Abstract
Background: The tumor microenvironment in pancreatic cancer has a multifaceted role in disease development and progression. Prolyl 4-hydroxylase subunit alpha 2 (P4HA2) and proteinase 3 (PRTN3) are involved in the synthesis and degradation of collagen in the tumor microenvironment and have been identified as prognostic biomarker candidates for pancreatic cancer in our previous mass spectrometric study. This study aimed at validating prognostic performance of P4HA2 and PRTN3 in a larger cohort of patients.
Methods: The expression of P4HA2 and PRTN3 was evaluated with tissue microarrays and immunohistochemistry in 140 patients with pancreatic cancer who underwent surgical resection. Kaplan–Meier and Cox proportional hazards regression modeling were used to explore the association of P4HA2 and PRTN3, either separately or combined, with clinicopathological factors and survival.
Results: Most tumors were positive for P4HA2 (133/140, 95%), whereas 77 tumors (55%) were positive for PRTN3. Expression levels of P4HA2 and PRTN3 did not separately correlate with disease-free or overall survival, in either uni- or multivariable analysis. However, a low P4HA2 and high PRTN3 expression correlated with shorter disease-free survival (median 7.0 vs. 13.4 months, adjusted HR 3.24, 95% CI: 1.13–9.25, p = .028) and overall survival (median 8.5 vs. 25.8 months, adjusted HR 8.14, 95% CI: 3.41–19.44, p < .001).
Conclusion: Our data show that a low P4HA2 and high PRTN3 expression correlates with poor survival in patients with pancreatic cancer, indicating the involvement of collagen deposition in the restraint of the tumor. The tumoral expression of PRTN3 reinforces the therapeutic potential of PR1-targeting immunotherapy in pancreatic cancer.
Introduction
Pancreatic cancer is a devastating malignancy with a dismal prognosis. With a 5-year survival rate in the single digits, pancreatic cancer has become the third cause of cancer-related mortality, after colorectal and lung cancer [Citation1]. The poor prognosis is mainly due to the lack of early detection tools and the resistance to current treatment modalities, including chemotherapy, radiotherapy and targeted therapies.
Development of new biomarkers may aid in clinical decision making. CA 19-9 is the only serum marker for pancreatic cancer, but lacks the necessary performance to be used as a screening tool. Although many tissue biomarkers have shown potential prognostic utility in pancreatic cancer [Citation2], few have been translated into the clinical setting and none for routine use. To overcome therapeutic resistance in pancreatic cancer, it has been proposed to subgroup patients based on biomarker profiles in tumor tissue [Citation3,Citation4]. Thus far, only hENT1 expression has been suggested by NCCN as a predictive marker in patients undergoing tumor resection and treatment with gemcitabine.
The pancreatic tumor microenvironment (TME) has attracted much interest in the past decade because of its crucial role in tumor progression and chemoresistance [Citation5]. The TME contains an abundant fibrotic stroma, which encompasses a variety of cellular and molecular entities, such as pancreatic stellate cells (PSCs), and extracellular matrix components (ECM), such as collagen, fibronectin and hyaluronic acid. The TME that interacts with tumor cells is dynamic. Activated PSCs are mainly responsible for deposition of collagen, which also undergoes degradation by PSCs, cancer cells and inflammatory cells through secretion of matrix metalloproteinases (MMPs) [Citation6]. It has been suggested that an activated stroma status relates to progression and consequently poor survival of pancreatic cancer [Citation7].
In a previous study, we discovered and verified several novel tissue biomarkers for pancreatic cancer utilizing mass spectrometry-based proteomics [Citation8]. We identified prolyl 4-hydroxylase subunit alpha 2 (P4HA2) and proteinase 3 (PRTN3) as biomarkers which are related to the TME. P4HA2 is a component of prolyl 4-hydroxylase (P4H), a key enzyme in collagen synthesis composed of two identical alpha subunits and two beta subunits [Citation9]. P4H catalyzes the formation of 4-hydroxyproline that is essential to the proper three-dimensional folding of newly synthesized procollagen chains. P4HA2 is one of the three P4HA isoforms that has been identified in human tissue (P4HA1, P4HA2 and P4HA3). Increased P4HA2 expression has been detected in breast cancer [Citation10], oral cavity squamous cell carcinoma [Citation11] and papillary thyroid cancer [Citation12].
PRTN3, also known as myeloblastin or c-ANCA (cytoplasmic pattern of antineutrophil cytoplasmic autoantibodies) antigen, is a serine protease secreted by cells of myeloid lineage [Citation13] and allocated to the cell surface of neutrophils [Citation14] and endothelial cells [Citation15]. PRTN3 is related to inflammatory processes, but its link to neoplasia is less understood. Sharing structural similarity with elastase, PRTN3 has an elastase-like specificity for small aliphatic residues (Ala, Val, Ser, Met) and degrades a variety of matrix proteins in vitro including fibronectin, laminin, vitronectin, and collagen [Citation16]. PRTN3 is also thought to be involved in MMP activation, hence potentially being involved in tumor invasion and metastasis [Citation17,Citation18]. Moreover, it has been found that PRTN3 induces phosphorylation and nuclear translocation of p44/p42 and JNK1, leading to cancer cell motility, through a nonproteolytic way [Citation19]. Notably, recent studies revealed that PRTN3 expressed by neutrophils within the TME can be taken up by breast cancer and melanoma cells, which in turn increase the susceptibility to PR1-targeting therapies [Citation14,Citation20].
Based on previous experience that P4HA2 and PRTN3, respectively, participate in the synthesis and degradation of collagen in ECM, we hypothesized that these two biomarkers may correlate with the survival of patients with pancreatic cancer, possibly by exerting an influence on the dynamics of the TME. The aim of this study was to validate the prognostic potential of P4HA2 and PRTN3 in a large cohort of patients with resectable pancreatic cancer.
Methods
Patients and samples
Formalin-fixed, paraffin-embedded tissue samples were collected from 140 patients with pancreatic cancer who underwent pancreatic resection at the Department of Surgery, Skåne University Hospital, Lund and Malmö, Sweden, between 1996 and 2017. Ethical approval was obtained from the local human ethics committee at Lund University (Ref 2017/320). Written informed consent was given by participants in this study. The REMARK guidelines were followed when possible throughout the whole study period [Citation21].
Tissue microarray
Tissue microarray (TMA) construction was applied to tumors with sufficient amount of material. Compared with whole sections, TMA has the advantage of a reduced consumption of both tissue and time, which enables studies of a larger scale with reduced experimental variability. Using an automated tissue arraying device (Minicore® 3, Alphelys, Plaisir, France), 4 cores ⊘ 2 mm of cancer tissues (marked by pathologist A.S.) from each specimen were stabilized into paraffin blocks. The TMA-blocks were sectioned to 3 µm thick slides for immunohistochemical analysis.
Immunohistochemistry
TMA-sections were heated in 60 °C for 1 hour and then cooled in room temperature (RT). Next, using automated PT Link (Dako, Glostrup, Denmark), deparaffinization, rehydration and antigen-retrieval were performed in EnVision FLEX Target Retrieval Solution pH = 6 (K800521-2, Dako) heated to 96 °C for 20 min. After three times of washing in phosphate-buffered saline for 5 min, sections were blocked against endogenous peroxidase activity with 0.3% H2O2 and 1% methanol in phosphate-buffered saline for 10 min. The specimens were then blocked with 5% goat serum for 1 hour at RT, followed by avidin/biotin blocking kit (SP-2001, Vector Laboratories, Burlingame, CA) for 15 min at RT. Subsequently, the sections were incubated with rabbit polyclonal antibody against human P4HA2 (Atlas Antibodies AB, Bromma, Sweden, cat no HPA016997, dilution 1:300) or PRTN3 (cat no HPA005938, dilution 1:300) at 4 °C overnight. Next, sections were incubated with biotinylated secondary goat anti-rabbit antibodies (BA-1000, dilution 1:200, Vector Laboratories) for 1 hour at RT. Following incubation with avidin–biotin–peroxidase complex (Vectastain Elite ABC-HRP Kit, PK-6100, Vector Laboratories) for 30 min at RT, the sections were incubated with chromogen diaminobenzidine (DAB) (SK-4100, Vector Laboratories) for 5 min. After washing in deionized water for 5 min, nuclei were counterstained with Mayer’s hematoxylin (Histolab, Gothenburg, Sweden) for 30 sec and washed in tap water for another 5 min. Finally, the specimens were dehydrated in graded alcohol and mounted using Pertex (Histolab). Negative controls were produced by omitting the primary antibodies. Regarding the positive controls, P4HA2 staining by western blot is remarkably reduced in cells with knock-down of P4HA2, while PRTN3 is highly stained in hematopoietic cells of bone marrow, according to the antibody provider and database ‘The Human Protein Atlas’ (www.proteinatlas.org) [Citation22]. Slides were scanned for evaluation using an Aperio scanscope scanner (Leica Biosystems, Wetzlar, Germany).
Scoring procedure
The immunostaining of P4HA2 and PRTN3 was assessed semi-quantitatively by an experienced pancreas pathologist (A.S.) blinded to the clinical outcome. The scoring algorithm was modified from Norihiro et al. [Citation23] and had taken into consideration the proportion of stained tumor cells and the intensity of the staining. Staining less than 10% of tumor cells was denoted as negative (0). When >10% of tumor cells were stained, the expression was considered positive and denoted as weak (1), moderate (2) or strong (3) depending on the intensity. Scoring with (0) and (1) was categorized as the low expression, while scoring with (2) and (3) was categorized as the high expression.
Statistical analysis
Comparisons of categorical data were performed using Chi-square test or Fisher’s exact test. Kaplan–Meier analysis and log rank test were used to illustrate differences in disease-free survival (DFS) and overall survival (OS). Cox regression proportional hazards regression models were used for estimation of hazard ratios (HRs) for recurrence and death according to P4HA2 and PRTN3 expression in both uni- and multivariable analysis, adjusted for age, gender, TNM status, differentiation grade, resection margin status and adjuvant chemotherapy. A p-value of <.05 was considered statistically significant. All the statistical analyses were performed with STAT MP 14.1.
Results
Patient cohort
Baseline characteristics of patients with pancreatic cancer are presented in . The median age was 68 years (interquartile range 63–73 years) and 72 patients (51.4%) were male. One hundred thirteen (80.7%) patients received adjuvant chemotherapy.
Table 1. Baseline characteristics of patients with pancreatic ductal adenocarcinoma.
P4HA2 expression in pancreatic cancer
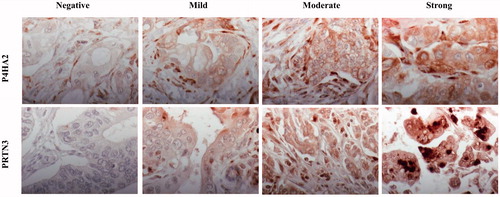
P4HA2 was positively labeled in the cytoplasm and cell membrane of tumor cells in 133 patients (95%). Weak staining was denoted in 32 cases (22.9%), moderate staining in 63 cases (45.0%) and strong staining in 38 cases (27.1%). Myofibroblasts were positive for P4HA2 in all patients. shows representative immunohistochemical images of P4HA2 expression in pancreatic cancer.
Association between P4HA2 and clinicopathological characteristics and survival
P4HA2 expression was not associated with any clinicopathological characteristics. P4HA2 expression did not significantly correlate with either DFS or OS, in either uni- or multivariable analysis (). The median DFS and OS were 17.8 and 21.9 months, respectively, in the low P4HA2 expression group, while they were 12.7 and 27.7 months in the high P4HA2 group.
Table 2. Cox survival analysis of P4HA2 and PRTN3 expression in pancreatic cancer.
PRTN3 expression in pancreatic cancer
The staining of PRTN3 was detected in the nuclei, cytoplasm and cell membrane of tumor cells and lymphocytes (). PRTN3 expression in tumor cells was considered positive in 77 cases (55%). Weak, moderate and strong staining of PRTN3 accounted for 40 (28.6%), 26 (18.6%) and 11 (7.9%) cases.
Association between PRTN3 and clinicopathological characteristics and survival
The high PRTN3 expression group had a higher frequency of adjuvant chemotherapy receipt (94.6% vs. 79.6%, p = .035). There were no association of PRTN3 expression with any other clinicopathological parameters, including age, gender, TNM stage, histological grade and resection margin status (p > .05). The median DFS and OS were 12.4 and 24.5 months in the low PRTN3 group while they were 15.5 and 25.8 months in the high PRTN3 group. PRTN3 expression did not correlate with DFS or OS ().
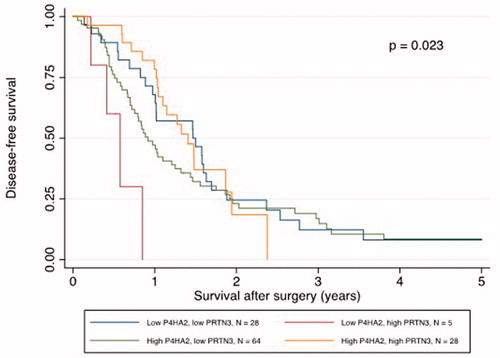
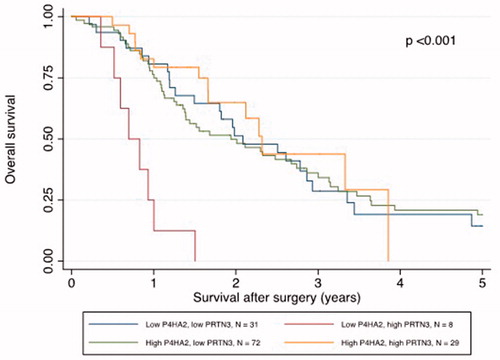
Low P4HA2 and high PRTN3 expression was correlated with poor survival
Kaplan–Meier analysis revealed that a low P4HA2 and high PRTN3 expression correlated with a significantly shorter DFS and OS ( and ). The median DFS was 7.0 months in patients with low P4HA2 and high PRTN3 expression pattern compared to 13.4 months in patients with other expression patterns (p = .004). The correlation with DFS was confirmed in univariable Cox regression analysis (HR 4.12, 95% CI: 1.46–11.63, p = .008) and remained significant in multivariable analysis (HR 3.24, 95% CI: 1.13–9.25, p = .028), adjusted for age, gender, TNM status, differentiation grade, resection margin status and adjuvant chemotherapy (). The median OS in patients with a low P4HA2 and high PRTN3 expression was shorter than those with other expression patterns (8.5 vs. 25.8 months, p < .001). The correlation with OS was also confirmed in univariable Cox regression analysis (HR 5.97, 95% CI: 2.77–12.85, p < .001), and remained significant in multivariable analysis after adjustment (HR 8.14, 95% CI: 3.41–19.44, p < .001) ().
Discussion
Our results demonstrate that a combination of low P4HA2 and high PRTN3 expression is associated with shorter DFS and OS. This is to our knowledge, the first report of using P4HA2 and PRTN3 as tissue biomarkers in a large number of patients from a well annotated clinical cohort.
P4HA2 and PRTN3 have been implicated in the formation and degradation of collagen, respectively. As the main constitute of ECM, collagen plays a key role in tumor progression [Citation24]. It has been shown that by regulating collagen deposition, P4HA2 promotes breast cancer progression and metastasis and correlates with a poor prognosis [Citation10]. The dynamics of collagen production and turnover involve various steps, such as activation of PSCs and participation of relavant enzymes. Moreover, heteregenity is strongly implicated in pancreatic cancer both in expressional signatures and therapeutic response [Citation4,Citation25,Citation26]. It seems reasonable that one biomarker such as P4HA2 or PRTN3 alone fail to capture the characteristics of ECM in the tumor. A combination of biomarkers for the signatures of the TME in pancreatic cancer may better predict the prognosis, as has been shown in several studies [Citation6,Citation27].
Our data indicated that a decrease of collagen in TME may favor progression of pancreatic cancer. Deposition of a collagen-rich TME around the tumor may act as a barrier to confine tumor progression. This is supported by the presence of a capsule around the metastasis correlated with a better prognosis in colorectal liver cancer metastases [Citation28]. Nevertheless, some studies suggested that both increased deposition of collagen in TME and a highly aligned stromal collagen were negative prognostic factors in pancreatic cancer [Citation7,Citation29]. The role of collagen in pancreatic cancer seems to be controversial. Our results were contradictory with previous study in which low mRNA expression of P4HA2 correlated with reduced collagen deposition and better survival of breast cancer [Citation10]. However, Erkan and colleagues have reported that low expression of collagen deposition correlated with poor survival of pancreatic cancer [Citation6]. In their study, using immunohistochemistry of α-SMA and collagen, the stroma in pancreatic cancer was classified according to four patterns of collagen deposition: inert, dormant, fibrogenic and fibrolytic. The fibrolytic stroma, characterized by high α-SMA and low collagen, was independently associated with the worse prognosis [Citation5,Citation6]. This was further confirmed by a recently published larger study [Citation27]. More studies will be needed to elucidate the mechanisms of collagen deposition and the link to the clinical outcome.
Interestingly, more than half of the patients displayed PRTN3 expression in the tumor cells. The absent or minimal RNA-seq expression of PRTN3 in pancreatic cancer cell lines from the Cancer Cell Line Encyclopedia [Citation30], together with the finding that the MIA PaCa-2 cell line was found to display an uptake of PRTN3 [Citation14], led us to the speculate that the tumoral expression of PRTN3 was derived from neutrophils within the TME of pancreatic cancer and then taken up by tumor cells, as has been reported in other tumors [Citation14,Citation20]. Tumor-associated antigen PR1, a peptide derived from PRTN3 and neutrophil elastase, has been shown to be cross-presented in breast cancer and melanoma cells, which in turn increased the susceptibility to PR1-targeting therapies [Citation14,Citation20]. Our results might shed light on the potential of PR1-targeting immunotherapy in patients with pancreatic cancer whose tumor cells cross-present PR1.
In conclusion, our study showed that a low P4HA2 and high PRTN3 expression correlates with poor survival in patients with pancreatic cancer. The finding highlights the involvement of collagen deposition in the restraint of the tumor progression. The tumoral expression of PRTN3 in the majority of pancreatic tumors also reinforces the potential of PR1-targeting immunotherapy. Further studies are needed to confirm this association as well as to investigate the deposition of collagen in TME and the activation status of PSCs in relation to P4HA2 and PRTN3 expression.
Disclosure statement
There are no conflicts of interest.
Additional information
Funding
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68:7–30.
- Ansari D, Rosendahl A, Elebro J, et al. Systematic review of immunohistochemical biomarkers to identify prognostic subgroups of patients with pancreatic cancer. Br J Surg. 2011;98:1041–1055.
- Neoptolemos JP, Kleeff J, Michl P, et al. Therapeutic developments in pancreatic cancer: current and future perspectives. Nat Rev Gastroenterol Hepatol. 2018;15:333–348.
- Waddell N, Pajic M, Patch AM, et al. Whole genomes redefine the mutational landscape of pancreatic cancer. Nature. 2015;518:495–501.
- Erkan M, Hausmann S, Michalski CW, et al. The role of stroma in pancreatic cancer: diagnostic and therapeutic implications. Nat Rev Gastroenterol Hepatol. 2012;9:454–467.
- Erkan M, Michalski CW, Rieder S, et al. The activated stroma index is a novel and independent prognostic marker in pancreatic ductal adenocarcinoma. Clin Gastroenterol Hepatol. 2008;6:1155–1161.
- Whatcott CJ, Diep CH, Jiang P, et al. Desmoplasia in primary tumors and metastatic lesions of pancreatic cancer. Clin Cancer Res. 2015;21:3561–3568.
- Hu D, Ansari D, Pawlowski K, et al. Proteomic analyses identify prognostic biomarkers for pancreatic ductal adenocarcinoma. Oncotarget. 2018;9:9789–9807.
- Myllyharju J. Prolyl 4-hydroxylases, the key enzymes of collagen biosynthesis. Matrix Biol. 2003; 22:15–24.
- Xiong G, Deng L, Zhu J, et al. Prolyl-4-hydroxylase alpha subunit 2 promotes breast cancer progression and metastasis by regulating collagen deposition. BMC Cancer. 2014; 14:1.
- Chang KP, Yu JS, Chien KY, et al. Identification of PRDX4 and P4HA2 as metastasis-associated proteins in oral cavity squamous cell carcinoma by comparative tissue proteomics of microdissected specimens using iTRAQ technology. J Proteome Res. 2011;10:4935–4947.
- Jarzab B, Wiench M, Fujarewicz K, et al. Gene expression profile of papillary thyroid cancer: sources of variability and diagnostic implications. Cancer Res. 2005;65:1587–1597.
- Csernok E, Ernst M, Schmitt W, et al. Activated neutrophils express proteinase 3 on their plasma membrane in vitro and in vivo. Clin Exp Immunol. 2008;95:244–250.
- Alatrash G, Mittendorf EA, Sergeeva A, et al. Broad cross-presentation of the hematopoietically derived PR1 antigen on solid tumors leads to susceptibility to PR1-targeted immunotherapy. J Immunol. 2012;189:5476–5484.
- Mayet WJ, Csernok E, Szymkowiak C, et al. Human endothelial cells express proteinase 3, the target antigen of anticytoplasmic antibodies in Wegener's granulomatosis. Blood. 1993;82:1221–1229.
- Rao NV, Wehner NG, Marshall BC, et al. Characterization of proteinase-3 (PR-3), a neutrophil serine proteinase. Structural and functional properties. J Biol Chem. 1991;266:9540–9548.
- Shamamian P, Schwartz JD, Pocock BJ, et al. Activation of progelatinase A (MMP-2) by neutrophil elastase, cathepsin G, and proteinase-3: a role for inflammatory cells in tumor invasion and angiogenesis. J Cell Physiol. 2001;189:197–206.
- Shamamian P, Pocock BJ, Schwartz JD, et al. Neutrophil-derived serine proteinases enhance membrane type-1 matrix metalloproteinase-dependent tumor cell invasion. Surgery. 2000;127:142–147.
- Kolonin MG, Sergeeva A, Staquicini DI, et al. Interaction between tumor cell surface receptor RAGE and proteinase 3 mediates prostate cancer metastasis to bone. Cancer Res. 2017;77:3144–3150.
- Peters HL, Tripathi SC, Kerros C, et al. Serine proteases enhance immunogenic antigen presentation on lung cancer cells. Cancer Immunol Res. 2017;5:319–329.
- McShane LM, Altman DG, Sauerbrei W, et al. REporting recommendations for tumour MARKer prognostic studies (REMARK). Br J Cancer. 2005;93:387–391.
- Uhlen M, Fagerberg L, Hallstrom BM, et al. Proteomics. Tissue-based map of the human proteome. Science. 2015;347:1260419.
- Sato N, Fukushima N, Maehara N, et al. SPARC/osteonectin is a frequent target for aberrant methylation in pancreatic adenocarcinoma and a mediator of tumor-stromal interactions. Oncogene. 2003;22:5021–5030.
- Hamada S, Masamune A. Elucidating the link between collagen and pancreatic cancer: what's next? Expert Rev Gastroenterol Hepatol. 2018;12:315–317.
- Bailey P, Chang DK, Nones K, et al. Genomic analyses identify molecular subtypes of pancreatic cancer. Nature. 2016;531:47–52.
- Collisson EA, Sadanandam A, Olson P, et al. Subtypes of pancreatic ductal adenocarcinoma and their differing responses to therapy. Nat Med. 2011;17:500–503.
- Mahajan UM, Langhoff E, Goni E, et al. Immune cell and stromal signature associated with progression-free survival of patients with resected pancreatic ductal adenocarcinoma. Gastroenterology. 2018;155:1625–1639.
- Lunevicius R, Nakanishi H, Ito S, et al. Clinicopathological significance of fibrotic capsule formation around liver metastasis from colorectal cancer. J Cancer Res Clin Oncol. 2001;127:193–199.
- Drifka CR, Loeffler AG, Mathewson K, et al. Highly aligned stromal collagen is a negative prognostic factor following pancreatic ductal adenocarcinoma resection. Oncotarget. 2016;7:76197–76213.
- Cancer Cell Line Encyclopedia C, Genomics of Drug Sensitivity in Cancer C. Pharmacogenomic agreement between two cancer cell line data sets. Nature. 2015;528:84–87.