Abstract
This study investigated the effects of hexahydrocannabinol (HHC) and other unclassified cannabinoids, which were recently introduced to the recreational drug market, on cannabis drug testing in urine and oral fluid samples. After the appearance of HHC in Sweden in 2022, the number of posts about HHC on an online drug discussion forum increased significantly in the spring of 2023, indicating increased interest and use. In parallel, the frequency of false positive screening tests for tetrahydrocannabinol (THC) in oral fluid, and for its carboxy metabolite (THC-COOH) in urine, rose from <2% to >10%. This suggested that HHC cross-reacted with the antibodies in the immunoassay screening, which was confirmed in spiking experiments with HHC, HHC-COOH, HHC acetate (HHC-O), hexahydrocannabihexol (HHC-H), hexahydrocannabiphorol (HHC-P), and THC-P. When HHC and HHC-P were classified as narcotics in Sweden on 11 July 2023, they disappeared from the online and street shops market and were replaced by other unregulated variants (e.g. HHC-O and THC-P). In urine samples submitted for routine cannabis drug testing, HHC-COOH concentrations up to 205 (mean 60, median 27) µg/L were observed. To conclude, cannabis drug testing cannot rely on results from immunoassay screening, as it cannot distinguish between different tetra- and hexahydrocannabinols, some being classified but others unregulated. The current trend for increased use of unregulated cannabinols will likely increase the proportion of positive cannabis screening results that need to be confirmed with mass spectrometric methods. However, the observed cross-reactivity also means a way to pick up use of new cannabinoids that otherwise risk going undetected.
Introduction
Laboratory testing for alcohol and drugs fulfills important functions for objective control of harmful or illegal substance use, and for follow-up of treatment efforts, in healthcare and workplace testing. For many decades, the use of illegal drugs mainly involved a few substances, typically amphetamines, cannabis, cocaine and heroin, and drug testing was consequently mainly focused on detecting these. Over the past ∼15 years, however, the supply of novel recreational drugs has increased sharply, including hundreds of new psychoactive substances (NPS; also called designer or internet drugs) and psychoactive medications (e.g. benzodiazepines, synthetic opioids and ADHD medications) [Citation1]. The conventional drugs still dominate the market, but drug testing has become much more extensive and complicated.
The NPS are structural variants of substances belonging to the traditional classes of psychoactives, such as stimulants, hallucinogens, dissociatives, benzodiazepines and cannabinoids [Citation1,Citation2], but they have been chemically modified to circumvent drug legislations, allowing for open sale, and avoid detection. At the same time, this has sometimes led to unpredicted side effects and increased health risks. For example, contrasting tetrahydrocannabinol (THC), the principal psychoactive constituent in the cannabis plant which is a partial agonist of the endocannabinoid type 1 receptor (CB1), the new synthetic cannabinoids are often full CB1 receptor agonists, explaining their generally higher potency and more serious toxidrome including fatalities, compared with smoking herbal cannabis (marijuana) [Citation3–5].
Cannabis usually refers to delta-9-THC which is the most abundant of seven THC double bond isomers in the cannabis plant. In recent years, however, other isomers, especially delta-8-THC, have emerged as alternatives to delta-9-THC [Citation6]. Delta-8-THC is a natural minor constituent of the plant, but it may also be synthesized from cannabidiol (CBD). A main reason for the increased popularity and rapid spread of delta-8-THC was its sometimes different or unclear legal status compared with delta-9-THC. In Sweden, which has ratified the 1971 UN Convention on Psychotropic Substances that controls the stereochemical variants of THC [Citation7], all double bond isomers are scheduled and therefore delta-8-THC soon disappeared from the market [Citation6].
More recently, hydrogenated THC derivatives, i.e. hexahydrocannabinol (HHC) and analogs, with cannabimimetic properties have emerged as recreational drugs [Citation8–11], sprayed on hemp materials, as liquids intended for e-cigarettes or vaping, and as gummy edibles [Citation8,Citation12,Citation13], and been widely discussed in drug chat forums [Citation14]. HHC may not occur naturally or in only trace amounts in the cannabis plant [Citation15,Citation16], but it too can be synthesized from CBD by chemical transformation and is hence called semi-synthetic. CBD is readily available at high concentration in “industrial” hemp, i.e. cannabis strains containing <0.2% (EU) or <0.3% (USA/Canada) delta-9-THC. The hexahydrocannabinols are not covered in the UN Convention on Psychotropic Substances [Citation7] but have to be evaluated separately for drug classification.
Due to the close structural similarity to THC (), HHC has been demonstrated to cross-react in immunoassay screening for delta-9-THC in oral fluid samples [Citation17,Citation18], and this was also expected to occur with immunoassays for the carboxylic acid metabolites (-COOH) [Citation8] which are analytical targets in urine drug screening. This may result in an increased incidence of false-positive THC and THC-COOH results in cannabis drug screening, which is used very often. However, due to the differences in structure and molecular mass compared to THC, HHC and analogs () can be separated and confirmed analytically using mass spectrometric (MS) analysis.
Figure 1. Chemical structures of delta-9-tetrahydrocannabinol (delta-9-THC), hexahydrocannabinol (HHC), its carboxylic acid metabolite (HHC-COOH), and the analogs hexahydrocannabiphorol (HHC-P), HHC acetate (HHC-O) and hexahydrocannabihexol (HHC-H).
This study focuses on the appearance of HHC and some related substances that have recently emerged as new recreational drugs in Sweden, and their implications for routine drug testing of cannabis use in urine and oral fluid samples, based on results from the Karolinska University Laboratory (Stockholm, Sweden).
Methods
Clinical samples and chemicals
The laboratory investigations were based on results with deidentified patient specimens submitted for routine drug testing at the Department of Clinical Pharmacology, Karolinska University Laboratory (Stockholm, Sweden). The selected oral fluid samples, obtained with the Quantisal collection device (Immunalysis Corp., Maam, The Netherlands), had tested positive for THC, and the urine samples positive for THC-COOH, in the immunoassay screening.
Reference materials for 9(R)-HHC (HHC), 11-nor-9(R)-carboxy-HHC (HHC-COOH), 9(R)-HHC acetate (HHC-O), 9(R)-hexahydrocannabihexol (HHC-H), 9(R)-hexahydrocannabiphorol (HHC-P) and delta-9-tetrahydrocannabiphorol (THC-P) () were obtained from Cayman Chemical Co. (Ann Arbor, MI, USA), and delta-9-THC, delta-9-THC-COOH and the corresponding deuterated internal standards from Cerilliant Co. (Round Rock, TX, USA). The materials were obtained as crystalline solid or solutions in acetonitrile or methanol, and dilutions were made in the same organic solvents. All other chemicals were of analytical or HPLC grade.
Routine drug testing for cannabis use
Immunoassay screening for THC-COOH in urine was performed with an accredited method, using the CEDIA Multi-Level THC Assay (Thermo Fisher Scientific, Fremont, CA, USA) according to the standard procedure [Citation19] on an AU680 analyzer (Beckman Coulter, Brea, CA, USA) and the nationally harmonized cut-off level of 25 µg/L [Citation20]. Screening for THC in oral fluid was performed with an accredited method, using the THC Oral Fluid HEIA (Immunalysis Corp.) according to the standard procedure on a DxC 700 AU analyzer (Beckman Coulter) and a cut-off level of 4.0 µg/L neat oral fluid.
In routine use, oral fluid and urine samples screening positive for THC and THC-COOH, respectively, are subjected to confirmatory liquid chromatography–tandem MS (LC–MS/MS) analysis, essentially as described elsewhere [Citation6]. THC-COOH is quantified in the free form following alkaline hydrolysis of THC-COOH-glucuronide in 1.3 mol/L ammonia for 30 min at 40 °C [Citation21]. The cut-off level for THC-COOH in urine was 10 µg/L, according to a national harmonization [Citation20], and 4 µg/L for THC in neat oral fluid (i.e. corrected for the 4-fold dilution of oral fluid with buffer in the Quantisal device).
LC–MS/MS analysis of HHC and HHC-COOH
For confirmation and quantification of HHC in oral fluid and HHC-COOH in urine, they were included in the routine LC–MS/MS detection methods for THC (Dionex Ultimate 3000 UHPLC coupled to a TSQ Quantiva MS; Thermo Fisher Scientific) and THC-COOH (AQUITY UPLC coupled to a Xevo TQ-S micro MS; Waters, Milford, MA, USA), respectively.
Chromatographic separation of HHC in oral fluid was achieved on a Hypersil GOLD VANQUISH column (2.1 × 100 mm, particle size 1.9 μm; Thermo Fisher Scientific), using gradient elution (mobile phase A: 1 mol/L ammonium formate and 0.5% formic acid; mobile phase B: methanol) at 0.6 mL/min. The MS was operated in positive mode and the ion transitions monitored for HHC were m/z 317.2 > 123.1 (quantifier) and m/z 317.2 > 193.2 (qualifier), and m/z 318.2 > 196.2 for THC-D3 (IS).
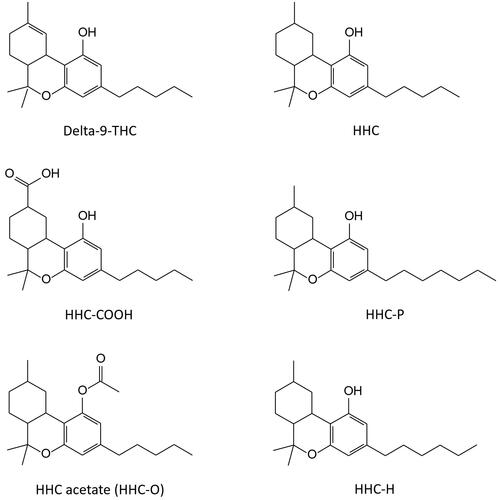
Chromatographic separation of HHC-COOH in urine samples was achieved on an AQUITY UPLC BEH C18 column (1.0 × 100 mm, particle size 1.7 μm; Waters), using gradient elution (mobile phase A: 0.1% formic acid; mobile phase B: acetonitrile) at 0.2 mL/min (). The MS was operated in negative mode and the ion transitions monitored for HHC-COOH were m/z 345.2 > 301.3 (quantifier) and m/z 317.2 > 191.2 (qualifier), and m/z 352.4 > 308.4 for THC-COOH-D9 (IS).
Figure 2. Chromatograms showing a patient urine sample testing positive for the carboxylic acid metabolite of delta-9-tetrahydrocannabinol (THC-COOH), a spiked urine standard for hexahydrocannabinol carboxylic acid (HHC-COOH), and a patient urine sample testing positive for HHC-COOH (22 µg/L).
Acceptance criteria for a positive identification are Gaussian peak shapes of analyte and IS, a relative retention time (RRT) versus calibrator within ± 0.5%, and ion ratios within ± 20%. The cut-off levels applied for HHC in oral fluid and HHC-COOH in urine were the same as for the tetrahydrocannabinols (i.e. 4 and 10 µg/L, respectively).
Results
Open online discussions about HHC on a drug chat forum
In October 2021, a discussion thread about HHC was started on a Swedish internet chat forum for psychoactive substances [Citation14]. Initial posts concerned the potency and legality of HHC, which was described as a weaker, legal alternative to THC, and from where it was obtained. During 2022, the number of posts remained at a low level () and mainly focused on personal experiences of the substance, and whether it would show up in a drug test. In November, it was also noted that HHC had been put under investigation for classification as a narcotic substance in Sweden by the Public Health Agency.
Figure 3. Monthly number of posts on hexahydrocannabinol (HHC) over two years until October 2023 on a Swedish open online discussion forum for psychoactive substances [Citation14]. After 11 July 2023 (broken line), when HHC was classified as a narcotic substance, the number of posts on the HHC thread decreased and eventually ended. Instead, new threads were started about the unregulated analogues HHC acetate (HHC-O) and tetrahydrocannabiphorol (THC-P).
![Figure 3. Monthly number of posts on hexahydrocannabinol (HHC) over two years until October 2023 on a Swedish open online discussion forum for psychoactive substances [Citation14]. After 11 July 2023 (broken line), when HHC was classified as a narcotic substance, the number of posts on the HHC thread decreased and eventually ended. Instead, new threads were started about the unregulated analogues HHC acetate (HHC-O) and tetrahydrocannabiphorol (THC-P).](/cms/asset/a4905a16-e082-4e83-aafc-01ca6753eaef/iclb_a_2340039_f0003_c.jpg)
In the spring of 2023, the number of posts about HHC increased sharply () and mainly included reviews of Swedish online drug dealers and products intended for smoking and vaping, as well as further discussions about the risk of testing positive in a cannabis drug test. However, there were also some posts about tolerance and negative effects. It was further noted that the Swedish customs had seized HHC products ordered from abroad, which is possible since 2011 for unregulated substances that are under investigation for classification. During the spring of 2023, HHC became available to buy also over the counter in street shops, after which its popularity greatly increased.
On 17 May 2023, the Swedish Public Health Agency recommended that HHC and the analogue HHC-P, having a longer carbon tail (), should be classified as narcotics in Sweden, and this became effective on 11 July through a government decision. In June, the upcoming classification was much debated, and the online vendors began selling out HHC. Other posts concerned which substances would follow HHC, mainly focusing on HHC acetate (also called HHC-O) and THC-P (). Eventually, separate threads about these substances were started and the one about HHC stopped being used ().
Analytical results with HHC in drug testing for cannabis use
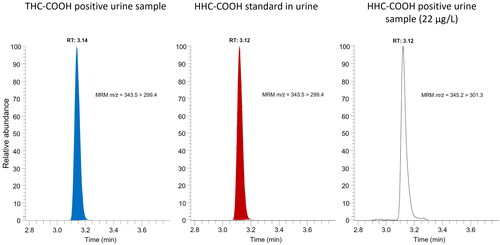
After observations in the fall of 2022 that HHC was sold openly on Swedish websites for unclassified recreational drugs, reference materials for HHC and its tentative urinary carboxy metabolite (HHC-COOH) () were purchased, spiked in blank samples, and tested for interferences in the routine immunoassay screening methods for cannabis use in urine (i.e. targeting THC-COOH) and oral fluid (i.e. THC) in December 2022. As shown in , HHC and HHC-COOH were found to give similar test responses as THC and THC-COOH, respectively.
Figure 4. Test responses for A) delta-9-tetrahydrocannabinol (THC) and hexahydrocannabinol (HHC) and B) their carboxylic acid metabolites, THC-COOH and HHC-COOH, respectively, in immunoassay screening for cannabis use in oral fluid (OF) (THC Oral Fluid HEIA, Immunalysis Corp.) and urine (CEDIA Multi-Level THC Assay, Thermo Fisher Scientific), respectively. The substances were diluted in acetonitrile or methanol. Blanks with solvent only generated negative results.
The LC-MS/MS methods used for routine identification and quantification of THC in oral fluid and THC-COOH in urine, respectively, also proved useful for inclusion of HHC and HHC-COOH ().
In a subset of 21 routine urine samples testing positive for HHC-COOH, the concentrations ranged 10–205 (mean 60, median 27) µg/L.
Frequency of false positive test results in cannabis drug screening
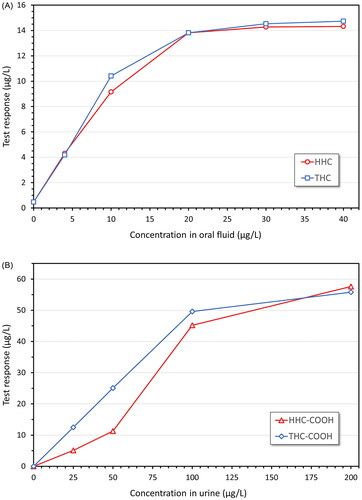
Since HHC was demonstrated to show high cross-reactivity in the cannabis screening tests (), and the interest in HHC as a recreational drug was indicated to increase much in Sweden in the spring of 2023 () when HHC was still unregulated, this was expected to cause an increased incidence of false positive screening results in the routine drug testing for cannabis use.
This was investigated by comparing the frequency of positive screening results for THC-COOH in urine with the corresponding results of the confirmatory analysis, among samples sent to the Karolinska University Laboratory for routine drug testing. As shown in , the relative proportion of false positive THC results was typically <2% until the spring of 2023 when it increased sharply to >10% in June. After a small drop in July, which coincided with the classification of HHC as a narcotic substance, the frequency of false positive screening results for cannabis use continued to increase ().
Figure 5. Frequency of urine samples testing preliminary positive for cannabis use (THC-COOH) in immunoassay screening, but confirmed negative by LC–MS/MS, among samples submitted for routine drug testing at the Karolinska University Laboratory (Stockholm, Sweden) in 2021–2023. On 11 July 2023, HHC was classified as a narcotic substance (broken line). The monthly number of samples that are screened for cannabis use is ∼10 000. Results are shown for urine samples confirmed to contain <5 µg/L THC-COOH, to exclude samples just below the 10 µg/L routine cut-off.
Screening results with other novel cannabinols
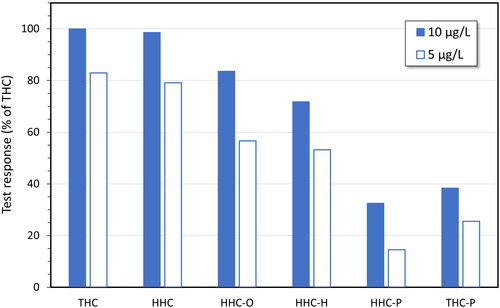
When the Swedish classification of HHC and HHC-P as narcotics became effective on 11 July 2023, the online drug vendors switched to selling other unclassified cannabinols, the most common being HHC acetate (also called HHC-O), HHC-H, and THC-P (). Since the frequency of false positive test results in the routine cannabis screening did not decrease but rather continued to rise (), it was investigated whether also the newly marketed cannabinols cross-reacted in the cannabis screening assays. This study was performed with the parent compounds spiked in oral fluid samples collected with the Quantisal device, as reference materials of the corresponding carboxylic acid metabolites were not yet commercially available. The results demonstrated that these substances also cross-reacted in the screening assay for THC in oral fluid, albeit at slightly lower levels than observed for HHC ().
Figure 6. Test responses for hexahydrocannabinol (HHC), HHC acetate (HHC-O), hexahydrocannabihexol (HHC-H), hexahydrocannabiphorol (HHC-P) and delta-9-tetrahydrocannabiphorol (THC-P), in comparison with delta-9-tetrahydrocannabinol (THC), in immunoassay screening for cannabis use in oral fluid (OF) (THC Oral Fluid HEIA, Immunalysis Corp.). The substances were diluted in acetonitrile or methanol and spiked in three different blank oral fluid samples collected with the Quantisal device (Immunalysis Corp.). Results are the mean values. Blanks with solvent only generated negative results.
Discussion
The results of this study highlighted serious practical implications for the routine drug testing of cannabis use, after a new group of unregulated cannabinoids, the hexahydrocannabinols which are structurally closely related to the principal psychoactive constituent THC and have similar cannabimimetic effects, were recently introduced as recreational drugs. Although HHC has been known for a long time [Citation8,Citation9,Citation22,Citation23], it has not previously been associated with drug use, while cannabis, i.e. plant materials containing delta-9-THC, is one of the most common drugs worldwide [Citation1]. Most importantly, and in agreement with previous observations [Citation17,Citation18,Citation24], high cross-reactivity was demonstrated for HHC and its carboxylic acid metabolite HHC-COOH in laboratory-based immunoassays for cannabis drug testing in oral fluid and urine samples, respectively.
The present study extended the knowledge about the analysis of new cannabinols by also demonstrating a high but variable cross-reactivity of some HHC analogs, i.e. HHC-O, HHC-H and HHC-P, as well as THC-P, in the oral fluid THC screening. However, there appears to be differences in test response between laboratory-based assays and point-of-care test strips [Citation24,Citation25]. Moreover, while HHC-H, HHC-P and THC-P differ from HHC and THC in the length of the carbon tail (), a possible bioanalytical problem with HHC-O is that it may undergo hydrolysis to the parent compound in the body which would complicate the interpretation of a drug test result [Citation26]. This is further supported by observations of a delayed bioactivity of THC acetates compared with the parent compounds, and less cross-reactivity in immunoassays [Citation8], although the present study showed no major difference between HHC and HHC-O (). As of January 16, 2024, however, HHC-O, as well as THC-O and THC-P (both the delta-9 and delta-8 variants), are classified as narcotics in Sweden.
The cannabinol concentrations tested for cross-reactivity in the screening assays were considered relevant, by referring to those observed for HHC-COOH in a subset of patient urine samples at the Karolinska University Laboratory (mean 60, median 27, and maximum 205 µg/L). For comparison, the yearly mean (median) THC-COOH concentrations in drug positive samples were 383 (90) µg/L (range = 10–28 300 µg/L). However, it should be noted that the immunoassays give a maximum test response already at relatively low substance concentrations (), e.g. typically around 100 µg/L for THC-COOH in urine samples and ∼20 µg/L for THC in oral fluid.
The present results supported that the parent compounds are suitable targets for drug testing of hexahydrocannabinols in oral fluid, while their carboxylic acid metabolites are useful for urine testing, in agreement with THC [Citation27]. Other studies have suggested the 11-OH-HHC metabolite [Citation24,Citation28] to be an alternative, major urinary target analyte [Citation29,Citation30], but it was not included here. It should also be noted that HHC and analogs occur as the 9R and 9S epimers [Citation24,Citation31], but no attempt was made to separate these in this study as it primarily focused on the impact on routine cannabis drug testing.
In Sweden, the classification of HHC and HHC-P as narcotic drugs in the summer of 2023 meant that the open sales, which took place both online and in street shops, shifted to other yet unclassified structural variants and trade now continues as before. This is consistent with the flexible online drug market and constantly changing drug trends noticed for hundreds of NPS over the past ∼15 years [Citation1,Citation2]. Accordingly, the demonstrated increased occurrence of positive screening results in routine drug testing for cannabis use, presently (early 2024) making up >12% of all positive findings at the Karolinska University Laboratory in Sweden, increases the burden on the more laborious confirmatory analysis.
Conclusions
The results of this study demonstrated that HHC and some structurally related substances, that were recently introduced in Sweden and have become popular as unclassified recreational drugs, cross-react in immunoassay screening tests for cannabis use, i.e. assays targeting THC in oral fluid and THC-COOH in urine samples. An apparent increased use of HHC in the spring of 2023, as reflected in a sharp rise in the number of HHC-related posts on an open online drug discussion forum, coincided in time with an increased frequency of false positive screening results for cannabis use in the routine drug testing, from typically <2% to >10%. After HHC and HHC-P were classified as narcotic substances in Sweden on 11 July 2023, other unclassified cannabinols were introduced, which in turn have been classified.
The observed cross-reactivity of HHC and analogs in the screening assays for cannabis is expected to have major practical implications for routine drug testing, because a positive screening result may be due to use of either classified or unclassified substances and thus cannot be relied upon alone. Accordingly, an increased use of unclassified cannabinoids will mean that more preliminary positive samples need to be confirmed with more laborious and expensive LC–MS-based methods. At the same time, the cross-reactivity means an analytical advantage by increasing the possibility of identifying use of new cannabinoids that would otherwise risk go undetected, since samples that screen negative are rarely subject to further investigation.
To conclude, cannabis drug testing cannot rely on test result from immunoassay screening, as these methods cannot distinguish between different subtypes of tetra- and hexahydrocannabinols, some of which are illegal but others unregulated. The current trend for an increasing use of novel unregulated cannabinoids means that the proportion of preliminary positive screening results that need to be confirmed is likely to increase. This also highlights the need for laboratories to keep up with the rapid changes in the recreational drugs market and regularly update their confirmation methods to cover the panel of recreational drugs currently on the market, otherwise the final test result will more often be false negative.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- EMCDDA. European Drug Report 2023: Trends and Developments. 2023. Available from: https://www.emcdda.europa.eu/publications/european-drug-report/2023_en.
- Helander A, Bäckberg M, Beck O. Drug trends and harm related to new psychoactive substances (NPS) in Sweden from 2010 to 2016: experiences from the STRIDA project. PLoS ONE. 2020;15(4):e0232038. doi: 10.1371/journal.pone.0232038.
- Lea Houston M, Morgan J, Kelso C. Narrative review of the pharmacodynamics, pharmacokinetics, and toxicities of illicit synthetic cannabinoid receptor agonists. Mini Rev Med Chem. 2024;24(1):92–109. doi: 10.2174/1389557523666230515163107.
- de Oliveira MC, Vides MC, Lassi DLS, et al. Toxicity of synthetic cannabinoids in K2/spice: a systematic review. Brain Sci. 2023;13(7):990. doi: 10.3390/brainsci13070990.
- Yoganathan P, Claridge H, Chester L, et al. Synthetic cannabinoid-related deaths in England, 2012-2019. Cannabis Cannabinoid Res. 2022;7(4):516–525. doi: 10.1089/can.2020.0161.
- Helander A, Johansson M, Andersson A, et al. Analytical and medico-legal problems linked to the presence of Delta-8-tetrahydrocannabinol (Delta-8-THC): results from urine drug testing in Sweden. Drug Test Anal. 2022;14(2):371–376. doi: 10.1002/dta.3190.
- United Nations. Convention on psychotropic substances; 1971. Available from: https://www.unodc.org/pdf/convention_1971_en.pdf.
- Ujváry I. Hexahydrocannabinol and closely related semi-synthetic cannabinoids: a comprehensive review. Drug Test Anal. 2023;16(2):127–161. doi: 10.1002/dta.3519.
- Adams R, Pease DC, Cain CK, et al. Structure of cannabidiol. VI. Isomerization of cannabidiol to tetrahydrocannabinol, a physiologically active product. Conversion of cannabidiol to cannabinol. J Am Chem Soc. 1940;62(9):2402–2405. doi: 10.1021/ja01866a040.
- Docampo-Palacios ML, Ramirez GA, Tesfatsion TT, et al. Saturated cannabinoids: update on synthesis strategies and biological studies of these emerging cannabinoid analogs. Molecules. 2023;28(17):6434. doi: 10.3390/molecules28176434.
- Graziano S, Varì MR, Pichini S, et al. Hexahydrocannabinol pharmacology, toxicology, and analysis: the first evidence for a recent new psychoactive substance. Curr Neuropharmacol. 2023;21(12):2424–2430. doi: 10.2174/1570159X21666230623104624.
- Casati S, Rota P, Bergamaschi RF, et al. Hexahydrocannabinol on the light cannabis market: the latest "new" entry. Cannabis Cannabinoid Res. 2022;9(2):622–628. doi: 10.1089/can.2022.0253.
- Tanaka R, Kikura-Hanajiri R. Identification of hexahydrocannabinol (HHC), dihydro-iso-tetrahydrocannabinol (dihydro-iso-THC) and hexahydrocannabiphorol (HHCP) in electronic cigarette cartridge products. Forensic Toxicol. 2023;42(1):71–81. doi: 10.1007/s11419-023-00667-9.
- Har någon erfarenhet av HHC/hexahydrocannabinol. Available from: https://www.flashback.org/p86324323#p86324323.
- Basas-Jaumandreu J, de Las Heras FXC. GC-MS metabolite profile and identification of unusual homologous cannabinoids in high potency cannabis sativa. Planta Med. 2020;86(5):338–347. doi: 10.1055/a-1110-1045.
- Hanuš LO, Meyer SM, Muñoz E, et al. Phytocannabinoids: a unified critical inventory. Nat Prod Rep. 2016;33(12):1357–1392. doi: 10.1039/c6np00074f.
- Williams PL, Moffat AC, King LJ. Combined high-performance liquid chromatography and radioimmunoassay method for the analysis of Delta 9-tetrahydrocannabinol metabolites in human urine. J Chromatogr. 1979;186:595–603. doi: 10.1016/s0021-9673(00)95280-4.
- Jones AB, ElSohly HN, ElSohly MA. Analysis of the major metabolite of Delta 9-tetrahydrocannabinol in urine. V. Cross-reactivity of selected compounds in a radioimmunoassay. J Anal Toxicol. 1984;8(6):252–254. doi: 10.1093/jat/8.6.252.
- Thermo Fisher Scientific. CEDIA Multi Level THC Assay. Available from: https://www.thermofisher.com/document-connect/document-connect.html?url=https%3A//assets.thermofisher.com/TFS-Assets/CDD/Package-Inserts/10006559-CEDIA-Multi-Level-THC-EN.pdf.
- Hansson T, Helander A, Beck O, et al. Uniform analyzes of drugs in urine needed for rule of law. Läkartidningen. 2015;112:DLHH.
- Stephanson N, Josefsson M, Kronstrand R, et al. Accurate identification and quantification of 11-nor-Delta(9)-tetrahydrocannabinol-9-carboxylic acid in urine drug testing: evaluation of a direct high efficiency liquid chromatographic-mass spectrometric method. J Chromatogr B Analyt Technol Biomed Life Sci. 2008;871(1):101–108. doi: 10.1016/j.jchromb.2008.06.047.
- Harvey DJ. The mass spectra of the trimethylsilyl derivatives of cis- and trans- hexahydrocannabinol and their hydroxy and acid analogues. Biomed Mass Spectrom. 1981;8(8):366–372. doi: 10.1002/bms.1200080810.
- Harvey DJ, Martin BR, Paton WD. Identification of metabolites of delta1- and delta1(6)-tetrahydrocannabinol containing a reduced double bond. J Pharm Pharmacol. 1977;29(8):495–497. doi: 10.1111/j.2042-7158.1977.tb11376.x.
- Höfert L, Becker S, Dreßler J, et al. Quantification of (9R)- and (9S)-hexahydrocannabinol (HHC) via GC-MS in serum/plasma samples from drivers suspected of cannabis consumption and immunological detection of HHC and related substances in serum, urine, and saliva. Drug Test Anal. 2023. doi: 10.1002/dta.3570.
- Derne AS, Pape E, Jouzeau JY, et al. Immunological detection of hexahydrocannabinol (HHC) in oral fluid. Drug Test Anal. 2023. doi: 10.1002/dta.3595.
- Watanabe K, Matsunaga T, Kimura T, et al. Stereospecific and regioselective hydrolysis of cannabinoid esters by ES46.5K, an esterase from mouse hepatic microsomes, and its differences from carboxylesterases of rabbit and porcine liver. Biol Pharm Bull. 2005;28(9):1743–1747. doi: 10.1248/bpb.28.1743.
- Huestis MA, Henningfield JE, Cone EJ. Blood cannabinoids. I. Absorption of THC and formation of 11-OH-THC and THCCOOH during and after smoking marijuana. J Anal Toxicol. 1992;16(5):276–282. doi: 10.1093/jat/16.5.276.
- Harvey DJ, Brown NK. In vitro metabolism of the equatorial C11-methyl isomer of hexahydrocannabinol in several mammalian species. Drug Metab Dispos. 1991;19(3):714–716.
- Schirmer W, Auwärter V, Kaudewitz J, et al. Identification of human hexahydrocannabinol metabolites in urine. Eur J Mass Spectrom (Chichester). 2023;29(5–6):326–337. doi: 10.1177/14690667231200139.
- Manier SK, Valdiviezo JA, Vollmer AC, et al. Analytical toxicology of the semi-synthetic cannabinoid hexahydrocannabinol studied in human samples, pooled human liver S9 fraction, rat samples, and drug product using HPLC-HRMS/MS. J Anal Toxicol. 2023;47(9):818–825. doi: 10.1093/jat/bkad079.
- Russo F, Vandelli MA, Biagini G, et al. Synthesis and pharmacological activity of the epimers of hexahydrocannabinol (HHC). Sci Rep. 2023;13(1):11061. doi: 10.1038/s41598-023-38188-5.

