively. The reference intervals in the third trimester were 2.7–16.1 1 µg/L, 0.1–1.4 mg/L, 2.9–12.9 TU/ml, and 1.9–8.0 1 µg/L, respectively. At pre–labor, the reference intervals were 4.8–32.9 1 µg/L, 0.2–1.9 mg/L, 4.2–12.6 TU/ml, and 2.8–15.4 1 µg/L, respectively. Gestational reference intervals for TAT, PIC, TM and tPAI-C in healthy pregnancies are provided, but only for TAT with increasing concentrations throughout pregnancy, the reference intervals for non-pregnant were not applicable.
Introduction
Pregnant women are at higher risk of developing venous thromboembolism (VTE) due to physiological hypercoagulability, and the diagnosis of VTE in pregnancy is challenging [Citation1]. Common clinical manifestations of VTE can also occur in healthy pregnant women, and therefore the ability to rapidly, noninvasively and harmlessly screen for VTE is of paramount importance [Citation2]. Several different markers have been found to be elevated during pregnancy [Citation1,Citation3,Citation4]. D-dimer is an epitope formed by the breakdown of cross-linked fibrin polymer by plasmin, making it a useful marker for blood coagulation and fibrinolysis [Citation3]. In clinical settings, the D-dimer test has a high negative predictive value and can be used to rule out thromboembolism [Citation5]. However, the use of D-dimer for VTE diagnosis in pregnancy is complicated due to physiological changes during pregnancy which lead to a gradual increase of D-dimer in healthy pregnancies [Citation3,Citation5–7]. D-dimer increases significantly during pregnancy and is correlated with the gestational period of pregnancy. Fibrin monomer (FM) is another marker which has been suggested to be an early marker of coagulation activation, as thrombin acts on fibrinogen to cleave peptide A and B to form FM. In healthy persons, there are very low levels of FM [Citation8,Citation9], and FM may increase early in VTE [Citation10]. FM remained relatively stable throughout pregnancy compared with other biomarkers [Citation8,Citation9], but increased significantly during DVT [Citation8]. FM could therefore be a potential marker for hypercoagulability in pregnancy [Citation8,Citation9], but this needs to be shown in larger clinical studies. Alternative biomarkers such as TAT, PIC, TM, and tPAI-C have been proposed as potential biomarkers for early diagnosis of thromboembolism or assessing thrombosis risk in different patient groups (e.g. DIC, cancer, sepsis) [Citation11–13]. In pregnancy, imbalance in hemostasis may lead to fibrin deposition, resulting in placental ischaemia and hypoxia, leading to preeclampsia, intrauterine growth retardation, and abortion [Citation14–16]. Biomarkers to be used for early detection or monitoring of disease could be useful. TAT is a marker of thrombin formation and coagulation activation and may increase thrombosis, recurrence and early-stage DIC [Citation17]. Elevated levels of TAT suggest an increased risk of thromboembolism [Citation17]. Plasma PIC indicates activation of the fibrinolytic system [Citation17] and TM levels indicate vascular endothelial injury [Citation18]. Elevated tPAI-C levels are seen in DIC, vascular endothelial cell injury, and thrombosis [Citation19–22]. These biomarkers have not been routinely used in clinical practice and there are few reference intervals available for pregnant women [Citation23]. Therefore, in this study, we established stratified reference intervals for TAT, PIC, TM and tPAI-C in healthy pregnant women during different trimesters according to the CLSI (Clinical Laboratory Standards Institute) document CA28-A3c [Citation24]. Our results may provide important information for future studies exploring the use of these biomarkers for VTE diagnosis in pregnancy.
Materials and methods
Patients and sample collection
A total of 496 healthy pregnant women and 103 healthy nonpregnant women were selected after being seen at the outpatient department of Shenzhen Bao’an Women’s and Children’s Hospital for a prenatal examination between October 2019 and April 2020. The criteria for enrolment were as follows: patients with (1) no history of cerebrovascular, liver, kidney and autoimmune diseases; (2) no coagulation disorder or haematological system disease; (3) no history of using drugs that may affect blood coagulation and fibrinolysis system; (4) no history of recurrent abortion or abnormal foetal development; and (5) no hereditary or acquired thrombophilia. According to the perinatal pregnancy classification criteria, the 496 enrolled patients were divided into four groups: first trimester (4+0–12+6 weeks), second trimester (14+0–26+6 weeks), third trimester (27+0–35+6 weeks) and pre-labor (24 h before labor).
Laboratory testing
A 2.7 mL venous blood sample was collected from all of the subjects, and the blood sample was added to a BD vacuum tube containing 0.3 mL sodium citrate (109 mmol/L), and then centrifuged at 1500 g for 15 mins. The plasma levels of TAT, PIC, TM and tPAI-C were measured on an automatic chemiluminescence enzyme immunoassay HISCL-5000 analyser (Sysmex Corporation, Kobe, Japan). The daily use of two quality controls was performed to ensure the stability of the system. The coefficient of variation of intra-assay and inter-assay of the four assays were less than 10%. All the samples were tested within 4 h. The manufacturer-specified reference intervals (Sysmex Corporation, Kobe, Japan) for TAT, TM, tPAI-C, and PIC were <4 µg/L, 3.8–13.3 TU/mL, <10.5 µg/L, and <0.8 mg/L, respectively.
Statistical analysis
The outliers were excluded based on the Dixon method proposed in C28-A3c (when D/R ≥ 1/3, the value should be regarded as an outlier, where “D” is the absolute difference between the maximum or minimum concentration and an adjacent concentration, R is the total range from the maximum to the minimum of all concentrations (including the extreme concentrations)). All parameters (TAT, TM, tPAI-C and PIC) are presented with the 2.5th and 97.5th percentiles. The differences between the groups were examined for statistical significance using a nonparametric test and the Mann–Whitney U test. A two-tailed p-value < 0.05 was considered to be statistically significant. All statistical analyses were performed using GraphPad Prism 8.0.1.
Results
The characteristics of the enrolled healthy women, including their numbers, ages and gestational weeks, in each group are described in . There was no significant difference in the mean ages between nonpregnant women and pregnant women and no significant difference among the proportion of women in the first trimester, second trimester, third trimester and pre-labor period.
Table 1. Characteristics of the enrolled healthy pregnant and non-pregnant women.
The medians and P2.5-P97.5 reference intervals of TAT, PIC, TM and tPAI-C for pregnant women in the trimester-specific groups are listed in . From the first trimester to the pre-labor period, the TAT level gradually increased and reached a peak during pre-labor. The differences between non-pregnant and pregnant women and between the different gestational periods were significant ( and ). There was no significant difference in the mean levels of PIC, TM and tPAI-C between the four trimester-specific groups and the nonpregnant group, and the values of PIC, TM and tPAI-C in these pregnant groups were mostly within the non-pregnant reference intervals ( and ). However, for PIC and tPAI-C, significant differences were found between the pre-labor group and the third-trimester group, and the ranges of the 95% reference intervals in the pre-labor group for PIC were 2.4 times ((97.5 percentile: 1.9 versus 0.8)) those in the nonpregnant group ().
Figure 1. Concentrations of thrombin-antithrombin complex (TAT), α2-plasmininhibitor-plasmin complex (PIC), thrombomodulin (TM) and tissue plasminogen activator-inhibitor complex (tPAI-C) in non-pregnant women (N), first trimester (F), second trimester (S), third trimester (T) and before labor (pre-L). The bars represent the 25th and 75th percentiles with the medians (horizontal lines). the end of the vertical lines represents the 2.5 and 97.5 percentiles. P: p-values.
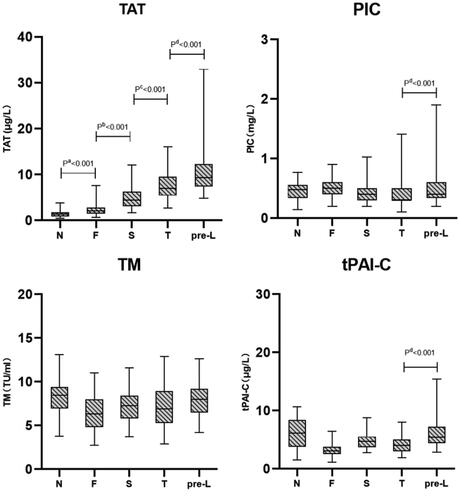
Table 2. Median and 95% reference intervals for TAT, PIC, TM and tPAI-C of pregnant women and non-pregnant women in trimester-specific groups.
Discussion
The purpose of this study was to establish trimester-specific reference intervals for TAT, PIC, TM and tPAI-C in healthy pregnant women. The PIC, TM and tPAI-C levels could be considered relatively stable during pregnancy. In addition, the three trimester’s reference intervals of TM, PIC and tPAI-C are all very close to or slightly exceed the non-pregnant reference interval in our study, similar to the results of Wu et al. [Citation23]. TAT showed an increasing trend through pregnancy and peaked in the pre-labor period, with a similar trend as shown for D-dimer in other studies [Citation25–26]. This is probably an indication of progressive increasing coagulation activation in pregnancy from the first trimester to the pre-labor period. Several studies have revealed that antithrombin (AT) decreases slightly during pregnancy [Citation25,Citation27], which may indicate increased antithrombin binding to thrombin and thereby increasing TAT [Citation23]. The reference intervals for TAT in nonpregnant women were not suitable for pregnant women, especially during pre-labor, and the levels were all above the non-pregnant reference intervals. TAT may be used as an early indicator of activated coagulation during pregnancy, but as its levels tend to increase with advancing gestational age, clinical studies will have to be performed to decide its usefulness in the diagnostic workup for thromboembolic diseases in pregnancy.
There was no significant difference in levels of PIC, TM and tPAI-C between the four gestational age groups and the non-pregnant group. However, significant differences existed between pre-labor group and third-trimester group for PIC and tPAI-C, and also the ranges of 95% reference intervals at pre-labor group for PIC were 2.4 times compared with non-pregnant group. PIC and tPAI-C, as molecular markers of fibrinolysis showed considerable individual variability for pre-labor women, and the reasons for individual variability need further exploration. In this paper, it was found that the increasing levels of PIC and tPAI-C in pregnant women occurred relatively late in pregnancy (pre-labor). The increased levels of PAI and tPAI-C in some pregnant women could be caused by increased placental production of plasminogen activator inhibitor type-2 (PAI-2), inhibiting the fibrinolytic system [Citation28]. In the third trimester, the placental location site may become a site for localized chronic DIC [Citation29]. If chronic localized intravascular coagulation at the placental interface occurs, the fibrin turnover rate in the plasma is accelerated and activation of the fibrinolytic system leads to an increase in PIC and tPAI-C levels. The well-known gradual increase in D-dimer levels in the third trimester may indicate a general fibrin deposition and simultaneously an enhanced fibrinolytic activity. Especially at pre-labor, the pregnant women tend to be at a hypercoagulable state, and coagulation and fibrinolysis systems are maintaining a state of dynamic equilibrium at a higher level than in the non-pregnant state. This physiological prothrombotic state of pregnancy facilitates effective hemostasis after delivery and enables rapid fibrin formation at the dissection surface of the placenta. The purpose of hemostasis is to clear the thrombus in the uterine snow arteries and venous sinuses, accelerate the regeneration and repair of the endometrium, and thus avoid the occurrence of thrombotic diseases [Citation30–31]. We speculated that the fibrinolytic system of healthy pregnant women was in a state of suppression before the third trimester, so the PIC and tPAI-C concentrations did not change significantly until pre-labor. TM, which is a marker of vascular endothelial cell damage, did not change during normal pregnancy, indicating that endothelial damage is not sufficient to upregulate TM levels. The reference intervals of nonpregnant women, therefore, were suitable for pregnant women.
Patients with VTE and DIC were excluded from the calculations of reference intervals in pregnancy. In a pregnant woman with confirmed PE, it was found abnormally elevated PIC and TAT levels suggesting hypercoagulability. While the establishment of reference intervals for TAT, PIC, TM, and tPAI-C may be useful in detecting coagulation disorders in pregnant women, further studies are needed to confirm the clinical significance and utility of these markers in the diagnosis and treatment of these conditions. These biomarkers need to be further studied in pathological pregnancy.
In conclusion, our study reported reference intervals of TAT, PIC, TM and tPAI-C in healthy pregnancies in trimester-specific groups. We confirm that TAT concentrations increase significantly during pregnancy. The normal reference intervals of PIC, TM, and tPAI-C in non-pregnant individuals were also applicable to pregnant women, but during the pre-labor period, larger inter-individual variability was observed in the PIC and tPAI-C values.
Ethical approval
The studies involving human participants were reviewed and approved by the ethics committee of the Shenzhen City Bao’an District Womens and Childrens Hospital, China (Project No. LLSCHY 2019-10-15-02). Written informed consent for participation was required for this study in accordance with the national legislation and the institutional requirements.
Disclosure statement
No potential conflicts of interest are reported by the authors.
Additional information
Funding
References
- Bremme KA. Haemostatic changes in pregnancy. Best Pract Res Clin Haematol. 2003;16(2):153–168. doi: 10.1016/s1521-6926(03)00021-5.
- James AH. Venous thromboembolism in pregnancy. Arterioscler Thromb Vasc Biol. 2009;29(3):326–331. doi: 10.1161/ATVBAHA.109.184127.
- Joly B, Barbay V, Borg JY, et al. Comparison of markers of coagulation activation and thrombin generation test in uncomplicated pregnancies. Thromb Res. 2013;132(3):386–391. doi: 10.1016/j.thromres.2013.07.022.
- Hedengran KK, Andersen MR, Stender S, et al. Large D-dimer fluctuation in normal pregnancy: a longitudinal cohort study of 4,117 samples from 714 healthy Danish women. Obstet Gynecol Int. 2016;2016:3561675. doi: 10.1155/2016/3561675.
- Kristoffersen AH, Ajzner E, Rogic D, et al. Is D-dimer used according to clinical algorithms in the diagnostic work-up of patients with suspicion of venous thromboembolism? A study in six European countries. Thromb Res. 2016;142:1–7. doi: 10.1016/j.thromres.2016.04.001.
- McLean KC, James AH. Diagnosis and management of VTE in pregnancy. Clin Obstet Gynecol. 2018;61(2):206–218. doi: 10.1097/GRF.0000000000000354.
- Sekiya A, Hayashi T, Kadohira Y, et al. Thrombosis prediction based on reference ranges of coagulation-related markers in different stages of pregnancy. Clin Appl Thromb Hemost. 2017;23(7):844–850. 15. doi: 10.1177/1076029616673732.
- Onishi H, Kaniyu K, Iwashita M, et al. Fibrin monomer complex in normal pregnant women: a potential thrombotic marker in pregnancy. Ann Clin Biochem. 2007;44(Pt 5):449–454. doi: 10.1258/000456307781646076.
- Kristoffersen AH, Petersen PH, Bjørge L, et al. Concentration of fibrin monomer in pregnancy and during the postpartum period. Ann Clin Biochem. 2019;56(6):692–700. doi: 10.1177/0004563219869732.
- Refaai MA, Riley P, Mardovina T, et al. The clinical significance of fibrin monomers. Thromb Haemost. 2018;118(11):1856–1866. doi: 10.1055/s-0038-1673684.
- Mei H, Jiang Y, Luo L, et al. Evaluation the combined diagnostic value of TAT, PIC, tPAIC, and sTM in disseminated intravascular coagulation: a multi-center prospective observational study. Thromb Res. 2019;173:20–26. doi: 10.1016/j.thromres.2018.11.010.
- Zhou K, Zhang J, Zheng Z-R, et al. Diagnostic and prognostic value of TAT, PIC, TM, and t-PAIC in malignant tumor patients with venous thrombosis. Clin Appl Thromb Hemost. 2020;26:107602962097104. doi: 10.1177/1076029620971041.
- Iba T, Thachil J. Clinical significance of measuring plasminogen activator inhibitor-1 in sepsis. J Intensive Care. 2017;5:56. doi: 10.1186/s40560-017-0250-z.
- Hayashi M, Hamada Y, Ohkura T. Thrombin-antithrombin complex and alpha2-plasmin inhibitor-plasmin complex levels after cesarean section in normal pregnancies and pre-eclampsia. Int J Gynaecol Obstet. 2003;82(2):213–216. doi: 10.1016/s0020-7292(03)00193-0.
- Shamshirsaz AA, Paidas M, Krikun G. Preeclampsia, hypoxia, thrombosis, and inflammation. J Pregnancy. 2012;2012:374047. doi: 10.1155/2012/374047.
- Ilkhchoui Y, Szabo EE, Gerstein NS, et al. Cerebral venous thrombosis complicating severe preeclampsia in the postpartum period: a diagnostic challenge. J Clin Anesth. 2014;26(2):143–146. doi: 10.1016/j.jclinane.2013.11.007.
- Asakura H, Takahashi H, Uchiyama T, et al. Proposal for new diagnostic criteria for DIC from the Japanese society on thrombosis and hemostasis. Thromb J. 2016;14:42.
- Kuryliszyn-Moskal A, Zarzycki W, Dubicki A, et al. Clinical usefulness of videocapillaroscopy and selected endothelial cell activation markers in people with type 1 diabetes mellitus complicated by microangiopathy. Adv Med Sci. 2017;62(2):368–373. doi: 10.1016/j.advms.2016.11.007.
- Tipoe TL, Wu WKK, Chung L, et al. Plasminogen activator inhibitor 1 for predicting sepsis severity and mortality outcomes: a systematic review and meta-analysis. Front Immunol. 2018;9:1218. doi: 10.3389/fimmu.2018.01218.
- Fowler AA, Truwit JD, Hite RD, et al. Effect of vitamin C infusion on organ failure and biomarkers of inflammation and vascular injury in patients with sepsis and severe acute respiratory failure: the CITRIS-ALI randomized clinical trial. JAMA. 2019;322(13):1261–1270. doi: 10.1001/jama.2019.11825.
- Ito T, Thachil J, Asakura H, et al. Thrombomodulin in disseminated intravascular coagulation and other critical conditions-a multi-faceted anticoagulant protein with therapeutic potential. Crit Care. 2019;23(1):280. doi: 10.1186/s13054-019-2552-0.
- Iba T, Thachil J. Present and future of anticoagulant therapy using antithrombin and thrombomodulin for sepsis-associated disseminated intravascular coagulation: a perspective from Japan. Int J Hematol. 2016;103(3):253–261. doi: 10.1007/s12185-015-1904-z.
- Wu YF, Qiao Y, Zhang YM, et al. Trimester-specific reference intervals of TAT, TM, tPAI-C and PIC for healthy Chinese pregnant women. J Obstet Gynaecol Res. 2021;47(1):368–374. doi: 10.1111/jog.14536.
- Boyd JC. Defining, establishing, and verifying reference intervals in the clinical laboratory; approved guidelines. 3rd Ed CLSI Document C28-A3c. 2010;28(3):15.
- Cui C, Yang S, Zhang J, et al. Trimester-specific coagulation and anticoagulation reference intervals for healthy pregnancy. Thromb Res. 2017;156:82–86. doi: 10.1016/j.thromres.2017.05.021.
- Haire G, Egan K, Parmar K, et al. Alterations in fibrin formation and fibrinolysis in early onset-preeclampsia: association with disease severity. Eur J Obstet Gynecol Reprod Biol. 2019;241:19–23. 26. doi: 10.1016/j.ejogrb.2019.07.035.
- Kristoffersen AH, Petersen PH, Røraas T, et al. Estimates of within-subject biological variation of protein C, antithrombin, protein S free, protein S activity, and activated protein C resistance in pregnant women. Clin Chem. 2017;63(4):898–907. doi: 10.1373/clinchem.2016.265900.
- Chapin JC, Hajjar KA. Fibrinolysis and the control of blood coagulation. Blood Rev. 2015;29(1):17–24. doi: 10.1016/j.blre.2014.09.003.
- Erez O. Disseminated intravascular coagulation in pregnancy: new insights. Thrombosis Update. 2022;6:100083. doi: 10.1016/j.tru.2021.100083.
- Edelstam G, Löwbeer C, Kral G, et al. New reference values for routine blood samples and human neutrophilic lipocalin during third-trimester pregnancy. Scand J Clin Lab Invest. 2001;61(8):583–592. doi: 10.1080/003655101753267937.
- O’Riordan MN, Higgins JR. Haemostasis in normal and abnormal pregnancy. Best Pract Res Clin Obstet Gynaecol. 2003;17(3):385–396. doi: 10.1016/s1521-6934(03)00019-1.
