Abstract
Regional variations in the prevalence of gestational diabetes mellitus (GDM) have been found across Denmark. The objectives of this exploratory survey were to evaluate adherence to the national guideline for screening and diagnosing GDM and to identify variations in pre-analytical or analytical factors, which could potentially contribute to variations in GDM prevalence across regions. In a national interview-based survey, obstetric departments and laboratories throughout Denmark handling GDM screening or diagnostic testing were invited to participate. Survey questionnaires were completed through personal interviews. In total, 21 of 22 identified obstetric departments and 44 of 45 identified laboratories participated. Adherence to guideline among obstetric departments ranged 67–100% and uniformity in laboratory procedures was high. However, the gestational age at the time of late diagnostic testing with oral glucose tolerance test (OGTT) varied considerably, with 48% (10/21) of departments testing outside the recommended 24–28 weeks’ gestation. Procedural heterogeneity was most pronounced for the parts not described in current guidelines, with choice of laboratory equipment being the most diverse factor ranging 3–39% nationally. In conclusion, the overall adherence to the national guidelines was high across regions, and obstetric departments and laboratories had high uniformity in the procedures for screening and diagnosing GDM. Uniformity was generally high for procedures included in the guideline and low if not included. However, a high proportion of GDM testing was performed outside the recommended gestational window in late pregnancy, which may be a pre-analytical contributor to regional differences in GDM prevalence.
Introduction
Adherence to clinical guidelines and agreement upon standardised clinical procedures are keystones to minimising bias and securing uniform diagnostic testing in a population. However, this task may be compromised when screening programs are complex and involve several stakeholders. In screening programs for gestational diabetes mellitus (GDM), diagnosis is based on an oral glucose tolerance test (OGTT), even though it has known limitations, e.g. poor reproducibility [Citation1]. Moreover, the OGTT-derived diagnosis relies solely on the measurement of glucose, which is sensitive and complex as it is influenced by several pre-analytical and analytical factors, including duration of fasting prior to OGTT, glucose load, blood specimen used for diagnosis, blood collection tubes and analytical method [Citation1–4]. Differences in such factors may result in unintended variations in GDM prevalence between sites but can possibly be minimised if using the same procedural guidelines for GDM diagnostics [Citation5].
In Denmark, GDM screening has been risk-factor based for more than 20 years and is managed in Primary or Secondary Care [Citation6]. Screening indications for the standard ‘late’ diagnostic testing (24–28 weeks’ gestation) include one or more of the following risk factors: GDM in a previous pregnancy, previous birth of a macrosomic infant (≥4,500 g), pre-pregnancy BMI ≥27 kg/m2, family history of diabetes, polycystic ovarian syndrome, current multiple pregnancy or glucosuria. Women with previous GDM or more than one of the other indications are also recommended early diagnostic testing (10–20 weeks’ gestation) [Citation6].
The first Danish national obstetric guideline for screening and diagnosing GDM was formulated in 2003, however, no national laboratory guideline on the OGTT procedure exists [Citation6]. Despite a long tradition of using uniform guidelines, we have previously described significant differences in GDM prevalence throughout the five administrative regions of Denmark, which was not explained by variations in well-known clinical risk factors [Citation7]. Such differences could occur with poor adherence to guidelines resulting in large variations in pre-analytical or analytical factors [Citation5].
No prior studies have investigated whether the national guidelines for GDM in Denmark are followed or how the screening and diagnostic procedures are performed [Citation8–12].
We aimed to evaluate the clinicians’ adherence to national guidelines on GDM screening and diagnosis and to identify variations in pre-analytical or analytical factors related to diagnostic procedures at obstetric departments and laboratories.
Materials and methods
In this national interview-based survey, we gathered information on the local screening procedure for GDM from obstetric departments and hospital or walk-in laboratories throughout Denmark. Laboratories were invited to participate if they performed OGTTs as part of the GDM testing or analysed glucose samples from such tests. The local management nominated the person(s) with the most knowledge of the practical procedure to complete the interview. When in doubt, the appointed person would address colleagues for help.
Two sets of unique surveys were developed using the secure web-based REDCap electronic data capture tools hosted at the Capital Region of Denmark for the obstetric departments and laboratories, respectively [Citation13,Citation14]. Data were collected by personal interviews online or by telephone. In case of supplemental questions after the interview, or if an interview was not feasible, the survey was completed by email correspondence. All interviews were completed by the same researcher (CS) from March 2022 to February 2023.
The survey conducted among the obstetric departments in the present study consisted of 14 questions divided into five topics: Indications for screening and timing of diagnostic testing (three questions); site of testing, diagnostic test and criteria (seven questions); use of repeat OGTTs (defined as an initial sub-diagnostic 2h glucose test result between 8.0 and 9.0 mmol/L at which the OGTT is repeated after 4–6 weeks, two questions); and initial postpartum follow-up (8–12 weeks postpartum, two questions). Patient information pamphlets or online material on OGTT in pregnancy were collected from all obstetric departments, from which patient instructions on the advised fasting duration and restrictions prior to OGTT were collected.
The survey conducted among laboratories consisted of 15 questions allocated to three topics: The OGTT procedure (three questions); analytical equipment and analyses (four questions); and storage and transportation (eight questions).
All participants were instructed to answer the questions in terms of the actual daily clinical practice and not based on the written local instructions. Answers from the surveys were compared with the two national guidelines on screening and diagnosis of GDM and GDM treatment by the Danish Society of Obstetrics and Gynecology (DSOG) [Citation6,Citation15]. The DSOG recommendations cover screening indications, gestational age at the time of testing (early and late), OGTT type, blood specimen for diagnosis and diagnostic criteria. However, the recommendations do not include laboratory procedures relating to the OGTT or patient instructions prior to the OGTT. Local procedures were divided into whether standards or recommendations were described in national guidelines, Yes vs. No. Data on the different procedures were reported from the obstetric departments or laboratories, respectively, as well as for each of the five Regions (Capital, Zealand, Southern, Central and North). Data from laboratory diagnostic testing and glucose analyses were reported separately as OGTT procedure and glucose analysis procedure, respectively, as not all laboratories conduct the analyses of samples. GDM prevalence from 2022 was obtained via the online Danish Medical Birth Registry [Citation16].
Statistical analyses
Data was organised in IBM SPSS Statistics, version 29.0.1.0. Descriptive statistics were used to describe the local procedures (percentage, number), and time variables of glucose analyses were presented as median, 25–75 percentiles.
Results
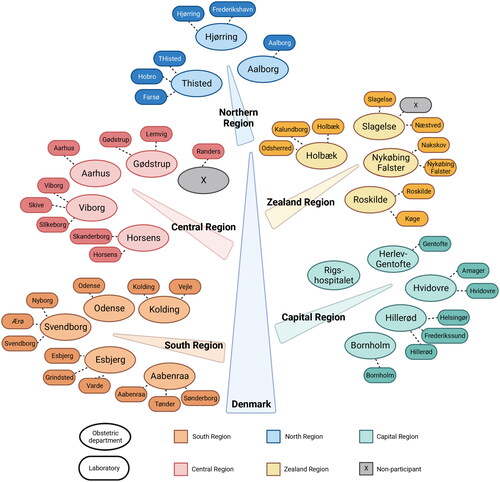
A total of 22 obstetric departments and 45 hospital- or walk-in laboratories were invited to participate, of which 95% (21/22) and 98% (44/45) accepted, respectively (). Of the 65 participants, 78% (51/65) completed the survey by personal interview, the remaining by an electronic survey.
Figure 1. Obstetric departments and laboratories performing OGTT in pregnancy. OGTT: Oral Glucose Tolerance Test. All except one obstetric department and all except one laboratory participated. Created with BioRender.com.
Generally, adherence to the national guideline was high, ranging 67–100%. The lowest adherence rate was for the type of postpartum follow-up ( and Citation2). Regarding screening indications, all obstetric departments followed the national recommendations, except 10% (2/21) that did not report multiple pregnancies as a screening indication. Further, 95% (20/21) of departments supplemented with other indications not featuring in the national guideline, and of these other indications, polyhydramnios and estimated fetal weight > +22% were the most frequently used. In contrast, procedures not described in the guidelines varied considerably between departments, including the recommended fasting duration and standards regarding restrictions prior to OGTT. Duration of fasting varied predominantly in the Capital Region and ranged from 8 to 12h, 8h being the most common recommendation (57%, 12/21).
Table 1. Indications for GDM screening and patient information (obstetric departments).
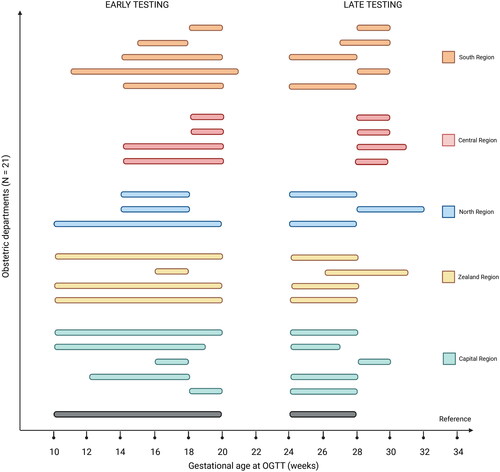
The timing of early and late testing is illustrated in . All departments except one (20/21) adhered to the interval of gestational age at which the national guideline recommends early testing resulting in an adherence rate of 95%. For late testing, the divergence was more pronounced, with a subsequent adherence rate of only 48%. Nationally, 38% (8/21) of departments booked women after 28 weeks’ gestation and thus later than recommended, and a total of 48% (10/21) booked at another interval than the recommended 24–28 weeks’ gestational window. Non-adherence to the guideline for late testing was most frequent in the Central Region.
Figure 2. Variations in gestational age-intervals of early and late GDM testing. GDM: gestational diabetes mellitus. OGTT: Oral Glucose Tolerance Test. The reference refers to the National Guideline on screening for and diagnosis of GDM, Danish Society of Obstetrics and Gynaecology (2014). Created with BioRender.com.
All obstetric departments (21/21) adhered to the recommendation of using a 2h 75 g OGTT as the diagnostic test with a 2h diagnostic criterion of ≥9.0 mmol/L (). However, 10% (2/21) reported performing additional measurement of fasting glucose, although this is not current recommendation. Nationally, 57% (12/21) of departments shared the responsibility of performing GDM diagnostic testing with general practitioners, with a more pronounced contribution from the general practitioners in the Central and North Regions. The use of repeat OGTTs, which is not part of current recommendations, was practiced at all departments in the Capital and Zealand Region. In contrast, such procedure was rarely used in the Southern, Central and North regions (20, 0 and 33%, respectively) ().
Table 2. Diagnostic test and criteria, repeat OGTT and postpartum follow-up (obstetric departments).
Initial postpartum follow-up was reported to be performed at the hospital by 48% (10/21) of departments, the remaining follow-up being handled by the general practitioner (). Regardless of location for follow-up, this most often included an OGTT 8–12 weeks postpartum as recommended (67%, 14/21), followed by HbA1c with/without fasting glucose in 24% (5/21) of the departments.
Overall, there was a high degree of uniformity in laboratory procedures, although the national guideline only includes recommendations regarding blood specimens. Of the 44 included laboratories performing diagnostic OGTT in pregnancy, 31 (70%) conducted the glucose analyses, and in 30% (13/44) of cases, the samples were transported to another location for analysis (). A monohydrate ready-to-use glucose solution was the most used glucose product for OGTT (95%, 42/44), and all laboratories but one (43/44) sampled venous blood in fluoride citrate (FC-mix) tubes. All laboratories stored the blood samples at 20–25 °C prior to centrifugation, and time estimates from sampling to analysis were in general higher in the Northern Region due to greater geographic distances. Overall, the Capital Region was the region with most heterogeneity in the glucose analysis procedures ().
Table 3. Diagnostic testing and glucose analyses (laboratories).
At 30 of 31 laboratories, plasma glucose concentration was measured by enzymatic analytical method, whereas one laboratory sampled capillary blood analysed with point-of-care testing (HemoCue Glucose 201+). Analytic equipment was supplied by five different manufacturers and reached the lowest procedural uniformity among laboratories, ranging 3–39% nationally ().
Discussion
Overall, there was a high degree of uniformity in the procedures for screening and diagnosing GDM across regions. Adherence to the national obstetric guidelines for screening and diagnosing GDM at the Danish obstetric departments was good, but with large variations in gestational age at time of late OGTT and in methods used for postpartum follow-up. Heterogeneity across departments and regions was, in general, more pronounced for procedures not described in the national guidelines, including the recommended fasting duration, the number of restrictions prior to OGTT and the use of repeat OGTTs. In contrast, despite the lack of clinical guidelines describing procedures for glucose analyses, we demonstrated high uniformity in glucose analysis procedures across laboratories.
The high adherence to the national guidelines observed in our survey is somewhat unexpected since it has previously been reported as being rather low for risk-factor based GDM screening [Citation8–10,Citation12]. However, despite a high adherence, we cannot determine from the present survey whether all women with risk factors were, in fact, screened according to the recommended clinical indications.
The gestational age at time of late GDM testing varied considerably between departments and regions. The postprandial glucose level in pregnant women increases during pregnancy as a normal physiological response to hormonal changes [Citation17]. GDM testing performed beyond 28 weeks’ gestation may therefore increase the rates of GDM diagnosis compared to testing in 24–28 weeks’ gestation [Citation18]. Thus, the large variations in the gestational age at which late testing was performed may contribute to regional differences in GDM prevalence, as departments performing OGTTs later than recommended would be prone to identify more women as having GDM. Nevertheless, the Central Region had the lowest GDM prevalence in 2022, even though it was the only region where all departments reported to perform the OGTT later than 28 weeks’ gestation [Citation16]. Such discrepancy could be partly due to unmeasured variance from OGTT procedures performed at general practitioners’ surgeries, which were widely used in the Central Region. OGTTs performed at these surgeries could be at a late gestational age in some regions while early in others and perhaps opposite to the time of testing performed at the hospital laboratories. The 48% of departments performing late testing outside the recommended gestational age interval highlights another but central challenge, as it may in part reflect poor guideline implementation: The interval used to be at 28–32 weeks’ gestation before the latest guideline revision in 2014.
Fasting duration is one of many pre-analytical factors potentially influencing the measured glucose values [Citation1]. Results of previous studies are inconsistent, as some have reported a significant increase in the 2h glucose with fasting exceeding 8h (up to 16-20h), while others observed no differences in glucose levels at fasting durations as low as 3h [Citation19–22]. Thus, the observed variations in fasting duration within the narrow range of 8–12h will most likely not have had a major effect on GDM prevalence. Another factor, which may affect the glucose values much more than the duration of fasting itself, is non-adherence to fasting instructions [Citation23]. Overall, patient adherence to recommendations, including screening, diagnosis, and treatment, may be just as influential as the clinicians’ guideline adherence and is reportedly important in improving health outcomes [Citation24]. However, neither of these aspects could be quantified in the present study.
An additional important pre-analytical factor in GDM diagnostics is the blood specimen used for glucose analyses. Compared to the glucose concentration obtained from venous plasma, capillary blood analysed by point-of-care method results in lower glucose concentrations when reported as whole blood equivalents and higher when reported as plasma equivalents [Citation25–27]. In the present survey, one laboratory used capillary whole blood equivalents for diagnosis, and consequently, as this department in 2022 handled 9% of births in the Capital Region, this may have contributed to the previously reported low regional GDM prevalence in the Capital Region [Citation16].
Nevertheless, despite not having a national laboratory guideline for the OGTT procedure, the overall uniformity of glucose analyses was very high. Further, the international recommendation as formulated by the American Diabetes Association, including the use of venous plasma and FC-Mix collection tubes, was followed at all sites but one [Citation28]. The use of FC-Mix glucose-stabilising tubes is reported to be the most efficient pre-analytical method to inhibit glycolysis [Citation28]. Thus, when using FC-Mix tubes, differences in storage temperature and time until centrifugation can be neglected as a cause of regional divergence in GDM prevalence. The present study did not include information regarding OGTT procedures at general practitioners’ surgeries. Since all departments in the Central Region have shared responsibility of OGTT in pregnancy with the general practitioners, unmeasured variance in procedures at these surgeries – including the use of capillary blood sampling or less glucose-stabilising collection tubes – may substantially influence the overall GDM prevalence and contribute to the low prevalence in the Central Region.
Postpartum follow-up has previously been demonstrated to be of rather poor heterogeneity and was the procedure with lowest adherence rate in our survey [Citation29,Citation30]. The choice of postpartum OGTT has been a tradition in Denmark for 20 years, but due to latest evidence on test sensitivity and local experiences of poor patient adherence, a new suggestion was proposed by the DSOG in April 2023. Thus, the future recommendations for postpartum follow-up in Denmark will most likely include measurement of HbA1c and fasting plasma glucose [Citation31].
Altogether, high adherence to guidelines and procedure uniformity is not enough to ensure perfect alignment. Even when using the same analytical equipment, significant differences in glucose values and the resulting GDM prevalence can occur [Citation32]. Further, the complexity of factors influencing the glucose level and the challenges in performing accurate glucose measurements leave room for a considerable number of other potential confounding factors as biological variation and analytical traceability, bias and imprecision, which we were unable to quantify in the present survey [Citation1,Citation2].
The main strengths of our exploratory survey are the high participation rate and high uniformity in data collection, as all interviews were completed by the same researcher ensuring a high response accuracy. Despite the high participation rate, the data presented is not a complete national report of GDM practices and procedures, as GDM diagnostic testing was also performed by the general practitioners. Further, the present survey did not include an evaluation of patient adherence, which would be essential in a full assessment of the impact of guideline adherence on the resulting GDM prevalence. Such assessment, including evidence of cause and effect, can, however, generally not be provided by a survey, why such assessment was beyond the scope of the present study.
The results of this study alone were not able to explain the regional divergence in GDM prevalence but did identify some pre-analytical differences across sites and regions, which may be contributable factors to regional differences. The previously unknown finding of poor guideline implementation further underlines the importance of a structured guideline implementation strategy to achieve high procedural uniformity and adherence to guidelines [Citation11,Citation23]. Such strategies could include adding currently undescribed procedures to future guidelines, establishing formal education practice on procedures, and ensure systematic audits with feedback schemes. Identifying inconsistencies as obtained by the present survey increases the awareness of unexpected gaps in practice and is of high clinical relevance to all countries using national guidelines that need local implementation.
In conclusion, obstetric departments and laboratories reported a high degree of uniformity in the procedures for screening and diagnosing GDM across regions, with low uniformity for procedures not specified in the national guideline. The overall adherence to the national guidelines was good, however, the gestational age at late diagnostic testing varied considerably across departments likely related to poor implementation. Such procedural heterogeneity may constitute pre-analytical factors that contribute to regional differences in GDM prevalence. These findings highlight the need for including all essential procedures in future guidelines and establishing structured implementation strategies including education and audit feedback to help optimise standardised diagnostic procedures.
Acknowledgments
The authors would like to thank all participating sites for their kind benevolence in partaking in the survey.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Additional information
Funding
References
- Bogdanet D, O’Shea P, Lyons C, et al. The oral glucose tolerance test-is it time for a change?-a literature review with an emphasis on pregnancy. J Clin Med. 2020;9(11):9–3451. doi: 10.3390/jcm9113451.
- Bruns DE, Metzger BE, Sacks DB. Diagnosis of gestational diabetes mellitus will be flawed until we can measure glucose. Clin Chem. 2020;66(2):265–267. doi: 10.1093/clinchem/hvz027.
- Song D, Lia M, Hurley JC. Recommended pre-analytical plasma glucose sampling methodology may distort gestational diabetes mellitus prevalence: implications for diagnostic thresholds. Diabet Med. 2019;36(10):1226–1233. doi: 10.1111/dme.14073.
- Potter JM, Hickman PE, Oakman C, et al. Strict preanalytical oral glucose tolerance test blood sample handling is essential for diagnosing gestational diabetes mellitus. Diabetes Care. 2020;43(7):1438–1441. doi: 10.2337/dc20-0304.
- Simundic A-M, Lippi G. Preanalytical phase – a continuous challenge for laboratory professionals. Biochem Med. 2012;22(2):145–149. doi: 10.11613/bm.2012.017.
- Gestational diabetes mellitus: screening and diagnosis [Internet]. Copenhagen (DK): Danish Society of Obstetrics and Gynaecology; 2014 Jan 25 [cited 2023 Mar 12]. Available from: https://static1.squarespace.com/static/5467abcce4b056d72594db79/t/65056123c668314d9aef86d7/1694851364506/GDM-Sandbjerg-2014-godkendt-2014.pdf
- Scheuer CM, Andersen MH, Mathiesen ER, et al. Regional divergence and time trends in the prevalence of gestational diabetes mellitus: a national Danish Cohort Study. Acta Diabetol. 2023;60(3):379–386. doi: 10.1007/s00592-022-02013-8.
- Murphy NM, McCarthy FP, Khashan AS, et al. Compliance with national institute of health and care excellence risk-based screening for gestational diabetes mellitus in nulliparous women. Eur J Obstet Gynecol Reprod Biol. 2016;199:60–65. doi: 10.1016/j.ejogrb.2016.01.044.
- Ruengkhachorn I, Sunsaneevithayakul P, Boriboonhirunsarn D. Non-compliance to clinical practice guideline for screening of gestational diabetes mellitus in Siriraj Hospital. J Med Assoc Thai. 2006;89:767–772.
- Persson M, Winkvist A, Mogren I. Surprisingly low compliance to local guidelines for risk factor based screening for gestational diabetes mellitus – a population-based study. BMC Preg Childbirth. 2009;9(1):53. doi: 10.1186/1471-2393-9-53.
- Salama NI, Abushaikha L. Adherence to clinical practice guidelines during antenatal management of gestational diabetes mellitus: an integrative review. OJN. 2018;08(10):758–770. doi: 10.4236/ojn.2018.810057.
- Grönvall L. Adherence to screening guidelines for gestational diabetes in pregnancy [master’s thesis]. MED-3950. Norway: Faculty of Health Sciences, UiT The Arctic University of Norway; 2020.
- Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381. doi: 10.1016/j.jbi.2008.08.010.
- Harris PA, Taylor R, Minor BL, et al. The REDCap consortium: building an international community of software platform partners. J Biomed Inform. 2019;95:103208. doi: 10.1016/j.jbi.2019.103208.
- Gestational Diabetes Mellitus: Treatment [Internet]. Danish Society of Obstetrics and Gynaecology; 2007. Available from: http://gynobsguideline.dk/sandbjerg/Behandling af gestationel diabetes mellitus.pdf.
- Danish Health Data Authority. The Danish medical birth register [Internet] [cited 2023 May 13]. Available from: https://www.esundhed.dk/Emner/Graviditet-foedsler-og-boern/Foedte-og-foedsler-1997-#tabpanel61119A72216248AC86DB508579760DED.
- Zannat MR, Nessa A, Hossain MM, et al. Serum glucose level in first and third trimester of pregnancy. Mymensingh Med J Bangladesh. 2016;25:211–214.
- Guedj A-M. When should screening be performed for gestational diabetes? Diabetes Metab. 2010;36(6 Pt 2):652–657. doi: 10.1016/j.diabet.2010.11.015.
- Clemmensen KKB, Quist JS, Vistisen D, et al. Role of fasting duration and weekday in incretin and glucose regulation. Endocr Connect. 2020;9(4):279–288. doi: 10.1530/EC-20-0009.
- Hulmán A, Færch K, Vistisen D, et al. Effect of time of day and fasting duration on measures of glycaemia: analysis from the Whitehall II study. Diabetologia. 2013;56(2):294–297. doi: 10.1007/s00125-012-2770-3.
- Moebus S, Göres L, Lösch C, et al. Impact of time since last caloric intake on blood glucose levels. Eur J Epidemiol. 2011;26(9):719–728. doi: 10.1007/s10654-011-9608-z.
- Emberson JR, Whincup PH, Walker M, et al. Biochemical measures in a population-based study: effect of fasting duration and time of day. Ann Clin Biochem. 2002;39(Pt 5):493–501. doi: 10.1258/000456302320314511.
- Kackov S, Simundic A, Gatti-Drnic A. Are patients well informed about the fasting requirements for laboratory blood testing? Biochem Med. 2013;23(3):326–331. doi: 10.11613/bm.2013.040.
- Mustafa ST, Harding JE, Wall CR, et al. Adherence to clinical practice guideline recommendations in women with gestational diabetes and associations with maternal and infant health—a cohort study. Nutrients. 2022;14(6):1274. doi: 10.3390/nu14061274.
- Stahl M, Brandslund I, Jørgensen LGM, et al. Can capillary whole blood glucose and venous plasma glucose measurements be used interchangeably in diagnosis of diabetes mellitus? Scand J Clin Lab Invest. 2002;62(2):159–166. doi: 10.1080/003655102753611799.
- Ignell C, Berntorp K. Evaluation of the relationship between capillary and venous plasma glucose concentrations obtained by the HemoCue glucose 201+ system during an oral glucose tolerance test. Scand J Clin Lab Invest. 2011;71(8):670–675. doi: 10.3109/00365513.2011.619703.
- Carstensen B, Lindström J, Sundvall J, et al. Measurement of blood glucose: comparison between different types of specimens. Ann Clin Biochem. 2008;45(Pt 2):140–148. doi: 10.1258/acb.2007.006212.[PMC]]
- Sacks DB, Arnold M, Bakris GL, et al. Guidelines and recommendations for laboratory analysis in the diagnosis and management of diabetes mellitus. Clin Chem. 2011;57(6):e1–e47. doi: 10.1373/clinchem.2010.161596.
- Benhalima K, Van Crombrugge P, Devlieger R, et al. Screening for pregestational and gestational diabetes in pregnancy: a survey of obstetrical centers in the Northern part of Belgium. Diabetol Metab Syndr. 2013;5(1):66. doi: 10.1186/1758-5996-5-66.
- Vercammen E, Van Hoof L, Vercammen C, et al. Screening and follow-up of pregestational diabetes and gestational diabetes mellitus: a survey of primary care physicians in Belgium. Prim Care Diabetes. 2020;14(6):628–632. doi: 10.1016/j.pcd.2020.04.006.
- Danish Society of Obstetrics and Gynaecology. Gestational diabetes mellitus (GDM) – screening og diagnose [Internet]; 2023 [cited 2023 Dec 3]. Available from: https://static1.squarespace.com/static/5467abcce4b056d72594db79/t/64920a8ca21e7c662dbb1d6c/1687292557843/Guideline_screening_GDM_Final_120623.pdf.
- Scheuer CM, Tvarnø CD, Gils C, et al. The impact of inter-laboratory glucose bias on the diagnosis of gestational diabetes mellitus: comparison of common automated central laboratory methods. Clin Chim Acta. 2023;546:117414. doi: 10.1016/j.cca.2023.117414.
