Abstract
The aim of the present study was to define pediatric reference intervals for serum cobalamin and folate utilizing data generated from a population not exposed to food fortified with folic acid. Folate and cobalamin results analyzed by electrochemiluminescence immunoassay (Roche Cobas) were obtained from 2375 children (2 months to 17.99 years of age). The serum samples were collected between 2011 and 2015 as part of the LIFE (Leipzig Research Centre for Civilization Diseases) Child cohort study in Germany, where folic acid fortification of food is not mandated. These results were used to generate age- and gender-specific reference intervals presented as non-parametric 2.5 and 97.5 percentiles. Because of a subsequent restandardisation of the Roche folate assay in 2016, folate values were recalculated accordingly for adaptation to results obtained using the present calibration. In both genders, folate concentrations decreased continuously with age, whereas cobalamin concentrations peaked at five years of age and then declined. Teenage females had higher concentrations of cobalamin in the age group 12–17.99 years.
Introduction
Reference intervals (RIs) are a vital part of the information provided by laboratories to support the interpretation of laboratory test results. RIs describe the typical distribution of results observed in a healthy population [Citation1]. In pediatric populations, the continuously changing physiology of growing children makes the establishment of RIs one of the most difficult tasks for clinical laboratories [Citation2].
The traditional method to establish RIs is to define discrete age groups and determine age- and gender-specific RIs [Citation3]. However, these discrete groups can only capture the underlying physiological dynamics up to a certain degree and may introduce unnatural, discontinuous transitions between age groups [Citation4]. To avoid this problem, the introduction of continuous RIs has been proposed [Citation5]. Currently used laboratory information systems are, however, unable to accommodate the mathematical equations required to allow test result interpretation based on continuous RIs specific for age and sex [Citation6].
Cobalamin and folate are essential B-vitamins that have important roles for cell functions [Citation7]. While several studies have been carried out on folate and cobalamin in European children, none of the studies included the full age spectrum from newborn to 18 years of age [Citation8,Citation9]. Another problem when calculating RIs for folate is that levels may be significantly higher in populations of the many countries where flour is fortified with folic acid [Citation10] compared to populations not exposed to folic acid fortification. In 1998, the United States Food and Drug Administration mandated that all enriched flour should be fortified with folic acid. Canada and Chile followed with similar mandated folate fortification of wheat flour [Citation11]. In the EU, any decision on mandatory folic acid fortification rests with the member states and so far, none of the countries have taken this population-wide measure [Citation12]. Thus, to be applicable in countries of the EU or other countries without folic acid fortification, folate RIs should be derived from a population likewise not exposed to such fortification.
The LIFE (Leipzig Research Centre for Civilization Diseases) Child Study is a rolling prospective, longitudinal, population-based cohort study with a life course approach to health and disease [Citation13,Citation14]. Data on serum folate and cobalamin in children from that study were previously presented as continuous RIs [Citation15] which, however, are often hard to apply to contemporary laboratory information systems. Thus, the aim of the present study was to complement the previously published data by generating traditionally partitioned discrete age- and gender-RIs, using the principles of the CLSI guidelines [Citation3].
Materials and methods
Study population
The present study is a sub-study of the LIFE (Leipzig Research Centre for Civilization Diseases) Child Study [Citation13]. LIFE Child started recruitment in 2011, and details of the study design have been previously described elsewhere [Citation14]. The study was approved by the Ethical Committee of the University of Leipzig (Reg. no. 264-10-19042010).
Participants and setting
Participants were between 2 months and 18 years of age and without chronic, chromosomal, or syndromal diseases. The dataset for folate and cobalamin consisted of 2357 and 2375 measurements, respectively, utilizing samples from only the first visit of each individual. For participants with more than one visit, laboratory test results from ensuing visits were excluded. However, calculated RIs differed negligibly when results from succeeding visits were included (data not shown). Since food is not fortified with folic acid in Germany, we assume that the children studied had not been exposed to high dietary folic acid levels.
Measurements
Cobalamin and folate were analyzed according to the manufacturer’s protocol for the in vitro quantitative determination of serum folate and cobalamin (Cobas e602, Roche Diagnostics, Mannheim, Germany), with measuring ranges of 1.36–45.4 nmol/L and 36.9–1476 pmol/L, respectively. Both assays were restandardised against the reference material WHO IS 03/178 in 2015. The cobalamin assay change was negligible and raw data were therefore not recalculated. However, the restandardisation of the Roche Elecsys Folate III assay yielded lower results, particularly for the lowest levels. Thus, all raw data on folate were recalculated in accordance with the equation new = 1.14 × old-1.97 [Citation16] after adjustment of ng/mL to nmol/L.
Statistical analysis
RI calculations were performed using Microsoft Excel, MedCalc® (Ostend, Belgium) and non-parametric 2.5 and 97.5 percentiles generated by the RefVal 4.0 software that is endorsed by the International Federation of Clinical Chemistry and Laboratory Medicine (IFCC) recommendations [Citation17]. Partitioning used the theory outlined by Lahti et al. [Citation18]. In a first step, ages that could potentially motivate partitioning were identified by ‘qualified guessing’ based on graphic visualization of raw data (), and the continuous RIs previously published [Citation15], as well as comparisons with other previous publications [Citation8,Citation9,Citation19–22]. In a second step, these age groups were evaluated for potential gender partitioning. The statistical outcome of gender portioning according to the Lahti principle is presented in the column ‘Suggested’ of , where raw data figures are also rounded to two significant figures. In addition, the table shows all gender-specific RIs, including for age groups where gender partitioning was not justified. Outliers identified by the RefVal software were eliminated before final calculations. This applied to only one entry, a male in the 1–2.99 years age group for cobalamin.
Figure 1. (A, B) Scatter plots showing trends for individual gender separated results for folate (A) and cobalamin (B) with age.
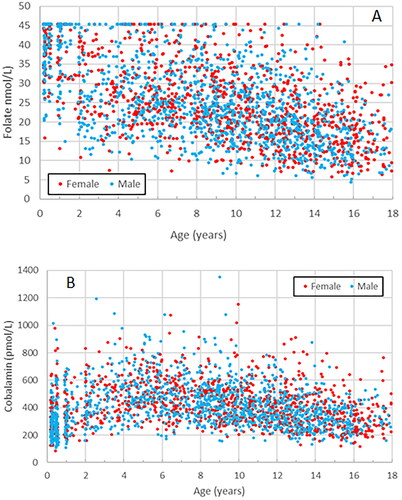
Table 1. Age- and gender specific pediatric reference interval limits for serum folate (nmol/L).
Results
Folate
Folate levels displayed a continuous decline between 2 months and 18 years of age, which, however, was steeper in the age group 2 months to 1.99 years. Particularly among the youngest children, a substantial portion had measurements over the upper limit of the measuring range, 45.4 nmol/L. Since such samples were not rerun after dilution, the upper limit of the RI is uncertain for this age group. From a clinical point of view, however, the upper limit is of minor importance for folate. In the whole cohort, 212 out of 2357 (9.0%) participants had readings over 45.4 nmol/L. In contrast, 61% of participants in the CALIPER study had readings exceeding 45.4 nmol/L using the same Roche method, which reflects the effect of folic acid fortification of food in Canada [Citation20].
Gender separation was not justified for folate. shows all raw data for genders separately as well as merged. The right-hand column ‘Suggested’ also shows upper and lower RI limits with numbers rounded off to two significant figures, and information on whether gender partitioning was justified or not.
Cobalamin
Cobalamin was partitioned into five discrete age groups, . Both lower and upper RI limits displayed a peak at about four to five years of age. Three age groups were also gender partitioned. Particularly among females in the oldest group, 12–17.99 years of age, the upper RI limit differed from that of males.
Table 2. Age- and gender specific pediatric reference interval limits for cobalamin (pmol/L).
Discussion
Several previous studies have shown that the restandardisation of the Roche folate assay in 2016, with traceability to the WHO international standard 03/178, resulted in lower test results, particularly at the lower reference limit [Citation23–26]. However, the new RIs generated in those studies were only applicable to adults. The present study provides RIs applicable to all age groups of children from 2 months to 18 years of age. For use as RIs, it is important that intervals cover the entire pediatric age range since age gaps lacking RIs create problems when evaluating patient test results.
The present finding of a steeper decline of folate levels between 2 months to 1.99 years of age could in part be attributable to the weaning from folate-rich breast milk [Citation15]. A similar pattern was also observed in some previous European studies [Citation27,Citation28] as well as some of those derived from the CALIPER project, which has been presented for the full pediatric and adolescent age ranges on different platforms from major manufacturers Abbott [Citation29], Beckman [Citation30], Roche [Citation20], and Siemens [Citation31]. However, whereas in some of the CALIPER studies [Citation20,Citation29,Citation31], folate levels appeared to increase again in early childhood, the levels in the present study continued to decline throughout childhood. This difference is likely due to the folic acid fortification of food in Canada, while such fortification is not mandated in Germany or other EU countries. Largely for the same reason, the percentage of children with folate readings above the upper measurement range of 45 nmol/L was substantially lower in the present study compared to finding in the CALIPER cohort using the Roche method. Moreover, the clinically important lower limits are considerably lower in the present study compared to all four CALIPER studies, confirming the need to establish RIs on a population without folic acid fortification to be applicable in countries of the EU or other countries where such fortification is not mandated. Several previous studies on European children, however, either included only younger [Citation21] or older children [Citation8] or utilized other assays than the presently used modern Roche assay [Citation8,Citation21,Citation27,Citation28], making direct comparisons uncertain. Using the Roche assay, the present study complements the previously presented continuous RIs [Citation15] with age- and gender-separated discrete RIs.
For cobalamin, the present finding of a physiological trend with low levels the first years, a peak at 4–5 years followed by slowly declining levels up to 18 years, could also be seen in a study with continuous RIs in Dutch children [Citation22]. The CALIPER data [Citation20] are similar to the RIs of the present study for the lower limit but show slightly higher figures for the upper limit. One reason for these differences could be the partitioning in age groups, where the present study used five age groups and CALIPER three. However, a Danish study on healthy children also showed slightly higher upper limits, but the lower RI limits were comparable to the present data [Citation9]. Comparisons to the Danish data are, however, complicated by the fact that these were derived using a different method, namely Siemens Advia.
A strength of the present study is the establishment of RIs for serum folate and cobalamin covering all ages of children from 2 months up to 18 years using principles of the CLSI guidelines. Moreover, disregarding any a posteriori studies based on extraction of patient test results from laboratory information systems, few, if any, previous studies provided practically applicable RIs for serum folate and cobalamin derived from such a large population of generally healthy children without exposure to folic acid fortification.
A limitation of the study is that many of the youngest children did have serum folate measurements above the measuring limit of the method, and the samples were not diluted. Thus, the true upper limit for folate is uncertain in the age group of 0.2 to 1.99 years, although the upper limit for folate is of minor clinical importance [Citation32]. Also, the present results are primarily applicable to Roche instruments.
Conclusion
In conclusion, to fit current laboratory information systems, we have generated discrete age- and gender-specific RIs for children aged 0.2–17.99 years for serum folate and cobalamin, and for folate using data applicable to the restandardised Roche Folate III assay. Laboratories in countries where folic acid fortification of food is not mandated should consider using serum folate RIs derived from a population without exposure to such fortification.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Ozarda Y, Sikaris K, Streichert T, et al. Distinguishing reference intervals and clinical decision limits - a review by the IFCC committee on reference intervals and decision limits. Crit Rev Clin Lab Sci. 2018;55(6):420–431. doi:10.1080/10408363.2018.1482256.
- Ceriotti F. Establishing pediatric reference intervals: a challenging task. Clin Chem. 2012;58(5):808–810. doi:10.1373/clinchem.2012.183483.
- CLSI. Defining, establishing, and verifying reference intervals in the clinical laboratory. 3rd ed. CLSI EPC28-A3c. Wayne (PA). Clin Lab Stand Inst; 2010.
- Ammer T, Schützenmeister A, Prokosch HU, et al. A pipeline for the fully automated estimation of continuous reference intervals using real-world data. Sci Rep. 2023;13(1):13440. doi:10.1038/s41598-023-40561-3.
- Zierk J, Hirschmann J, Toddenroth D, et al. Next-generation reference intervals for pediatric hematology. Clin Chem Lab Med. 2019;57(10):1595–1607. doi:10.1515/cclm-2018-1236.
- Higgins V, Adeli K. Advances in pediatric reference intervals: from discrete to continuous. J Lab Precis Med. 2018;3:3–3. doi:10.21037/jlpm.2018.01.02.
- de Benoist B. Conclusions of a WHO technical consultation on folate and vitamin B12 deficiencies. Food Nutr Bull. 2008;29: S238–S244. doi:10.1177/15648265080292S129.
- González-Gross M, Benser J, Breidenassel C, et al. Gender and age influence blood folate, vitamin B12, vitamin B6, and homocysteine levels in European adolescents: the Helena study. Nutr Res. 2012;32(11):817–826. doi:10.1016/j.nutres.2012.09.016.
- Abildgaard A, Knudsen CS, Hoejskov CS, et al. Reference intervals for plasma vitamin B12 and plasma/serum methylmalonic acid in Danish children, adults and elderly. Clin Chim Acta. 2022;525:62–68. doi:10.1016/j.cca.2021.12.015.
- Pfeiffer CM, Sternberg MR, Fazili Z, et al. Unmetabolized folic acid is detected in nearly all serum samples from US children, adolescents, and adults. J Nutr. 2015;145(3):520–531. doi:10.3945/jn.114.201210.
- Oakley GP, Tulchinsky TH. Folic acid and vitamin B12 fortification of flour: a global basic food security requirement. Public Health Rev. 2010;32(1):284–295. doi:10.1007/BF03391603.
- Morris JK, Addor M-C, Ballardini E, et al. Prevention of neural tube defects in Europe: a public health failure. Front Pediatr. 2021;9:647038. doi:10.3389/fped.2021.647038.
- Quante M, Hesse M, Döhnert M, et al. The LIFE child study: a life course approach to disease and health. BMC Public Health. 2012;12(1):1021. doi:10.1186/1471-2458-12-1021.
- Poulain T, Baber R, Vogel M, et al. The LIFE child study: a population-based perinatal and pediatric cohort in Germany. Eur J Epidemiol. 2017;32(2):145–158. doi:10.1007/s10654-016-0216-9.
- Kreusler P, Vogel M, Willenberg A, et al. Folate and cobalamin serum levels in healthy children and adolescents and their association with age, sex, BMI and socioeconomic status. Nutrients. 2021;13(2):546. doi:10.3390/nu13020546.
- Woldeyohannes M, Girma M, Petros A, et al. Ethiopia national food and nutrition survey to inform the Ethiopian national food and nutrition strategy: a study protocol. BMJ Open. 2023;13(4):e067641. doi:10.1136/bmjopen-2022-067641.
- Solberg HE. RefVal: a program implementing the recommendations of the international federation of clinical chemistry on the statistical treatment of reference values. Comput Methods Programs Biomed. 1995;48(3):247–256. doi:10.1016/0169-2607(95)01697-x.
- Lahti A, Hyltoft Petersen P, Boyd JC, et al. Objective criteria for partitioning Gaussian-distributed reference values into subgroups. Clin Chem. 2002;48(2):338–352. doi:10.1093/clinchem/48.2.338.
- Evliyaoglu O, van Helden J, Imöhl M, et al. Mining the age-dependent reference intervals of B vitamins from routine laboratory test results. Lab Med. 2019;50(1):54–63. doi:10.1093/labmed/lmy045.
- Bohn MK, Higgins V, Asgari S, et al. Paediatric reference intervals for 17 Roche cobas 8000 e602 immunoassays in the CALIPER cohort of healthy children and adolescents. Clin Chem Lab Med. 2019;57(12):1968–1979. doi:10.1515/cclm-2019-0707.
- Gómez-Bueno S, Vázquez-López MA, García-Escobar I, et al. Status of folate in healthy children in almeria. Eur J Pediatr. 2021;180(6):1825–1832. doi:10.1007/s00431-020-03902-2.
- Heiner-Fokkema MR, Riphagen IJ, Wiersema NS, et al. Age dependency of plasma vitamin B12 status markers in Dutch children and adolescents. Pediatr Res. 2021;90(5):1058–1064. doi:10.1038/s41390-021-01372-2.
- Ferraro S, Panzeri A, Borille S, et al. Estimation of the reference interval for serum folate measured with assays traceable to the WHO international standard. Clin Chem Lab Med. 2017;55:e195–e196.
- Hepburn S, Likhari T, Twomey PJ. Roche serum folate assay restandardization: an estimate of the new reference interval. Ann Clin Biochem. 2019;56(1):183–184. doi:10.1177/0004563218793159.
- Solé-Enrech G, San-José P, Aliste-Fernández M, et al. Vitamin B12 and folate levels in a healthy population: establishing reference intervals. Clin Chem Lab Med. 2019;57(8):e173–e175. doi:10.1515/cclm-2018-1080.
- Cluitmans JCA, van den Ouweland JMW. Reference values of a new serum folate assay traceable to the WHO international standard. Clin Chem Lab Med. 2019;57(8):e176–e178. doi:10.1515/cclm-2018-1229.
- Hay G, Johnston C, Whitelaw A, et al. Folate and cobalamin status in relation to breastfeeding and weaning in healthy infants. Am J Clin Nutr. 2008;88(1):105–114. doi:10.1093/ajcn/88.1.105.
- Monsen AL, Refsum H, Markestad T, et al. Cobalamin status and its biochemical markers methylmalonic acid and homocysteine in different age groups from 4 days to 19 years. Clin Chem. 2003;49(12):2067–2075. doi:10.1373/clinchem.2003.019869.
- Bailey D, Colantonio D, Kyriakopoulou L, et al. Marked biological variance in endocrine and biochemical markers in childhood: establishment of pediatric reference intervals using healthy community children from the CALIPER cohort. Clin Chem. 2013;59(9):1393–1405. doi:10.1373/clinchem.2013.204222.
- Karbasy K, Lin DC, Stoianov A, et al. Pediatric reference value distributions and covariate-stratified reference intervals for 29 endocrine and special chemistry biomarkers on the Beckman coulter immunoassay systems: a CALIPER study of healthy community children. Clin Chem Lab Med. 2016;54:643–657.
- Bohn MK, Horn P, League D, et al. Pediatric reference intervals for endocrine markers and fertility hormones in healthy children and adolescents on the Siemens Healthineers Atellica immunoassay system. Clin Chem Lab Med. 2021;59(8):1421–1430. doi:10.1515/cclm-2021-0050.
- Ferraro S, Panteghini M. Folate and vitamin B12 assays after recalibration to the WHO international standard 03/178: making the interpretation as simple as possible, but not simpler. Clin Chem Lab Med. 2019;57(8):1112–1114. doi:10.1515/cclm-2019-0050.
