Abstract
Background: Galectins are a group of carbohydrate-binding proteins that are involved in neoplastic development and progression. In a previous mass spectrometry-based study, we identified galectin 4 as a down-regulated protein in short-term survivors of pancreatic cancer. This study was performed to validate the prognostic value of galectin 4 in a larger cohort of pancreatic cancer patients undergoing surgical resection.
Methods: Galectin 4 expression was evaluated by tissue microarrays and immunohistochemistry in 140 patients with surgically resected pancreatic cancer. Kaplan-Meier and Cox proportional hazards modeling were used to explore the association between galectin 4 and survival.
Results: Galectin 4 staining expression was positive in 111 cases (79.3%). The expression of galectin 4 was significantly associated with tumor size (p = .008) and differentiation (p = .001). Galectin 4 expression was significantly correlated with disease recurrence within 1 year of surgery (adjusted HR 0.485, p = .027). There was also a significant association between galectin 4 and overall survival at 1 year (adjusted HR 0.482, p = .047) and at 3 years (adjusted HR 0.550, p = .025).
Conclusion: Galectin 4 expression is a novel biomarker for early recurrence and mortality after surgical resection for pancreatic cancer.
Introduction
Pancreatic ductal adenocarcinoma is currently the third leading cause of cancer-related mortality and the most lethal cancer in the digestive system [Citation1]. Survival rates remain poor, with <7% of patients surviving beyond 5-years after diagnosis [Citation2]. The main contributors to this poor prognosis are lack of early detection strategies and effective-targeted treatments, as well as the aggressive tumor biology per se.
Surgical resection is the only potentially curative treatment option for pancreatic cancer. However, even patients that are staged with a locally, resectable tumor, may already have micro-metastasis at the time of diagnosis and recurrence within the first year of surgery is common [Citation3]. Tumor size, lymph node involvement, grade and margin status are important prognostic factors, but are insufficient to predict early disease recurrence and survival after surgical resection [Citation4]. No biomarker is yet available to help guide prognosis and treatment selection in pancreatic cancer patients. A search for novel biomarkers at tissue level can thus lead to the development of tumor-derived serum biomarkers that can facilitate clinical decision making.
Galectins, localized both intra- and extra-cellularly, are a family of lectins that have affinity for β-galactosides. Based on their structure and carbohydrate-recognition domains, they are classified into three groups, including prototype galectins (-1, -2, -5, -7, -10, -11, -13, -14), chimera type galectin (-3), and tandem repeat type galectins (-4, -6, -8, -9, -12) [Citation5]. Galectins bind to a wide array of glycoproteins and glycolipids both on the cell surface and in extracellular matrices [Citation6]. Through cell-to-cell and cell-to-extracellular matrix adhesion and triggering signals intracellularly, galectins participate in cell proliferation, apoptosis, adhesion and immune response, thus acting as modulators in cancer [Citation6]. The most well-studied galectins are galectin-1 and galectin-3. Galectin 1 has been found to be implicated in pancreatic cancer pathophysiology, including tumor cell proliferation, invasion, angiogenesis, inflammation, and metastasis [Citation7]. Galectin 3 has recently been demonstrated to interact with KRAS and mediate tumor cell-stroma interactions in pancreatic cancer [Citation8,Citation9].
In a previous mass spectrometry-based study, we identified galectin 4 as a down-regulated protein in short-term survivors of pancreatic cancer [Citation10]. The present study aimed to elucidate the prognostic role of galectin 4 in a large cohort of patients with resectable pancreatic cancer.
Methods
Patients and samples
We collected formalin-fixed, paraffin-embedded tissue samples from 140 patients with pancreatic cancer who underwent surgical resection at the Department of Surgery, Skåne University Hospital, Lund and Malmö, Sweden, between 1996 and 2017. Ethical approval was obtained from the local human ethics committee at Lund University (Ref 2017/320). The REMARK guidelines were followed when possible throughout the whole study period [Citation11]. Disease-free survival (DFS) was defined as the time from surgery to the first event of either disease recurrence or death due to any cause. Overall survival (OS) was defined as the time from surgery to death due to any cause.
Tissue microarray
Exploiting an automated tissue arraying device (Minicore® 3, Alphelys, Plaisir, France), tissue microarray (TMA) construction was applied to tumors by stabilizing 4 cores ø 2 mm of cancerous tissues (marked by pathologist A.S.) from each tumor into paraffin blocks. The TMA-blocks were then sectioned to 3-µm-thick slides.
Immunohistochemistry
After incubation in 60 °C for 1 h and cool down in room temperature (RT), TMA-sections underwent a deparaffinization, rehydration and antigen-retrieval procedure in EnVision FLEX Target Retrieval Solution pH = 6 (K800521-2, Dako) heated to 96 °C for 20 min, using automated PT Link (Dako, Glostrup, Denmark). After three times of washing in phosphate-buffered saline for 5 min, slides were blocked against endogenous peroxidase activity with 0.3% H2O2 and 1% methanol in phosphate-buffered saline for 10 min. Next, the specimens were blocked with 5% goat serum at RT for 1 h, followed by application of avidin/biotin blocking kit (SP-2001, Vector Laboratories, Burlingame, CA) at RT for 15 min. The sections were then incubated with rabbit polyclonal antibody against human galectin 4 (Atlas Antibodies AB, Bromma, Sweden, cat no HPA031184, dilution 1:100) at 4 °C overnight. Next, sections were incubated with biotinylated secondary goat anti-rabbit antibody (BA-1000, dilution 1:200, Vector Laboratories) at RT for 1 h. After a 30-min incubation with avidin-biotin-peroxidase complex (Vectastain Elite ABC-HRP Kit, PK-6100, Vector Laboratories) at RT, the sections were incubated with chromogen diaminobenzidine (DAB) (SK-4100, Vector Laboratories) for 5 min. After washing in deionized water for 5 min, nuclei were counterstained with Mayer’s hematoxylin (Histolab, Gothenburg, Sweden) for 30 s, and washed in tap water for another 5 min. Finally, the specimens were dehydrated in graded alcohol and mounted by Pertex (Histolab). Negative controls were produced by omitting the primary antibodies. Slides were scanned for evaluation using an Aperio scanscope scanner (Leica Biosystems, Wetzlar, Germany).
Scoring procedure
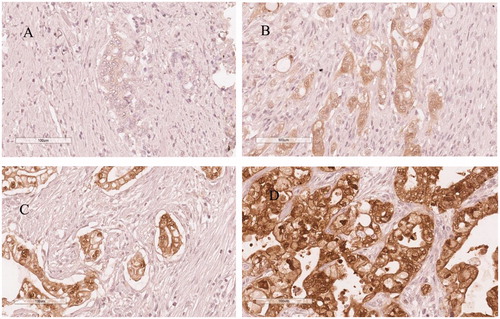
The immunostainings of galectin 4 were assessed semi-quantitatively by an experienced pancreas pathologist (A.S.) blinded to the clinical outcome. The determination of galectin positivity was based on the definition by Hayashi et al. [Citation12]. If more than 10% of tumor cells were stained, expression was considered positive and denoted as weak (1), moderate (2) or strong (3) depending on the intensity. Staining below 10% was denoted as negative (0).
Statistical analysis
Comparisons of categorical and continuous data were performed using Chi-square test or Mann Whitney U test. Kaplan–Meier analysis and log rank and Breslow tests were used to illustrate differences in DFS and OS. Cox regression proportional hazards models were used for estimation of hazard ratios (HRs) for recurrence and death according to galectin 4 expression in both uni- and multivariable analysis, adjusted for age, gender, AJCC stage and resection margin status. Tumor size, differentiation and adjuvant chemotherapy were not included in the multivariable model due to significant correlations to galectin 4 expression. A p-value of <.05 was considered statistically significant. All the statistics were performed with STATA MP 14.1.
Results
Patient cohort
The clinical characteristics of patients with pancreatic cancer are presented in . The median age was 69 years (interquartile range 63–73 years) and 66 patients (47.1%) were female. The estimated median DFS was 13.2 months while the estimated median OS was 24.1 months. One hundred thirteen (80.7%) of the patients received adjuvant chemotherapy.
Table 1. Baseline clinical characteristics stratified according to galectin 4 expression.
Galectin 4 expression in pancreatic cancer
Galectin 4 was positively labeled in cytoplasmic/membranous and nuclear compartments of tumor cells in 111 cases (79.3%). Staining was classified as weak in 32 patients (22.9%), moderate in 51 patients (36.4%) and strong in 28 patients (20.0%). shows representative immunohistochemical images of galectin 4 expression in pancreatic cancer. Expression of galectin 4 was significantly associated with tumor differentiation (p = .001), tumor size (p = .008) and adjuvant chemotherapy (p = .019).
Association between galectin 4 expression and disease-free survival
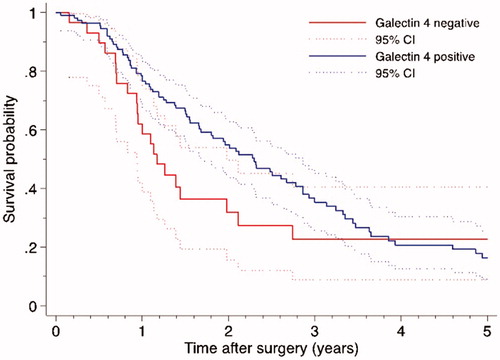
Galectin 4 expression was significantly correlated with disease recurrence within the first year of surgery (p = .014). Multivariable analysis confirmed the results (adjusted HR 0.485, p = .027). Galectin 4 expression was not correlated to 3- or 5-year DFS (). The median DFS was 10.4 months in patients with galectin 4-negativity and 15.9 months in patients with galectin 4-positivity, as estimated by the Kaplan–Meier method (log-rank p = .224, Breslow p = .087), .
Figure 2. Disease-free survival curves by galectin 4 expression in patients with surgically resected pancreatic cancer (log-rank p = .224, Breslow p = .087).
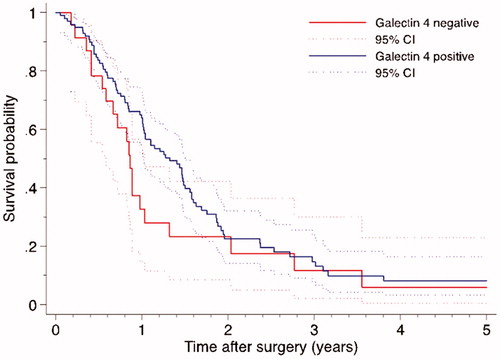
Table 2. Univariable and multivariable Cox survival analyses.
Association between galectin 4 expression and overall survival
Galectin 4 significantly correlated to 1-year OS (p = .036), which was confirmed in multivariable analysis (adjusted HR 0.482, p = .047). Expression of galectin 4 also correlated to 3-year OS in univariable analysis (p = .031) and multivariable analysis (adjusted HR 0.550, p = .025). Galectin 4 did not correlate to 5-year OS. The median OS was 14.0 months in patients with lack of galectin 4 expression and 27.6 months in patients with tumors that expressed galectin 4 (log-rank p = .118, Breslow p = .021), .
Discussion
To our knowledge, this is the first study to report the prognostic utility of galectin 4 in a large cohort of patients with pancreatic cancer. We found that lack of galectin 4 expression is an independent marker for early recurrence and death, defined as occurring within 12 months after curatively aimed surgery. The ability to identify resectable patients that are subjected to early recurrence is a worthy objective, as these selected patients may benefit from other treatment plans, such as neoadjuvant chemotherapy before surgery.
The data in our study are in line with previous experimental studies on the tumor suppressor properties of galectin 4 in pancreatic cancer. It has been demonstrated that galectin 4 inhibits migration and metastasis formation in pancreatic cancer cells both in vitro and in vivo [Citation13]. Further studies have revealed that galectin 4 markedly reduced cytoplasmic β-catenin levels, counteracted with the function of Wnt signaling, and sensitized pancreatic cancer cells to Wnt inhibitors [Citation14]. Interestingly, β-catenin was also highlighted as an upstream regulator in pancreatic cancer patients with poor survival in our previous study [Citation10]. As Wnt/β-catenin signaling is crucial for the development of pancreatic cancer [Citation15], galectin 4 expression might provide additional information to Wnt/β-catenin signaling targeted treatment in pancreatic cancer.
Our results demonstrated that galectin 4 was labeled in the tumor cells, rather than the stromal cells. In contrast, galectin 3 and galectin 1, which have been reported to correlate with survival in pancreatic cancer, are mainly expressed in stromal cells [Citation16–18]. Global gene expression profiling has proved useful for subtype identification in many human tumor types, including pancreatic cancer. Collisson and colleagues have classified pancreatic cancer into three molecular subtypes, that is, classical, exocrine-like and quasi-mesenchymal, with each subtype presenting different response rates to therapy and survival [Citation19]. Notably, by a recent proteomics approach, galectin 4 was identified as a biomarker for the exocrine-like subtype, characterized by resistance to tyrosine kinase inhibitors and paclitaxel [Citation20]. This information has high clinical relevance as it indicates that galectin 4 expression may be used to predict the response to chemotherapy.
In the present study, galectin 4 expression was associated with tumor size and histopathological differentiation. This indicated that galectin 4 expression may be related to pancreatic tumor biology. Galectin 4 has been regarded as a differentiation biomarker in colon cancer [Citation21]. The loss of galectin 4 expression is also linked to increased tumor size and tumor differentiation in hepatocellular carcinoma and lung cancer [Citation12,Citation22]. Most patients received adjuvant chemotherapy in the present study. There are many limitations associated with the retrospective analysis of chemotherapy data and the association between galectin 4 expression and adjuvant chemotherapy receipt found in our study remains speculative.
In summary, the results from this study suggest that galectin 4 expression is associated with early recurrence and death after resection for pancreatic cancer. Further studies are needed to determine whether galectin 4 expression can be included in treatment algorithms to stratify patients for neoadjuvant-direct approaches in resectable cases. Additional studies are also needed to elucidate the exact mechanisms by which galectin 4 exerts its tumor suppressive properties.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68:7–30.
- Kleeff J, Korc M, Apte M, et al. Pancreatic cancer. Nat Rev Dis Primers. 2016;2:16022.
- Rhim AD, Mirek ET, Aiello NM, et al. EMT and dissemination precede pancreatic tumor formation. Cell. 2012;148:349–361.
- Barhli A, Cros J, Bartholin L, et al. Prognostic stratification of resected pancreatic ductal adenocarcinoma: past, present, and future. Dig Liver Dis. 2018;50:979–990.
- Cao ZQ, Guo XL. The role of galectin-4 in physiology and diseases. Protein Cell. 2016;7:314–324.
- Liu FT, Rabinovich GA. Galectins as modulators of tumour progression. Nat Rev Cancer. 2005;5:29–41.
- Orozco CA, Martinez-Bosch N, Guerrero PE, et al. Targeting galectin-1 inhibits pancreatic cancer progression by modulating tumor-stroma crosstalk. Proc Natl Acad Sci USA. 2018;115:E3769–E3E78.
- Seguin L, Camargo MF, Wettersten HI, et al. Galectin-3, a druggable vulnerability for KRAS-addicted cancers. Cancer Discov. 2017;7:1464–1479.
- Zhao W, Ajani JA, Sushovan G, et al. Galectin-3 mediates tumor cell-stroma interactions by activating pancreatic stellate cells to produce cytokines via integrin signaling. Gastroenterology 2018;154:1524–1537. e6.
- Hu D, Ansari D, Pawlowski K, et al. Proteomic analyses identify prognostic biomarkers for pancreatic ductal adenocarcinoma. Oncotarget 2018;9:9789–9807.
- McShane LM, Altman DG, Sauerbrei W, et al. REporting recommendations for tumour MARKer prognostic studies (REMARK). Br J Cancer. 2005;93:387–391.
- Hayashi T, Saito T, Fujimura T, et al. Galectin-4, a novel predictor for lymph node metastasis in lung adenocarcinoma. PLoS One. 2013;8:e81883.
- Belo AI, van der Sar AM, Tefsen B, et al. Galectin-4 reduces migration and metastasis formation of pancreatic cancer cells. PLoS One. 2013;8:e65957.
- Maftouh M, Belo AI, Avan A, et al. Galectin-4 expression is associated with reduced lymph node metastasis and modulation of Wnt/beta-catenin signalling in pancreatic adenocarcinoma. Oncotarget 2014;5:5335–5349.
- Zhang Y, Morris JPT, Yan W, et al. Canonical wnt signaling is required for pancreatic carcinogenesis. Cancer Res. 2013;73:4909–4922.
- Shimamura T, Sakamoto M, Ino Y, et al. Clinicopathological significance of galectin-3 expression in ductal adenocarcinoma of the pancreas. Clin Cancer Res. 2002;8:2570–2575.
- Chen R, Pan S, Ottenhof NA, et al. Stromal galectin-1 expression is associated with long-term survival in resectable pancreatic ductal adenocarcinoma. Cancer Biol Ther. 2012;13:899–907.
- Tang D, Zhang J, Yuan Z, et al. Pancreatic satellite cells derived galectin-1 increase the progression and less survival of pancreatic ductal adenocarcinoma. PLoS One. 2014;9:e90476.
- Collisson EA, Sadanandam A, Olson P, et al. Subtypes of pancreatic ductal adenocarcinoma and their differing responses to therapy. Nat Med. 2011;17:500–503.
- Kuhlmann L, Nadler WM, Kerner A, et al. Identification and validation of novel subtype-specific protein biomarkers in pancreatic ductal adenocarcinoma. Pancreas 2017;46:311–322.
- van de Wetering M, Sancho E, Verweij C, et al. The beta-catenin/TCF-4 complex imposes a crypt progenitor phenotype on colorectal cancer cells. Cell. 2002;111:241–250.
- Cai Z, Zeng Y, Xu B, et al. Galectin-4 serves as a prognostic biomarker for the early recurrence/metastasis of hepatocellular carcinoma. Cancer Sci. 2014;105:1510–1517.