Abstract
Objectives
There appears to be marked discrepancies between total IgE reference intervals (RIs) in use by many laboratories and those recommended by published studies. The aim of this study was therefore to review total IgE RIs currently reported by Scandinavian and British laboratories and to compare these to published RIs identified by a literature review.
Methods
Relevant laboratories were identified by test directories provided by the national accreditation bodies in Norway, Sweden, Denmark and the UK. Total IgE RIs and their sources were acquired by accessing laboratory user handbooks or by an electronic survey. In addition a literature review of published total IgE RI studies was performed.
Results
From 172 accredited laboratories providing total IgE analysis, data was acquired from 122 laboratories. An adult upper reference limit between 81 to 150 kU/L was reported by 89% of these. Denmark and Sweden reported the most harmonised RIs whilst Norway and the UK exhibited the least degree of harmonisation. Published adult (n = 6) and paediatric (n = 6) RI studies reported markedly higher upper limits than those currently in use by the laboratories included in this study. There were also large variations in the number of age strata in use for paediatric RIs.
Conclusion
This study demonstrates large variations in currently utilised IgE RIs by Scandinavian and British accredited laboratories and most report markedly lower RIs than those recommended by recent RI publications. Many laboratories likely utilise outdated RIs and should consider critically reviewing and updating their RIs.
Introduction
Atopic diseases are one of the most common disorders worldwide, potentially affecting up to 30% of the European population [Citation1]. As a part of the diagnostic work-up of these diseases, serum total IgE is frequently measured [Citation2,Citation3]. Commercial assays for total IgE are widely available on allergy-focused platforms such as the Phadia ImmunoCAPTM or Siemens ImmuliteTM systems, as well as on general clinical chemistry platforms such as the Roche CobasTM, Abbott ArchitectTM or Siemens Advia Centaur/AttelicaTM systems and on some smaller instruments such as the Siemens BNIITM Nephelometer. The many different total IgE methods have been harmonised to a WHO IgE International Reference Preparation [Citation4], and investigations by external quality assessment (EQA) schemes demonstrate that commercially available assays report harmonised total IgE levels, with the possible exception of the Hycor HytecTM IgE-assay [Citation5]. There exists no specific cut off value able to discriminate between patients with atopic disease from those without, as there is considerable overlap between healthy and atopic populations [Citation3,Citation6]. High total IgE values may be seen in a wide range of non-atopic conditions including neoplastic disease, primary immunodeficiencies, and parasitic or inflammatory diseases [Citation3], whilst the clinical significance of low values remains uncertain and requires further research [Citation7].
During the past two decades multiple studies reporting total IgE reference intervals (RIs) in adult and paediatric populations have been published [Citation8–17]. However, the manufacturers’ package inserts [Citation18–25] typically refer to RIs based on more historical studies [Citation26–29], which differ markedly from the more recent estimates [Citation8–17]. It is therefore of concern whether laboratories may be reporting outdated or inappropriate RIs for total IgE. Adding to this concern, a recent verification study on an Austrian population [Citation30] reported that the total IgE RI derived from the manufacturer’s package insert [Citation24] was not suitable for transference. The aims of our study were therefore to review paediatric and adult total IgE RIs currently in use by Scandinavian and British medical laboratories and to identify their sources. In addition, we performed a review of the literature in order to compare published RIs to those currently in use by Scandinavian and British medical laboratories.
Methods and materials
Selection of included countries
All three Scandinavian countries (Norway, Sweden and Denmark), in addition to the United Kingdom were chosen due to freely available directories over ISO15189-accredited medical testing laboratories provided by the respective national accreditation bodies [Citation31–34]. In addition, the selected countries allowed for querying of the openly available laboratory user handbooks without the need for translation to the investigators.
Acquisition of results and data handling
The initial set of medical laboratories was compiled on September 1st 2022, after accessing the national directories of accredited laboratories [Citation31–34]. For the UK, the accredited laboratories were compiled by combining the two categories clinical biochemistry and immunology as presented by the British accreditation body (UKAS) [Citation32]. From the initial list, duplicates were removed. Laboratories specialising in medical genetics, microbiology, toxicology, criminology, cyto-/histopathology or reproductive testing were excluded, as well as laboratories confirming they did not provide total IgE analysis (). The directory provided by the Swedish accreditation body (Swedac) [Citation34] provided 20 distinct accredited organisations (laboratory regions), which in turn serves 77 hospital locations. Of these, 17 of 20 laboratory regions were confirmed to offer total IgE analysis. As each region provides RIs to the respective hospitals, each region was therefore considered as a single instance of a RI, and not as 77 distinct sets. For Denmark and Norway relevant laboratories were identified from the Norwegian (NA) [Citation31] and Danish (DANAK) [Citation33] accreditation bodies, after non-relevant laboratories were excluded as defined by the abovementioned criteria ().
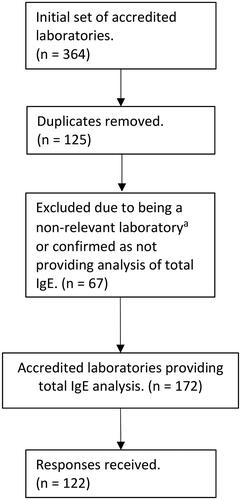
Figure 1. Data handling flowchart. a) Non-relevant laboratories were defined as accredited laboratories specialising in medical genetics, microbiology, toxicology, criminology, cyto-/histopathology or reproductive testing.
Data collection was performed from 15th September throughout 27th December 2022. A maximum of approximately 20 mins was allocated per laboratory in order to manually obtain RIs from the laboratory user handbook provided by the respective health trust’s or hospital’s website. If unsuccessfully obtained within this time scope, a query was electronically sent to the quality manager and/or the laboratory director. If no reply to the initial request was received, a new request was sent after 3-4 weeks. The data point was considered missing if no further response was received.
The data survey retrieved details, if relevant, on age and sex-partitioned adult and paediatric RIs with upper and lower limits, what limit was in use (e.g. a 95th or 97.5th percentile) and the source of these RIs. As there was in most cases a lack of information on the exact percentile being reported, all results for the upper reference limit (URL) were pooled together. Identification of the RI sources were in most cases achieved based on the age-stratification of paediatric RIs being identical to a handful of confirmed sources from the package inserts, local or regional RI investigations.
Review of published adult and total IgE reference interval studies
Medline, Embase and the Cochrane Library databases were searched from the time of database inception to 27th December 2022. In addition, relevant book chapters in the field of clinical chemistry and clinical immunology/allergy were reviewed. The search terms consisted of: ‘IgE’, ‘immunoglobulin E’, ‘immunoglobuline E’, ‘immuno-globulin E’, ‘immuno globulin E’ or ‘Ig E’, combined with the following: ‘reference interval’, ‘reference range’, ‘reference value’, ‘reference level’, ‘normal interval’, ‘normal range’, ‘normal value’ or ‘normal level’. The utilised search terms are provided in the Supplementary materials. Only publications in English were retrieved. All publications reporting at least one of the following 2.5th, 90th, 95th, 97.5th or 99th percentile reference limits/intervals were included. Studies utilising non-immunoassay methods were excluded, with the exception of the most recent publication performed using paper radioimmunosorbent test (PRIST) by Simoni et al. (2001) [Citation13].
Results
The initial set provided by the test directories yielded 364 accredited laboratories (43 Norwegian, 20 Swedish, 46 Danish and 255 British) [Citation31–34]. Following removal of duplicates (n = 125) and exclusion of non-relevant laboratories or laboratories confirming that they did not offer total IgE analysis (n = 67), the remaining laboratories (n = 172) were considered to constitute the set of current ISO15189 accredited medical laboratories providing total IgE testing in the UK and Scandinavia. The majority of duplicates were due to tests being listed under both categories (clinical biochemistry and immunology) in the UK or the same organisation or trust being listed several times due to multiple issued accreditations to the same organisation. From the 172 total IgE providers, information on total IgE RIs was identified from 122 laboratories (). Excellent acquisition rates were achieved from the Scandinavian countries with 17 of 17 from Norway (100%), 16 of 17 from Sweden (94%) and 22 of 26 from Denmark (85%). A good response rate was achieved from the UK with 67 of 112 (60%), in total conferring a response rate of 71% across all regions. Almost all laboratories provided both dedicated paediatric and adult RI sets. An overview of methods reported in use by laboratories, grouped by country, is provided in the Supplementary table 1.
Adult reference intervals
In descriptives and identified sources of upper adult RI limits identified in our study are provided. The median upper limit was closely matched throughout Scandinavia (114 – 115 kU/L) and was somewhat higher compared to the British median upper reference limit (81 kU/L) (). The limit of 81 kU/L was utilised by 33 (49%) of laboratories in the UK, whilst upper limits of 100 − 120 kU/L were reported by 23 (34%) British laboratories. Three British laboratories provided upper limits of 2, 15 or 30 kU/L in their laboratory user handbooks, which should be speculated if might constitute clerical errors. In Scandinavia, the upper limits of 114, 115, 120 and 150 kU/L were the most abundant, although four Norwegian laboratories reported a significantly higher upper limit of 297 kU/L. Reported upper limits varied between the different countries included in our survey; range (min – max) with Sweden 44 kU/L (85 − 129) and Denmark 50 kU/L (100 − 150) displaying the narrowest ranges, whilst Norway 210 kU/L (87 − 297) and the UK 248 kU/L (2 – 250) exhibited the widest ranges.
Figure 2. Adult total IgE reference intervals (only upper limits are shown) utilised in Scandinavia and the UK, compared to published upper reference limits (n=11 solid red points derived from 6 publications).
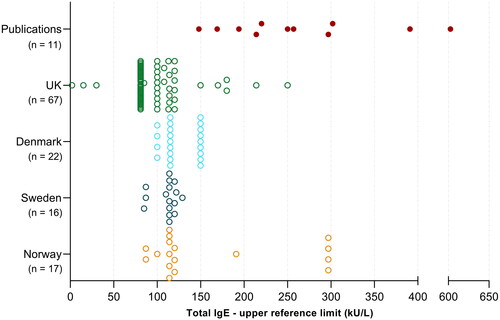
Table 1. Descriptives for acquired data sets, reference interval sources and adult total IgE upper reference limits reported by medical laboratories in Norway, Sweden, Denmark and the UK.
Overall, 89% of the included laboratories applied an adult upper limit clustered between 81 to 150 kU/L. No laboratories were found to report adult gender-partitioned RIs. All surveyed laboratories utilised lower limits in close agreement with the literature, ranging from 0 - 20 kU/L [Citation9–11,Citation14,Citation16]. Therefore, lower limits for total IgE are not further detailed in the results. The original sources of RIs varied significantly between the surveyed regions. The manufacturer’s package inserts were most frequently used in Sweden (88%) and Norway (65%), whilst a local or regional RI study was most frequently cited in Denmark (59%) [Citation35] and the UK (49%) [Citation36].
Paediatric reference intervals
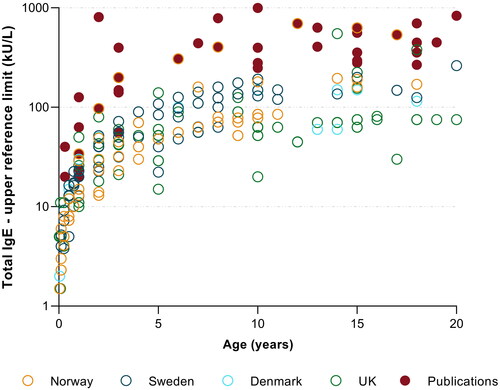
In total, 28 distinct age-strata were found to be in use by the different laboratories. A few laboratories only reported stratified RIs up to the age groups of 9, 10, 12 or 15 years of age, while concurrently not reporting any adult RI. Two laboratories used the age-strata of 19 years, and two laboratories provided RIs for the age-strata of 20 years of age. Generally, there was a low degree of harmonisation in regard to age-stratification, with the number of age-partitions used by a single laboratory varying from 3 and up to 16 age-partitions at the most. The paediatric upper reference limits also varied widely and are presented in .
Figure 3. Paediatric total IgE reference interval upper limits, reported by medical laboratories (n = 122) in Scandinavia and the UK and those reported in the literature (solid red points, derived from six publications). The y-axis displays reported IgE upper reference limits on a logarithmic scale.
Literature review of published adult and paediatric reference interval studies
The literature review identified (n = 6) publications reporting paediatric RIs and (n = 6) publications reporting adult RIs based on immunoassays ( and ). Adult upper reference limits varied between 148 − 603 kU/L, depending if reporting an upper 95%, 97.5% or 99% limit. All studies were performed using immunoassays with the exception of Simoni et al. (2001) [Citation13] utilising PRIST. The included studies were published between 1995 and 2022, with the majority published after 2010 (82%). Five studies were performed in North America, three in Europe and the remaining three in Asia. The largest available adult European study (n = 6670) found a 95th percentile of 169 kU/L in males and 148 kU/L in females, with 99th percentiles of 341 and 300 kU/L in males and females, respectively [Citation8].
Table 2. Summary of adult total IgE reference interval studies published after 1990 utilising immunoassays, with the exception of Simoni et al. (2001) [Citation13] utilising paper radio immunosorbent test (PRIST).
Table 3. Summary of published paediatric total IgE reference interval studies performed using immunoassays identified by the literature review.
With regard to paediatric RIs, CALIPER (greater Toronto area) found upper 97.5th limits of 440 kU/L (90%CI 322, 1000) for 0 to 6 years, and 450 kU/L (90%CI 380, 484) for 7–18 years on the Abbott ArchitectTM platform [Citation10]. RIs with (90% CIs) were also established using the Beckman Coulter platform yielding upper limits of 809 kU/L (173, 1230) for 0 to <2 years, 786 kU/L (317, 1705) for 2 to <8 years and 833 kU/L (571, 1292) for 8 to <19 years [Citation9]. It should be noted that RIs established by O.P. Soldin (2008) [Citation14] consisted of approximately 60% paediatric participants of African-American background, therefore the results might be less applicable for transference to a Northern European population. Martins et al. [Citation11] included 1376 healthy children from the Salt Lake City area, and found similar paediatric upper limits, peaking at 696 kU/L from 9–12 years.
The number of utilised age-strata differed markedly, with the CALIPER publications reporting only 2 or 3 age-strata [Citation9,Citation10], while Martins et al. [Citation11] and O.P. Soldin et al. [Citation14] reported 7 and 8 age-strata, respectively. Sacco et al. presents longitudinal total IgE growth curves following a German cohort of 1314 children over twenty years, divided into atopic and never-atopic groups [Citation37]. This publication does not provide CLSI-calculated RIs, however based on their data, an upper 97th limit for the never-atopic group (n = 466) was approximately 300 kU/L, peaking during 13–15 years of age. summarises RIs provided in the package inserts by market leaders of total IgE analysis in Europe [Citation18–25].
Table 4. Reference intervals provided in the package insert by market leaders of total IgE analysis in Europe with information on the international reference preparation (IRP), type of upper limit and source of data.
Discussion
This is the first study to systematically survey and document total IgE RIs in use by accredited laboratories in Northern Europe. Total IgE is a frequently requested analyte, particular in primary health care. Therefore, appropriately provided RIs is important for facilitating correct clinical interpretation and to avoid unnecessary referrals for further resource intensive investigations. Our study finds a wide range of different paediatric and adult RIs currently in use, in adults ranging from 87−297 kU/L () and in children, at for instance 5 years of age, ranging from 15–140 kU/L (). Most laboratories reported considerably lower upper limits than those reported by the recent literature ( and ).
Generally, there were some differences between total IgE RI limits reported by the four countries included in our survey. The upper limits of 114 or 120 kU/L for adults were used by around two thirds of the Swedish laboratory regions, whilst 81 kU/L was used by more than half of the included British laboratories (). This is likely explained by the laboratories in these countries relying on a few selected sources. In the UK, the PRU Handbook of Clinical Immunology was the most referenced source, citing an adult upper limit of <81 kU/L [Citation36]. In Sweden the upper limit of 114 kU/L can be traced back to the manufacturer’s package insert [Citation18], which in turn cites Zetterstrøm et al. (1981) [Citation27].
A geographic variation in the sources used for the transferred IgE RIs was exhibited. The manufacturer’s package inserts were most frequently used in Sweden (88%) and Norway (65%), whilst local or regional RI studies were most frequent in Denmark (59%) [Citation35] and the UK (49%) [Citation36] (). Most laboratories were unable to state (neither in their laboratory user handbook, or by survey) if their utilised upper limits represented 95th or 97.5th percentiles. In addition, some laboratories chose to report geometric means + 1 SD, which may be prone to misinterpretation by clinicians as this is a less standard approach. A few laboratories reported two-sided RIs. It remains debatable if it is most useful to report total IgE-measurements with a one-sided (0–95th percentile), or a two-sided (2.5 − 97.5th) RI. Presently, as the clinical significance of low total IgE values still remains uncertain [Citation7,Citation38], it could be argued that a one-sided 95th percentile may be clinically the most appropriate.
The results of our survey also highlight variations in selected age-strata for paediatric total IgE upper reference limits. Age-strata are currently poorly harmonised, with some laboratories reporting up to 16 distinct age-strata, whilst some laboratories have opted to report as few as 3 age-strata. Published studies range from reporting 8 strata at the most by Martins et al. [Citation11], with CALIPER reporting only 3 strata [Citation9,Citation10]. Total IgE concentrations are low at birth and during the first year of life, with upper limits of approximately 50–100 kU/L. Concentrations continue to increase during later childhood with the upper limit peaking at approximately 500 − 800 kU/L during 8 - 13 years of age [Citation11,Citation14]. Thus, it is important for clinicians to be cognisant of the fact that total IgE concentrations are largely age-dependent, which must be taken into account when interpreting results in children. Paediatric growth curves might be a more practical and intuitive means of presenting RIs in paediatric populations [Citation39–41].
As summarised in , the manufacturers’ provided RIs can mostly be traced back to studies from the 70s and 80s [Citation26–29]. Although the 1st WHO standard for total IgE (69/204) was established in 1973, it is difficult to ascertain if the methods used by the older publications [Citation26–29] were traceable to the WHO-standard. Moreover, concerns remain as to whether the older publications provide RIs calculated in accordance to current conventions/recommendations [Citation42] which may contribute towards explaining why many currently utilised upper RI limits are low compared to RI limits recommended by the more recent literature ( and ). The highly cited study by Zetterstrøm et al. (1981) reports a geometric mean (not to be confused with the arithmetic mean) with a ± 2 SD range, and the package insert for the ImmunoCAPTM platform [Citation18] provides a table of paediatric geometric means +1 SD. These should not be directly transferred as 95% or 97.5% RIs. Furthermore, if interpreting the geometric means + 1 SD as upper reference limits as presented by ImmunoCAP’s package insert [Citation18], this would directly infer a gradual and continuous increase of physiological IgE from birth until adulthood, as also could be interpreted from the package inserts from the Siemens Immunlite [Citation19] and BN2 systems [Citation22]. It has, however, been widely shown that this is not the case, as total IgE peaks during late childhood [Citation2,Citation10,Citation11,Citation14,Citation16,Citation37].
Laboratories’ responsibility in verifying transferred reference intervals
It is important for medical laboratories to provide updated RIs [Citation42–44], and it is generally agreed upon that optimum practice recommends each laboratory to establish its own RI. However, this is resource demanding and it may therefore not always be practical for each laboratory to establish RI by the direct method; i.e. performing sampling studies on healthy participants as detailed by the Clinical & Laboratory Standards Institute (CLSI) guidelines [Citation42]. Therefore, clinical laboratories often utilise RIs from the literature or as suggested by the manufacturer’s package insert. However, in such cases it is generally advisable to verify transferred RIs by performing smaller scale verification studies [Citation30,Citation44–46]. Leitner-Ferenc et al. recently published the results of such a verification of manufacturer-provided RIs from the package inserts on the Roche CobasTM platform [Citation30]. In their study, they found 10 of 40 total IgE values (25%) fell above the manufacturer-provided RI of 0–100 kU/L [Citation24] and therefore concluded the provided RI was not suitable for transference [Citation30]. This and other recent publications of total IgE RIs [Citation8,Citation11–13,Citation47] indicate that frequently used upper reference limits of 87–114 kU/L in adults are too low, and thus results from healthy individuals will unnecessarily fall outside of this interval. At our tertiary centre total IgE has been requested on average approximately 23 000 times annually the last four years, with 5 850 results per year falling above a limit of 114 kU/L. If, however, utilising an upper 95th percentile of 302 kU/L [Citation17], 55% fewer test results would fall above the URL.
The most recent RI studies ( and ) were performed using assays traceable to the 2nd (75/502) or the 3rd WHO (11/234) total IgE standard. The assigned value of the current 3rd WHO reference material established in 2014 (11/234) was demonstrated to closely match the 2nd WHO standard (75/502), established over three decades ago in 1980 [Citation4]. In addition, EQA scheme results show that currently used immunoassays provide similar results, with the possible exception of the Hycor HytecTM system which displayed somewhat lower levels compared to the other methods [Citation5]. However, none of the investigated British and Scandinavian laboratories in our study were found to use the Hytec HycorTM total IgE method. It would therefore be reasonable to assume the trueness of total IgE-measurements would be well-managed and unlikely to cause of the large differences in the reported RIs.
It must however, be considered that there may be marked differences in circulating total IgE levels between different populations. In a large study of n = 13 883 individuals from 37 centres Burney et al. found a 2 – 3 fold difference in the geometric means between different European countries [Citation48]. Iceland, Sweden and Norway displayed the lowest levels, whilst Ireland, Italy, France and Greece displayed the highest levels. Factors such as smoking status, ethnicity, socioeconomic status, obesity and level of alcohol consumption have been shown to affect the circulating total IgE levels [Citation6, Citation49]. Complicating matters further, environmental factors such as occupational dust or gas exposure have also been found to be independent predictors of higher total IgE levels [Citation50]. The many influencing factors of circulating total IgE levels should be taken into account both in the selection of RI study participants and in the interpretation of published RIs. Moreover, these factors would likely make a global harmonisation of total IgE RIs unfeasible. Nevertheless, further efforts are recommended towards harmonising both paediatric and adult total IgE RIs provided by medical laboratories investigated in our study.
Strengths and limitations of the study
The study had a response rate of 71% which may be considered a good response rate for an unsolicited survey. For Norway, the study was able to provide a complete set of RIs and for the other Scandinavian countries the study includes a near-complete set of RIs by laboratories providing medical testing of total IgE. Only laboratories accredited by ISO15189 were included. However, data from 2016 by Boursier et al. showed at least 80% of medical laboratories in the UK and 88% in Sweden had received ISO 15189 accreditation, and further laboratories were at the time working towards accreditation [Citation51]. Our survey is therefore able to provide a comprehensive representation of currently utilised total IgE RIs in these Northern-European countries. A limitation is that we were in most cases unable to collect exact documentation of the traceability in regard to the sources of RIs used by the reporting laboratories. However, in most cases identification could with high likelihood be achieved on the basis of the age-stratification and the RIs themselves being identical to a handful of known sources, either the RI sets provided by the manufacturer [Citation18–25] or from regional RI studies [Citation35,Citation36]. Furthermore, we did not have information on whether laboratories had verified their RIs prior to implementation.
Conclusion
In this study we demonstrate large variations in currently utilised total IgE RIs by Norwegian, Swedish, Danish and British accredited laboratories in which most report upper reference limits markedly lower than those recommended in recent RI publications. It appears likely that many laboratories have not performed verification investigations when transferring RIs from the manufacturers’ package inserts. We therefore recommend that laboratories critically review their total IgE RIs and their selected age-strata for paediatric RIs and consider updating these in accordance to more recent publications. On a final note, this study may serve as a reminder and underpin the general importance of performing smaller scale verification studies when transferring RIs provided by the manufacturers’ package inserts or from the literature in general.
Author contributions
EWV conceptualised the study, performed data collection/analysis, and drafted the manuscript. AKA contributed to data analysis and writing. AKA, TA and MA provided supervision as senior scientists in laboratory medicine and clinical allergy. EWV and ISKS performed search of the literature. All authors contributed to interpretation and critical review of the manuscript and approved the final draft.
Ethical statment
The study was deemed exempt from review by the Regional Ethics Review Board.
Supplemental Material
Download PDF (127.6 KB)Disclosure statement
The authors have no conflicts of interests to declare.
References
- Agache I, Annesi-Maesano I, Bonertz A, et al. Prioritizing research challenges and funding for allergy and asthma and the need for translational research – the European strategic forum on allergic diseases. Allergy. 2019;74(11):2064–2076. doi: 10.1111/all.13856.
- Ansotegui IJ, Melioli G, Canonica GW, et al. IgE allergy diagnostics and other relevant tests in allergy, a world allergy organization position paper. World Allergy Organ J. 2020;13(2):1–50.
- Stokes J, Casale TB. The relationship between IgE and allergic disease. UpToDate®. 2023 [cited 2023 Feb 19]. Available from: https://www.uptodate.com/contents/the-relationship-between-ige-and-allergic-disease.
- Thorpe SJ, Heath A, Fox B, et al. The 3rd international standard for serum IgE: international collaborative study to evaluate a candidate preparation. Clin Chem Lab Med. 2014;52(9):1283–1289.
- Kleine-Tebbe J, Poulsen LK, Hamilton RG. Quality management in IgE-based allergy diagnostics. J Lab Med. 2016;40(2):81–96. doi: 10.1515/labmed-2016-0013.
- Gergen PJ, Arbes SJ, Jr., Calatroni A, et al. Total IgE levels and asthma prevalence in the US population: results from the national health and nutrition examination survey 2005-2006. J Allergy Clin Immunol. 2009;124(3):447–453. doi: 10.1016/j.jaci.2009.06.011.
- Al S, Asilsoy S, Uzuner N, et al. Is there a clinical significance of very low serum immunoglobulin E level? J Clin Immunol. 2021;41(8):1893–1901. doi: 10.1007/s10875-021-01127-y.
- Carosso A, Bugiani M, Migliore E, et al. Reference values of total serum IgE and their significance in the diagnosis of allergy in young european adults. Int Arch Allergy Immunol. 2007;142(3):230–238. doi: 10.1159/000097025.
- Karbasy K, Lin DCC, Stoianov A, et al. Pediatric reference value distributions and covariate-stratified reference intervals for 29 endocrine and special chemistry biomarkers on the Beckman Coulter immunoassay systems: a CALIPER study of healthy community children. Clin Chem Lab Med. 2016;54(4):643–657.
- Kelly J, Raizman JE, Bevilacqua V, et al. Complex reference value distributions and partitioned reference intervals across the pediatric age range for 14 specialized biochemical markers in the CALIPER cohort of healthy community children and adolescents. Clin Chim Acta. 2015;450:196–202. doi: 10.1016/j.cca.2015.08.020.
- Martins TB, Bandhauer ME, Bunker AM, et al. New childhood and adult reference intervals for total IgE. J Allergy Clin Immunol. 2014;133(2):589–591. doi: 10.1016/j.jaci.2013.08.037.
- Shoormasti RS, Pourpak Z, Eshraghian M, et al. The study of total IgE reference range in healthy adults in Tehran, Iran. Iran J Public Health. 2010;39(3):32–36.
- Simoni M, Biavati P, Baldacci S, et al. The po river Delta epidemiological survey: reference values of total serum IgE levels in a normal population sample of North Italy (8–78 yrs). Eur J Epidemiol. 2001;17(3):231–239. doi: 10.1023/a:1017929831911.
- Soldin OP, Dahlin JR, Gresham EG, et al. IMMULITE 2000 age and sex-specific reference intervals for alpha fetoprotein, homocysteine, insulin, insulin-like growth factor-1, insulin-like growth factor binding protein-3, C-peptide, immunoglobulin E and intact parathyroid hormone. Clin Biochem. 2008;41(12):937–942. doi: 10.1016/j.clinbiochem.2008.04.025.
- Kim HY, Choi J, Ahn K, et al. Reference values and utility of serum total immunoglobulin E for predicting atopy and allergic diseases in Korean schoolchildren. J Korean Med Sci. 2017;32(5):803–809. doi: 10.3346/jkms.2017.32.5.803.
- Soldin SJ, Morales A, Albalos F, et al. Pediatric reference ranges on the Abbott IMx for FSH, LH, prolactin, TSH, T4, T3, free T4, free T3, T-uptake, IgE, and ferritin. Clin Biochem. 1995;28(6):603–606. doi: 10.1016/0009-9120(95)00038-5.
- Vinnes EW, Skarbø B, Wentzel‐Larsen T, et al. Updated total IgE reference intervals in norwegian adults. Immun Inflamm Dis. 2023;11:e751.
- Thermo Fisher - Phadia AB. Uppsala, Sweden - ImmunoCAP total IgE. Directions for use 52-5292-EN/08. 2018.
- Siemens Healthcare Diagnostics Inc - IMMULITE 2000 Total IgE. 2018.
- Siemens Healthcare Diagnostics Inc - Total IgE (tIgE) ADVIA Centaur XP/XPT. 10629879_EN Rev U, 2020-03. 2020.
- Siemens Healthcare Diagnostics Inc - Atellica IM - Totalt IgE (tIgE). 10995366_EN Rev 03, 2020-03. 2020.
- Siemens Healthineers - BN II System - N - Latex IgE mono. 2021.
- Beckman Coulter - IMMAGE Immunochemistry Systems - Total Immunoglobulin E. Chemistry Information Sheet 988635 AR - English IGE. 2021.
- Roche Diagnostics - Elecsys IgE II, V 10.0 English. 2022.
- Abbott. Quantia IgE - Directions for use with ARCHITECT - Ref. 6K42-02. 2020.
- Kjellman NM, Johansson SG, Roth A. Serum IgE levels in healthy children quantified by a sandwich technique (PRIST). Clin Allergy. 1976;6(1):51–59. doi: 10.1111/j.1365-2222.1976.tb01411.x.
- Zetterström O, Johansson SG. IgE concentrations measured by PRIST in serum of healthy adults and in patients with respiratory allergy. A diagnostic approach. Allergy. 1981;36(8):537–547. doi: 10.1111/j.1398-9995.1981.tb01871.x.
- Dati F, Ringel K editors. Reference values for serum IgE in healthy non-atopic children and adults. Clin Chem. 1982;28(7):1556.
- Bhalla R, Rappaport I, De Filippi I, et al. Serum IgE levels in a northeast United States caucasian population. Advanced interpretation of clinical laboratory data., Marcel Dekker, Inc, New York. 1982;295–305.
- Leitner-Ferenc V, Atamaniuk J, Jansen-Skoupy S, et al. CLSI-Based validation of Manufacturer-Derived reference intervals on the cobas 8000 platform. Lab Med. 2017;48(2):e30–e35. doi: 10.1093/labmed/lmx020.
- Norwegian Accreditation (NA) - National accreditation body for technical accreditation. [cited 2022 Sept 1]. Available from: https://www.akkreditert.no/en/akkrediterte-organisasjoner/?scope=MedLab.
- The United Kingdom Accreditation Service (UKAS) - Accredited Organisations - Medical Laboratories. [cited 2022 Sept 1]. Available from: https://www.ukas.com/find-an-organisation/browse-by-category/#orgtype-275.
- Danish Accreditation Fund (DANAK) - Accreditation for medical examination. [cited 2022 Sept 1]. Available from: https://registry.danak.dk/6310.
- Swedish accreditation (Swedac) - Database on accredited bodies - EN ISO15189:2012. [cited 2022 Sept 1]. Available from: https://search.swedac.se/en/accreditations.
- Referenceintervaller for de klinisk-biokemiske afdelinger i Stor København. Sundhedsfagligt Råd for Klinisk Biokemi. 1998.
- Milford-Ward A, Sheldon J, Rowbottom A, et al. PRU handbook of clinical immunochemistry. 9th ed. Sheffield (UK): PRU Press; 2007.
- Sacco C, Perna S, Vicari D, et al. Growth curves of “normal” serum total IgE levels throughout childhood: a quantile analysis in a birth cohort. Pediatr Allergy Immunol. 2017;28(6):525–534. doi: 10.1111/pai.12738.
- Elkuch M, Greiff V, Berger CT, et al. Low immunoglobulin E flags two distinct types of immune dysregulation. Clin Exp Immunol. 2017;187(3):345–352. doi: 10.1111/cei.12885.
- Madsen A, Almås B, Bruserud IS, et al. Reference curves for pediatric endocrinology: leveraging biomarker Z-Scores for clinical classifications. J Clin Endocrinol Metab. 2022;107(7):2004–2015. doi: 10.1210/clinem/dgac155.
- Goldman J, Becker ML, Jones B, et al. Development of biomarkers to optimize pediatric patient management: what makes children different? Biomark Med. 2011;5(6):781–794. doi: 10.2217/bmm.11.96.
- Higgins V, Adeli K. Advances in pediatric reference intervals: from discrete to continuous. J Lab Precis Med. 2018;3:3–3. doi: 10.21037/jlpm.2018.01.02.
- Horowitz G. Defining, establishing, and verifying reference intervals in the clinical laboratory. EP28A3C. Clinical and Laboratory Standards Institute (CLSI). 3rd Edition. 2010;28
- Jones G, Barker A. Reference intervals. Clin Biochem Rev. 2008;29:93–97.
- Płaczkowska S, Terpińska M, Piwowar A. The importance of establishing reference intervals – is it still a current problem for laboratory and doctors? Clin Lab. 2020;66(8):1429–1438. doi: 10.7754/Clin.Lab.2020.191120.
- Ozarda Y, Higgins V, Adeli K. Verification of reference intervals in routine clinical laboratories: practical challenges and recommendations. Clin Chem Lab Med. 2018;57(1):30–37. doi: 10.1515/cclm-2018-0059.
- Jones GRD, Haeckel R, Loh TP, et al. Indirect methods for reference interval determination – review and recommendations. Clin Chem Lab Med. 2018;57(1):20–29. doi: 10.1515/cclm-2018-0073.
- Ezeamuzie CI, Al-Ali SF, Al-Dowaisan A, et al. Reference values of total serum IgE and their significance in the diagnosis of allergy among the young adult kuwaiti population. Clin Exp Allergy. 1999;29(3):375–381. doi: 10.1046/j.1365-2222.1999.00463.x.
- Burney P, Malmberg E, Chinn S, et al. The distribution of total and specific serum IgE in the european community respiratory health survey. J Allergy Clin Immunol. 1997;99(3):314–322. doi: 10.1016/s0091-6749(97)70048-4.
- Litonjua AA, Celedón JC, Hausmann J, et al. Variation in total and specific IgE: effects of ethnicity and socioeconomic status. J Allergy Clin Immunol. 2005;115(4):751–757. doi: 10.1016/j.jaci.2004.12.1138.
- Omenaas E, Bakke P, Elsayed S, et al. Total and specific serum IgE levels in adults: relationship to sex, age and environmental factors. Clin Exp Allergy. 1994; 24(6):530–539. doi: 10.1111/j.1365-2222.1994.tb00950.x.
- Boursier G, Vukasovic I, Brguljan PM, et al. Accreditation process in european countries – an EFLM survey. Clin Chem Lab Med. 2016;54(4):545–551.
