Abstract
This study aimed to describe differences in prevalence and the long-term presence of nucleocapsid antibodies (N-antibodies) elicited by SARS-CoV-2 infection in a Swedish blood donor population not subjected to lockdown. We tested 20,651 blood donor samples for nucleocapsid antibodies from the beginning of March 2020 and 27 months onwards using the Roche Elecsys Anti-SARS-CoV-2 assay. The proportion of positive SARS-CoV-2 antibody samples was determined each week. After the exclusions of one-time donors and subjects with incomplete data, 19,726 samples from 4003 donors remained. Differences in antibody prevalences stratified for age, sex, and blood groups (ABO and RhD) were determined, as well as antibody loss and recovery. Lower antibody prevalence was seen for older donors, blood group AB, and RhD-negative subjects. A significant decrease in antibody titer between the first and the second antibody-positive donation was seen for the whole study group, females, older subjects, blood group O, AB, and RhD-positive subjects. The titer waned below the detection limit in 60 (3.0%) of 1983 N-antibody-positive donors, and for 18 of these donors, a second episode with antibodies was detected. We showed that N-antibodies persist for months or years and that surprisingly few antibody-positive donors lost their antibodies. We also conclude that antibody prevalence in a Swedish population never subject to lockdown did not apparently differ from populations that were subject to stricter regulations.
Introduction
Dispersion of the SARS-CoV-2 virus in Sweden was initially observed in March 2020 [Citation1]. As the pandemic developed, authorities took various actions to control the spread of the virus. For example, Sweden was never subject to total lockdown compared to many other countries. Thus, the long-term antibody prevalence in the Swedish population, compared to other populations, is of interest. Studies on blood donors in Stockholm, as well as a national Swedish study, investigated the point prevalence of spike antibodies during a short period [Citation2,Citation3]. Another short-term study explored seropositivity in transport workers in Stockholm using an assay detecting both SARS-CoV-2 spike (S-antibodies) and nucleocapsid antibodies (N-antibodies) [Citation4]. In contrast, our study focused on the long-term seroprevalence of N-antibodies among blood donors in a non-metropolitan Swedish county.
At the time of virus dispersion, antibody testing of blood donors was introduced in Kronoberg, as one of the first counties in Sweden. The information on antibody prevalence in Kronoberg County was used to monitor the pandemic progression and to get an indication of the immunity status of the population. The collected data also guided the national public health authorities regarding prevention strategies such as SARS-CoV-2 testing and vaccination [Citation5,Citation6]. In addition, the use of convalescent plasma was suggested at the beginning of the pandemic when evidence-based treatment was not fully established. The FDA and the European Commission issued emergency use authorizations for convalescent plasma [Citation7,Citation8], and the antibody titer was used to select suitable plasma donors. As COVID-19 treatment strategies developed, the use of convalescent plasma declined, and SARS-CoV-2 antibodies predominantly became a marker of previous infection and an indication of possible immunity [Citation6,Citation9].
Several studies on antibody prevalence have been performed [Citation10–12]. SARS-CoV-2 elicited antibodies can be detected by various assays with different performance characteristics [Citation13,Citation14]. Since a decline in antibody titer might affect the risk of re-infection with SARS-CoV-2, and disease severity, it is interesting to define the persistence of antibodies [Citation15]. Also, blood groups have been suggested as a possible risk factor for developing severe disease. Thus, studying blood donors is of interest, as the blood group is well defined, and comorbidities are rare [Citation16]. Most studies conclude that blood type A predisposes to infection, while type O and RhD-negative blood groups are preventive. Conclusions regarding severe illness and death are more diverging [Citation17–21].
For 27 months, starting in mid-March 2020, we tested all blood donors in Kronoberg County, Sweden, for SARS-CoV-2 antibodies. This study aimed to describe the prevalence and titers of antibodies elicited by a previously unknown virus in a blood donor population not subjected to lockdown. We also aimed to investigate the antibody presence and possible differences between donor subgroups such as age, sex, and blood groups.
Materials and methods
Cohort description
Kronoberg County is situated in Southern Sweden and has approximately 200,000 inhabitants. There are two secondary health care centers, each with a blood donor center.
Serum samples were collected in Vacutainer SST II Advance 3.5 mL tubes, REF 368498 (Becton Dickinson, Franklin Lakes, NJ, USA) at every blood, platelet, and plasma donation from mid-March 2020 through June 2022 (n = 20,651). The samples were collected at the blood donor centers in Kronoberg County, Sweden, after consent to store and use for research. In general, the blood donation interval for men was three months, and four months for women, which is common practice at Swedish blood establishments.
All samples were analyzed with the Elecsys Anti-SARS-CoV-2 assay on the Cobas 8000 e801 multianalyzer (Roche Diagnostics GmbH, Mannheim, Germany), as previously described [Citation13]. This assay detects antibodies (including IgG) binding to the SARS-CoV-2 nucleocapsid (N) protein, thus only detecting antibodies elicited by infection [Citation22]. A previous study concluded that the sensitivity was 98.7% (95% CI 95.3–99.8), and the specificity was 98.7 (95% CI 95.3–99.8%) [Citation13]. According to the manufacturer, the test is qualitative and reports samples as positive if the titer is ≥1.0 arbitrary units. Thus, all samples <1.0 were regarded as negative. Numerical values were recorded for the antibody-positive samples as an approximate measure of antibody titers [Citation14]. The assay was subject to the laboratory quality assurance system, including quality controls and proficiency testing. All results, including antibody status, blood group, sex, birth date, and sample collection date, were retrieved from the ProSang blood donor laboratory system (OMDA AS, Oslo, Norway). One-time donors were excluded from the longitudinal prevalence data. In total, 19,726 samples from 4003 donors were thus included for further analysis. Of the 19,726 samples, 0.9% came from plasma donations and thrombocytaphereses. The blood group frequencies were consistent with the general population in Sweden.
Statistical analysis
The chi-square test was used to define differences between groups in nominal variables, and the Mann–Whitney U-test for continuous variables. The Related-Samples Wilcoxon Signed Rank Test was used to determine titer differences between the first and the second antibody-positive donations, stratified for subgroups. Kruskal–Wallis H-test was used to determine differences between donors at the first antibody-positive donation. Non-parametric methods were used due to highly skewed variables.
A Kaplan–Meier survival plot was constructed to describe antibody presence. An episode was approximated as the time between the first and last antibody-positive donation plus half of the time between the previous and subsequent negative donation. This was to adjust for not knowing the exact time of antibody gain and loss. If the first donation was antibody-positive, that time point was considered the start of the episode.
Differences were regarded as significant when p < 0.05. SPSS version 28 (IBM Corp., Armonk, NY, USA) was used for all calculations.
The study design was approved by the Swedish Ethical Review Authority (Dnr 2021-03377).
Results
Antibody prevalence
The main characteristics of the study population is presented in . The increase in antibody-positive samples over time is presented in , where all donors (including one-time donors) were included. The average number of samples analyzed each week was 171.
Figure 1. The proportion of positive SARS-CoV-2 antibody samples each week among blood donors. Total number of samples n = 20,651, positive number of samples n = 4439.
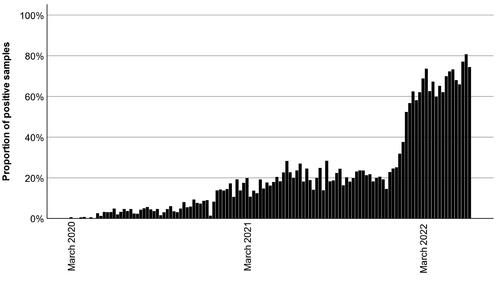
Table 1. SARS-CoV-2 N-antibody prevalence in the study population. The Chi-square test determined the p of the categorical variables; the Mann–Whitney U-test was used for continuous variables.
The age was lower for donors who acquired SARS-CoV-2 antibodies, but no significant difference in prevalence was seen between the sexes. A lower proportion of RhD-negative, and a higher proportion of RhD-positive donors had N-antibodies.
A significant difference in the overall distribution of ABO blood groups was seen between donors with and without antibodies, p = 0.002 ().
Post-hoc analysis showed significantly fewer donors with AB blood type among those with antibodies (p = 0.0019), but no significant differences were seen for other ABO blood types. There was no significant difference in antibody prevalence between O vs. non-O donors (p = 0.99).
Antibody titers, loss, and recovery of antibodies
An episode was defined as consecutive positive titers without intermittent seronegativity. Antibody titers at the first and the second positive donation during the first episode are presented in . Unlike male subjects, female antibody-positive subjects had significantly lower antibody titers at the second donation. Subjects older than the median age (44.5 years) had lower titers at the second donation. Diverging results were seen for ABO blood groups; O and AB subjects had a lower titer at the second donation. No differences were seen for blood group A and B subjects. RhD-positive subjects had a significantly lower antibody titer at the second donation, whereas no significant difference could be shown for RhD-negative subjects.
Table 2. Pairwise comparison of N-antibody titers between first and second antibody-positive donation during the first episode. The p-values were determined using the Related-Samples Wilcoxon signed rank test.
Female donors (p = <0.001) and donors over the median age of 44.5 years (p = 0.041) had significantly higher titers at first antibody-positive donation than males and younger subjects, respectively. No significant differences in titer at the first donation were seen for different ABO blood groups, or for RhD-positive and negative donors (Kruskall–Wallis H-test) (data not shown).
Of the antibody-positive donors (49.6%, n = 1983), 60 (3.0%) donors lost their antibodies during the study period (). The median duration of antibody presence among these donors was 133 days (25th–75th percentiles 70-302) (). Sex and age did not seem to be associated with antibody loss, however, blood group A seemed to be overrepresented. Only the first episode was included in the Kaplan–Meier analysis.
Figure 2. Kaplan–Meier survival curve describing antibody presence in donors whose N-antibodies waned below the detection limit of the assay (n = 60). Only the first episode of antibody positivity was included.
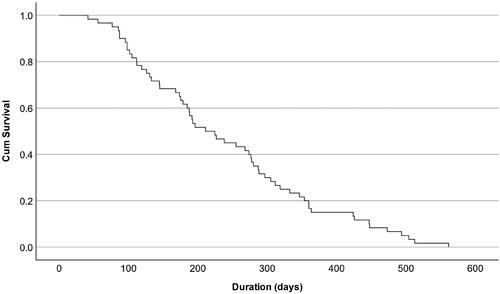
Table 3. Description of donors who lost their N-antibodies. The proportion of donors who lost their N-antibodies, as percent of the antibody-positive donors of each subgroup.
Eighteen of the 60 donors had two episodes of antibody positivity. The first median titer (n = 18) of the first episode was 2.5 (25th–75th percentiles 1.7-5.1). The second median titer of the first episode (n = 13) was 2.5 (25th–75th percentiles 1.3-7.2).
The first median titer (n = 18) of the second episode was 156.0 (25th–75th percentiles 77.9–205.8). The second median titer of the second episode (n = 6) was 88.5 (25th–75th percentiles 19.6–175.3). We had no subsequent titers for the remaining 12 donors due to closing of the study.
Discussion
As part of the immune system, antibodies indicate various degrees of immunity. This study focused on SARS-CoV-2 N-antibodies, which reflect immunity by infection, not vaccination. Sweden was never subject to lockdown, in contrast to many countries that had stricter regulations, for example, Great Britain, and Denmark. These are countries similar to Sweden, but with higher excess death rates during 2020–2021 [Citation23]. The mortality rate in Kronoberg did not differ markedly from other areas in Sweden, possibly excluding the greater Stockholm area. Thus, our results might be representative of Sweden in total [Citation24], and could mirror one effect of the coping strategies introduced by Swedish authorities.
Antibody prevalence
Over time, there was a cumulative increase of antibodies in the blood donor population (), which might reflect the antibody prevalence in the general population. In combination with, e.g., vaccinations, it could explain the diminished severity of disease, and lower mortality due to SARS-CoV-2 infection at the end of the pandemic than in the beginning [Citation25]. Although Sweden was subject to pandemic coping strategies differing from many other countries, the seroprevalence was similar to an Italian blood donor cohort in March 2020 [Citation26], as well as an Austrian blood donor study from June 2020 to March 2021 [Citation27]. A large American cross-sectional study had similar seroprevalences from November 2020 to January 2021 () [Citation12]. Thus, diverging coping strategies had seemingly little effect on the seroprevalence.
We also found that younger subjects had a higher prevalence of antibodies. This agrees with several other studies [Citation12,Citation28,Citation29]. These findings may be due to different risk awareness, protective measures, disease biology, or more social interactions among younger adults.
The results on prevalence between sexes are diverging. Some studies found no differences, while others indicated a higher proportion of seropositivity in men, albeit many of these studies were conducted during the early stages of the pandemic [Citation29–35]. Sexual discrepancies could result from cultural differences or other environmental factors. Our long-term longitudinal study could not demonstrate a different prevalence between sexes.
Many studies have investigated the association between COVID-19 severity, mortality, and blood group [Citation36]. Non-O individuals have been suggested to have a higher prevalence of antibodies [Citation37], increased susceptibility to SARS-CoV-2 infection [Citation38], and severe disease [Citation39]. It has been shown that the natural anti-A and anti-B antibodies block the interaction between the virus and the ACE2 receptors on the cell surfaces, thus hindering infection [Citation40]. Theories also include the presentation of glycosylated A and B epitopes on the viral S-protein, making the virus susceptible to natural anti-A and anti-B present in blood group O individuals [Citation40].
Findings on the relationship between ABO, RhD blood groups, and antibody prevalence are not consistent. As opposed to a previous study by Valenti et al. showing a higher risk of seropositivity in non-O donors [Citation26], our study could not demonstrate a significant difference between O and non-O donors. However, when comparing blood groups separately, we found a significantly higher proportion of seronegative AB donors. Different settings could explain the discrepancies: population, study period, antibody specificity, and assay [Citation41]. Blood donors are a healthy selection of the normal population and are not subject to severe disease. Also, our study exclusively reports N-antibodies’ presence over an extended period. One cannot exclude that mutations in the virus over time may have impacted the results.
Optimal activation of the immune response is crucial for effective viral clearance of SARS-CoV-2. Since individuals with blood groups A, B, and AB seem to be more susceptible to infection and severe disease, one might speculate that the immune system in these individuals is not as effectively modulated, resulting in the lower prevalence of N-antibodies seen in our study for AB subjects [Citation42]. This might also be in concordance with the fact that patients with severe COVID-19 show immune downregulation and immunosuppression [Citation43].
Antibody titers, loss, and recovery of antibodies
A significant decrease in antibody titers was seen for the whole study group, females, older subjects, blood group O, AB, and RhD-positive subjects between the first and the second antibody-positive donation (). Chia et al. found that younger subjects lost their neutralizing antibodies faster but found no differences between sexes regarding antibody waning [Citation44]. Markmann et al. found higher N-antibody titers in male blood donors that waned faster than in female donors [Citation45]. Navaratnam et al. found that younger subjects had faster N-antibody waning. However, they excluded subclinical infections [Citation46]. They also found that women had lower titers that waned faster. Nunhofer et al. found no differences in decline for sex or age [Citation47].
The discrepancies between the results might be attributed to different timeframes. Antibody response is a dynamic course, thus displaying different titers and waning, depending on the point in time [Citation46].
In our study, subjects with the B-antigen (blood groups B and AB) had a higher titer at the first positive donation at the first episode. Bloch et al. found higher titers of neutralizing antibodies in blood group B-subjects [Citation48]. However, contrasting findings were presented by Nunhofer et al., showing that AB donors had the lowest N-antibody titers [Citation47].
We found that O, AB, and Rh + donors significantly decreased N-antibodies between the first and second positive donations. We also found that O and A donors were overrepresented among donors who lost their antibodies. This concurs with Martin et al., where only O and A donors lost their antibodies [Citation49]. Also, they noted that those who lost their antibodies had initially lower titers. It cannot be excluded that donors with different blood groups have a different dynamic in acquiring and waning N-antibodies. Further studies are needed as the number of antibody-positive AB donors was low in our study, and AB donors generally have a slightly longer donation interval.
Titers were generally higher during the second episode than the first, which follows a classical humoral immune response to a secondary antigen exposure. The number of seropositive subjects where the antibody titer later abated below the detection limit (n = 60) was surprisingly low. In concordance with our findings () Nunhofer et al. found that N-antibodies had a significantly lower but detectable titer nine months after infection in 95% of the subjects [Citation47]. However, data on antibody waning is diverging, and other studies concluded that N-antibodies wane fast and can only be used to detect a recent infection [Citation50,Citation51]. Findings on S-antibodies also diverge. Gallian et al. showed that S-antibodies were undetectable 4-5 months post-infection in 50% of the subjects [Citation52], whereas Forgacs et al. concluded that S-antibodies remained constant over 14 months after infection [Citation53].
Strengths and limitations
There were several strengths of this study. The sample size was large, the study period was considerable, and it included the very beginning of the pandemic, although it was not long enough to detect a general decline in antibody prevalence in the blood donor population. Also, the subjects were from a healthy Swedish population without severe symptoms of COVID-19. The assay specifically recognized antibodies against wild-type viruses, not those elicited by vaccine.
A limitation was that we did not specifically identify neutralizing antibodies. However, previous studies have concluded that samples with the strongest inhibition of virus replication had the highest levels of N-antibodies, and the neutralization efficiency correlated with the levels of antibodies [Citation54,Citation55]. There was also a bias toward younger ages, although there was no upper age limit for blood donation. Also, we could not account for possible re-infections in subjects with persistent antibodies. In addition, we only compared seropositivity between the first and second donations because subsequent donations were too few for subgroup analysis.
Conclusions
We conclude that antibody prevalence was higher in younger subjects and lower for blood group AB and RhD-negative donors. There were differences regarding N-antibody waning for sex, age, and blood group. We also conclude that N-antibodies generally persist for months or even years in the blood donor population and that the divergent Swedish national pandemic coping strategy had a minor effect on the seroprevalence.
Acknowledgements
We thank the staff at the Department of Clinical Chemistry and Transfusion Medicine, Växjö Central Hospital, for excellent technical assistance and collaboration. We also thank Anna Lindgren at the Center for Mathematical Sciences, Lund University, for outstanding statistical advice.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- The Public Health Agency of Sweden. Analys av första covid-19-vågen– produktion, köer och väntetider i vården. 2020 [cited 2023 16 May 2023]. Available from: https://www.socialstyrelsen.se/globalassets/sharepoint-dokument/artikelkatalog/ovrigt/2020-11-7065.pdf.
- Castro Dopico X, Muschiol S, Christian M, et al. Seropositivity in blood donors and pregnant women during the first year of SARS-CoV-2 transmission in Stockholm, Sweden. J Intern Med. 2021;290(3):666–676. doi: 10.1111/joim.13304.
- Beser J, Galanis I, Enkirch T, et al. Seroprevalence of SARS-CoV-2 in Sweden, April 26 to May 9, 2021. Sci Rep. 2022;12(1):10816. doi: 10.1038/s41598-022-15183-w.
- Sjörs Dahlman A, Anund A. Seroprevalence of SARS-CoV-2 antibodies among public transport workers in Sweden. J Transp Health. 2022;27:101508. doi: 10.1016/j.jth.2022.101508.
- Ibrahim NK. Epidemiologic surveillance for controlling Covid-19 pandemic: types, challenges and implications. J Infect Public Health. 2020;13(11):1630–1638. doi: 10.1016/j.jiph.2020.07.019.
- Peeling RW, Heymann DL, Teo YY, et al. Diagnostics for COVID-19: moving from pandemic response to control. Lancet. 2022;399(10326):757–768.
- O’Shaughnessy J. COVID-19 convalescent plasma. 2021. Available from: https://www.fda.gov/media/141477/download.
- European Commission. An EU programme of COVID-19 convalescent plasma collection and transfusion - Guidance on collection, testing, processing, storage, distribution and monitored use version 4.0. Brussels: European Commission; 2021.
- Lamontagne F, Agarwal A, Rochwerg B, et al. A living WHO guideline on drugs for covid-19. BMJ. 2020;370:m3379.
- Terpos E, Stellas D, Rosati M, et al. SARS-CoV-2 antibody kinetics eight months from COVID-19 onset: persistence of spike antibodies but loss of neutralizing antibodies in 24% of convalescent plasma donors. Eur J Intern Med. 2021. 2021;89:87–96.
- Basto-Abreu A, Carnalla M, Torres-Ibarra L, et al. Nationally representative SARS-CoV-2 antibody prevalence estimates after the first epidemic wave in Mexico. Nat Commun. 2022;13(1):589. doi: 10.1038/s41467-022-28232-9.
- Wiegand RE, Deng Y, Deng X, et al. Estimated SARS-CoV-2 antibody seroprevalence trends and relationship to reported case prevalence from a repeated, cross-sectional study in the 50 states and the District Of Columbia, United States-October 25, 2020-February 26, 2022. Lancet Reg Health Am. 2023;18:100403.
- Ekelund O, Ekblom K, Somajo S, et al. High-throughput immunoassays for SARS-CoV-2 - considerable differences in performance when comparing three methods. Infect Dis (Lond). 2021;53(10):805–810.
- Perkmann T, Perkmann-Nagele N, Breyer MK, et al. Side-by-Side comparison of three fully automated SARS-CoV-2 antibody assays with a focus on specificity. Clin Chem. 2020;66(11):1405–1413. doi: 10.1093/clinchem/hvaa198.
- Chen Q, Zhu K, Liu X, et al. The protection of naturally acquired antibodies Against subsequent SARS-CoV-2 infection: a systematic review and meta-analysis. Emerg Microbes Infect. 2022;11:793–803.
- Atsma F, Veldhuizen I, Verbeek A, et al. Healthy donor effect: its magnitude in health research among blood donors. Transfusion. 2011;51(8):1820–1828. doi: 10.1111/j.1537-2995.2010.03055.x.
- Kim Y, Latz CA, DeCarlo CS, et al. Relationship between blood type and outcomes following COVID-19 infection. Semin Vasc Surg. 2021;34:125–131.
- Hindawi S, Daghistani S, Elgemmezi T, et al. Association of blood group with COVID-19 disease susceptibility and severity in Saudi Arabia. Transfusion. 2023;63 Suppl 1:S3–S9. doi: 10.1111/trf.17202.
- Palmos AB, Millischer V, Menon DK, et al. Proteome-wide Mendelian randomization identifies causal links between blood proteins and severe COVID-19. PLoS Genet. 2022;18(3):e1010042. doi: 10.1371/journal.pgen.1010042.
- Goel R, Bloch EM, Pirenne F, et al. ABO blood group and COVID-19: a review on behalf of the ISBT COVID-19 Working Group. Vox Sang 2021;116:849–861.
- Jawdat D, Hajeer A, Massadeh S, et al. Correlation between ABO blood group phenotype and the risk of COVID-19 infection and severity of disease in a Saudi Arabian cohort. J Epidemiol Glob Health. 2022;12(1):85–91. doi: 10.1007/s44197-021-00023-3.
- Heinz FX, Stiasny K. Distinguishing features of current COVID-19 vaccines: knowns and unknowns of antigen presentation and modes of action. NPJ Vaccines. 2021;6(1):104. doi: 10.1038/s41541-021-00369-6.
- Collaborators C-EM. Estimating excess mortality due to the COVID-19 pandemic: a systematic analysis of COVID-19-related mortality, 2020. Lancet. 2022;399(21):1513–1536.
- Wixe S, Lobo J, Mellander C, et al. Evidence of COVID-19 fatalities in swedish neighborhoods from a full population study. Sci Rep. 2024;14(1):2998. doi: 10.1038/s41598-024-52988-3.
- Marziano V, Guzzetta G, Menegale F, et al. Estimating SARS-CoV-2 infections and associated changes in COVID-19 severity and fatality. Influenza Other Respir Viruses. 2023;17:e13181.
- Valenti L, Pelusi S, Cherubini A, et al. Trends and risk factors of SARS-CoV-2 infection in asymptomatic blood donors. Transfusion. 2021;61(12):3381–3389. doi: 10.1111/trf.16693.
- Siller A, Seekircher L, Wachter GA, et al. Seroprevalence, waning and correlates of Anti-SARS-CoV-2 IgG antibodies in Tyrol, Austria: large-Scale study of 35,193 blood donors conducted between June 2020 and September 2021. Viruses. 2022;14(3):568. doi: 10.3390/v14030568.
- O’Brien SF, Caffrey N, Yi QL, et al. SARS-CoV-2 seroprevalence among Canadian blood donors: the advance of Omicron. Viruses. 2022;14(11):2336. doi: 10.3390/v14112336.
- Prete CA, Jr., Buss LF, Whittaker C, et al. SARS-CoV-2 antibody dynamics in blood donors and COVID-19 epidemiology in eight Brazilian state capitals: a serial cross-sectional study. Elife. 2022;11:e78233. doi: 10.7554/eLife.78233.
- Hayes C, Rubenstein W, Gibb D, et al. Blood group O convalescent plasma donations have significantly lower levels of SARS-CoV-2 IgG antibodies compared to blood group A donations. Transfusion. 2021;61(8):2245–2249. doi: 10.1111/trf.16524.
- Chaves DG, Takahashi RHC, Campelo F, et al. SARS-CoV-2 IgG seroprevalence among blood donors as a monitor of the COVID-19 epidemic, Brazil. Emerg Infect Dis. 2022;28:734–742.
- Valenti L, Bergna A, Pelusi S, et al. SARS-CoV-2 seroprevalence trends in healthy blood donors during the COVID-19 outbreak in Milan. Blood Transfus. 2021;19:181–189.
- Slot E, Hogema BM, Reusken C, et al. Low SARS-CoV-2 seroprevalence in blood donors in the early COVID-19 epidemic in The Netherlands. Nat Commun. 2020;11(1):5744. doi: 10.1038/s41467-020-19481-7.
- Stone M, Di Germanio C, Wright DJ, et al. Use of US blood donors for national serosurveillance of severe acute respiratory syndrome coronavirus 2 antibodies: basis for an expanded national donor serosurveillance program. Clin Infect Dis. 2022;74(5):871–881. doi: 10.1093/cid/ciab537.
- Vassallo RR, Dumont LJ, Bravo MD, et al. Progression and predictors of SARS-CoV-2 antibody seroreactivity In US blood donors. Transfus Med Rev. 2021;35:8–15.
- Pereira E, Felipe S, de Freitas R, et al. ABO blood group and link to COVID-19: a comprehensive review of the reported associations and their possible underlying mechanisms. Microb Pathog. 2022;169:105658.
- Deschasaux-Tanguy M, Szabo de Edelenyi F, Druesne-Pecollo N, et al. ABO blood types and SARS-CoV-2 infection assessed using seroprevalence data in a large population-based sample: the SAPRIS-SERO multi-cohort study. Sci Rep. 2023;13(1):4775. doi: 10.1038/s41598-023-30714-9.
- Mortensen SJ, Gjerding LAM, Exsteen MB, et al. Reduced susceptibility to COVID-19 associated with ABO blood group and pre-existing anti-A and anti-B antibodies. Immunobiology. 2023;228(4):152399. doi: 10.1016/j.imbio.2023.152399.
- Wu SC, Arthur CM, Jan HM, et al. Blood group A enhances SARS-CoV-2 infection. Blood. 2023;142(8):742–747. doi: 10.1182/blood.2022018903.
- Tamayo-Velasco A, Penarrubia-Ponce MJ, Alvarez FJ, et al. ABO blood system and COVID-19 susceptibility: anti-A and anti-B antibodies are the key points. Front Med (Lausanne). 2022;9:882477. doi: 10.3389/fmed.2022.882477.
- Hasan M, Moiz B, Qaiser S, et al. IgG antibodies to SARS-CoV-2 in asymptomatic blood donors at two time points in Karachi. PLoS One. 2022;17(8):e0271259. doi: 10.1371/journal.pone.0271259.
- Muniz-Diaz E, Llopis J, Parra R, et al. Relationship between the ABO blood group and COVID-19 susceptibility, severity and mortality in two cohorts of patients. Blood Transfus. 2021;19:54–63.
- Davitt E, Davitt C, Mazer MB, et al. COVID-19 disease and immune dysregulation. Best Pract Res Clin Haematol. 2022;35:101401.
- Chia WN, Zhu F, Ong SWX, et al. Dynamics of SARS-CoV-2 neutralising antibody responses and duration of immunity: a longitudinal study. Lancet Microbe. 2021;2:e240–e249.
- Markmann AJ, Giallourou N, Bhowmik DR, et al. Sex disparities and neutralizing-antibody durability to SARS-CoV-2 infection in convalescent individuals. mSphere. 2021;6(5):e0027521. doi: 10.1128/mSphere.00736-21.
- Navaratnam AMD, Shrotri M, Nguyen V, et al. Nucleocapsid and spike antibody responses following virologically confirmed SARS-CoV-2 infection: an observational analysis in the Virus Watch community cohort. Int J Infect Dis. 2022;123:104–111.
- Nunhofer V, Weidner L, Hoeggerl AD, et al. Persistence of naturally acquired and functional SARS-CoV-2 antibodies in blood donors one year after infection. Viruses. 2022;14(3):637. doi: 10.3390/v14030637.
- Bloch EM, Patel EU, Marshall C, et al. ABO blood group and SARS-CoV-2 antibody response in a convalescent donor population. Vox Sang. 2021;116:766–773.
- Martin MC, Jimenez A, Ortega N, et al. Persistence of SARS-CoV-2 total immunoglobulins in a series of convalescent plasma and blood donors. PLoS One. 2022;17(2):e0264124. doi: 10.1371/journal.pone.0264124.
- Fenwick C, Croxatto A, Coste AT, et al. Changes in SARS-CoV-2 spike versus nucleoprotein antibody responses impact the estimates of infections in population-based seroprevalence studies. J Virol. 2021;95(3):e01828-20. doi: 10.1128/JVI.01828-20.
- Germain M, Lewin A, Bazin R, et al. Cohort profile: a Quebec-based plasma donor biobank to study COVID-19 immunity (PlasCoV). BMJ Open. 2023;13(2):e068803. doi: 10.1136/bmjopen-2022-068803.
- Gallian P, Hozé N, Brisbarre N, et al. SARS-CoV-2 IgG seroprevalence surveys in blood donors before the vaccination campaign, France 2020-2021. iScience. 2023;26(4):106222. doi: 10.1016/j.isci.2023.106222.
- Forgacs D, Silva-Moraes V, Sautto GA, et al. The effect of waning on antibody levels and memory B cell recall following SARS-CoV-2 infection or vaccination. Vaccines (Basel). 2022;10(5):696. doi: 10.3390/vaccines10050696.
- Castillo-Olivares J, Wells DA, Ferrari M, et al. Analysis of serological biomarkers of SARS-CoV-2 infection in convalescent samples from severe, moderate and mild COVID-19 cases. Front Immunol. 2021;12:748291. doi: 10.3389/fimmu.2021.748291.
- Sudhakar R, Dontamala S, Bingi TC, et al. A comparative study of antibody response, virus neutralization efficiency & metabolites in SARS-CoV-2-infected adults & children. Indian J Med Res. 2022;156(4&5):659–668. doi: 10.4103/ijmr.ijmr_3475_21.
