Abstract
Objectives: Nonalcoholic steatohepatitis (NASH), which is a common and increasing indication for liver transplantation (LT), is known to recur after LT. Since the recurrence of NASH can lead to graft failure, the identification of predictive factors is needed and preventive strategies should be implemented.
Methods: We retrospectively examined 95 patients who had undergone LT for NASH or alcoholic liver disease (ALD) as a primary indication. We evaluated peritransplant characteristics and histological findings 1-year post LT among liver transplant patients due to NASH or ALD.
Results: Pre-LT body mass index (BMI) was higher and pre-LT diabetes was more prevalent in NASH patients than in ALD patients (p < .01). The difference of BMI persisted at 3 months and 1 year after LT. There were no differences between the groups regarding histopathological findings including the degree of steatosis and fibrosis in 1-year biopsy. In multivariate analysis, recipient age and 1-year BMI were independent risk factors for post-LT fatty liver disease development. Regarding predictive factors of NASH recurrence, the prevalence of pre-LT insulin-dependent diabetes was significantly higher in patients who developed NASH recurrence than those who did not. The increase of HbA1c at 1-year post-LT checkup was higher in patients who developed recurrence than those who did not, although the difference did not reach statistical significance.
Conclusions: The results of this study suggest that insulin-dependent diabetes has detrimental effects on NASH recurrence following LT. Optimal glycemic control should be recommended, but studies are needed to prove its preventive effect on NASH recurrence.
Introduction
With the increasing number of obese people, nonalcoholic fatty liver disease (NAFLD) has become a most common liver disease [Citation1]. Recent reports have shown that the prevalence of nonalcoholic steatohepatitis (NASH), which is commonly considered to be the progressive form of NAFLD, is 3–5% of the general population [Citation2,Citation3]. Further progression to advanced fibrosis and cirrhosis is documented in 9–20% of NASH patients [Citation4]. Accordingly, the number of liver transplantation (LT) for NASH-related cirrhosis has been increasing [Citation5,Citation6]. The outcome of LT for NASH-related cirrhosis has been considered to be comparable to LT for the other etiologies [Citation6–8]. However, NASH recurrence post-LT is shown to be not uncommon [Citation9–11] and results in severe liver graft fibrosis and cirrhosis [Citation7,Citation11,Citation12]. Therefore, the identification of risk factors for NASH recurrence and implementation of preventive strategies are required. Sourianarayanane et al. recently reported that post-LT graft fibrosis among NASH patients predominantly progressed during the first year after transplantation [Citation10], which indicated the importance of introducing preventive strategies early after LT. In our institution, protocol biopsies were routinely performed 1 year after LT. Therefore, by assessing histopathological findings obtained from the 1-year biopsy, we sought to clarify the characteristics and predictive factors of fatty liver disease development and NASH recurrence during the first year after transplantation.
First, pre-LT clinical characteristics and post-LT histological findings among NASH patients were evaluated in comparison with patients who underwent LT due to alcoholic liver disease (ALD), which is appropriate as a control group because of the similar immunosuppression regimen applied and no specific treatment required. Second, predictive factors of fatty liver disease development were investigated among NASH and ALD patients for whom data of 1-year protocol biopsy was obtained. Lastly, risk factors for NASH recurrence after LT were assessed among NASH patients.
Patients and methods
Patient selection
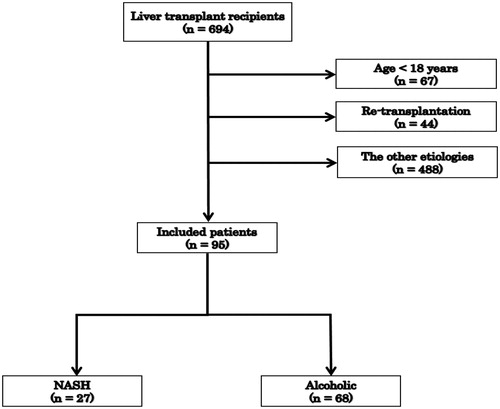
We retrospectively identified 694 consecutive patients who had undergone LT at Karolinska University Hospital between June 2007 and February 2017. Of these, 67 patients who were <18 years old and 44 patients who had undergone re-transplantation were excluded, and 95 patients who had received LT for NASH or ALD as a primary indication were included in the study (). Pretransplant NASH diagnosis was made based on hepatologists’ examination often verified with pretransplant liver biopsy. Pathological examinations of explant (patient’s native liver) were performed in all cases for verification of the diagnosis. In NASH group, we included all patients with NASH diagnosis registered before or after transplantation. Criteria for NASH diagnosis were based on the diagnostic algorithm by Bedossa and Consortium [Citation13]. Diagnosis of ALD was made before the transplantation based on hepatologists’ examination and psychiatric evaluation for abuse diseases. ALD was defined as liver disease in a person drinking more than 30 g of pure alcohol per day in men and 20 g/day in women. Patients with diagnosis of cryptogenic liver cirrhosis were excluded from the study to specifically focus on the post-transplant outcomes among NASH and ALD patients. Patients who were concomitantly infected with hepatitis B or C virus were also excluded. Clinical data were collected retrospectively from our registry database as well as from electronic clinical records. Protocol graft biopsy was routinely performed intraoperatively at the end of transplantation procedure and at 1-year follow up after LT. The data on the degrees of macrovesicular steatosis, perisinusoidal fibrosis, hepatocyte ballooning and acute rejection with rejection activity index were retrospectively collected. In the first analysis of the data, we investigated predictive factors for post-LT fatty liver disease development among pre-LT, post-LT and donor variables in patients from both groups using univariate and multivariate logistic regression models. In the second analysis, we compared peritransplant clinical variables including the donor demographics between patients who developed NASH recurrence and those who did not. These factors were selected based on theoretical considerations and relevant literature [Citation9,10,14,15]. The recurrence of NASH was defined as macrovesicular steatosis with perisinusoidal fibrosis or hepatocyte ballooning based on the criteria recently proposed by Younossi et al. [Citation16]. In our study, the lower threshold for steatosis was set at 5% of hepatocytes in which fat droplets accumulation resulted in displacement of the nucleus. Alcohol relapse was screened for all the patients at 1-year post-LT and recorded on the electronic medical record by specialized transplant coordinators. In our institution, alcohol relapse is monitored based on regular contacts with department of psychiatry, regular questionnaire and ad-hoc blood test for phosphatidylethanol (PEth). All patients are screened for alcohol consumption with PEth at one-year control checkups despite the indication for transplantation. All patients with positive PEth at any time after transplantation are regarded as alcohol users. In case of patients transplanted due to ALD, it is registered as alcoholism relapse based on our ‘no tolerance’ policy for alcohol use for this group of patients. This study and described procedures were conducted in accordance with the Helsinki Declaration.
Immunosuppressive regimens
All patients included in this study received induction immunosuppressive treatment consisting of steroids with basiliximab. Solumedrol was started at 1 g on the day of the operation and tapered to 40 mg on postoperative day 5. From postoperative day 6, 20 mg of prednisolone was administered orally, with subsequent tapering to 5–10 mg at 2 months and 2.5–5 mg at 1 year after transplantation. Tacrolimus was initiated on postoperative day 4 and adjusted so as to maintain the initial trough level of 6–10 ng/mL until 3 months after LT and 4–8 ng/mL thereafter. Mycophenolate mofetil was started on postoperative day 1, tapered and discontinued around 6–12 months after LT, depending on the patient’s state. mTOR inhibitors, sirolimus or everolimus, were used when the toxicity of tacrolimus was observed regardless of HCC diagnosis. However, there were no differences between the groups in creatinine levels at the checkup time points included in the study (data not shown).
Statistical analysis
Data are expressed as means (± standard deviations [SDs]) or medians (range) as appropriate. Statistically significant differences were determined using Student t-tests for normally distributed data, Wilcoxon’s signed-rank tests for skewed data, and Fisher’s exact tests or chi-squared tests for dichotomous data. Univariate and multivariate logistic regression analyses were used to assess the association of several parameters with post-LT development of fatty liver disease. Analyses were performed using JMP Pro 13 (SAS Institute, Cary, NC). A p value of less than .05 was considered to be significant.
Results
Peritransplant demographics of NASH and ALD patients
The mean age of NASH patients was 59.7 (range, 38–69) years, which was comparable to that of ALD patients (). The mean body mass index (BMI) was higher for NASH patients than ALD patients (31.3 vs. 28.6 kg/m2, p = .01). Both non-insulin-dependent and insulin-dependent diabetes were more prevalent in NASH patients (non-insulin-dependent diabetes, 26 vs. 3%; insulin-dependent diabetes, 56 vs. 21%, p < .001). In both groups, pre-LT median triglyceride and cholesterol levels were within the normal range, and only seven patients (8%) and one (1%) patient presented hypertriglyceridemia (≥150 mg/dL) and hypercholesterolemia (≥240 mg/dL), respectively. Higher frequency of hepatocellular cancer (HCC) occurrence was observed among NASH patients (p < .01).
Table 1. Pretransplant characteristics of 95 LT recipients.
Demographics at 3 months and 1-year checkup after LT
One-year patients’ survival in NASH and ALD groups was 89 and 91%, respectively. Among the 95 patients, histopathological findings using 1-year protocol biopsy were obtained for 22 NASH patients and 52 ALD patients. and show post-LT demographics and histological findings of NASH and ALD patients. The difference in BMI persisted at 3 months and 1 year after LT (at 3 months, 29.1 vs. 25.7 kg/m2, p = .001; at 1 year, 30.9 vs. 26.9 kg/m2, p < .001). Regarding the change in BMI over time, BMI at 1-year post-LT was lower than pre-LT BMI in ALD patients (p = .005), while post-LT BMI in NASH patients was stable (p = .71). There were no differences between NASH and ALD groups regarding triglyceride and total cholesterol levels at 3 months post-LT. However, at 1-year post-LT, the triglyceride level was significantly higher and total cholesterol level lower in NASH patients than in ALD patients (triglyceride, 195 vs. 133 mg/dL, p = .049; cholesterol, 139 vs. 179 mg/dL, p = .006). In both NASH patients and ALD patients, post-LT triglyceride level significantly increased (p < .001). There was a tendency to more often use of statins in the NASH group (27%) than in the ALD group (8%; p = .06). The prevalence of hypertriglyceridemia increased after LT both in NASH patients (pre-LT, 16%; 1-year post-LT, 71%) and ALD patients (Pre-LT, 5%; 1-year post-LT, 40%). The 1-year HbA1c level remained higher in NASH patients than in ALD patients, although the difference did not reach statistical significance (47.6 vs. 41.8 mmol/mol, p = .06). No patients in the NASH group drank alcohol at 1-year post-LT. In the NASH group, seven patients (32%) were diagnosed with moderate or severe macrovesicular steatosis and 11 patients (50%) had perisinusoidal fibrosis. There were no statistical differences regarding histopathological findings including the degree of steatosis and fibrosis between NASH and ALD patient groups.
Table 2. Biochemistry at 3 months after LT.
Table 3. Biochemistry and histological findings at one year after LT.
Risk factors in fatty liver disease development in both groups of patients, ALD and NASH
Univariate logistic regression analysis revealed that recipient age at the time of LT (p = .047), higher BMI at 1-year post-LT (p = .007), and the use of statin (p = .005) were significantly associated with the development of fatty liver disease (). In addition to these three factors, HbA1c at 1-year post-LT (p < .1), were included in multivariate analysis. In the multivariate logistic regression analysis, recipient age and higher BMI remained as independent risk factors for fatty liver disease development. Diabetes, dyslipidemia and graft steatosis at the time of LT were not associated with fatty liver disease development. Regarding subgroup analysis on the ALD cohort, alcohol relapse was not associated with post-LT steatosis (OR, 5.4; p = .10).
Table 4. Risk factors for post-LT development of fatty liver disease in 74 patients with NASH and ALD: univariate and multivariate analyses.
Risk factors of NASH recurrence
Out of 22 patients in the NASH group, nine developed NASH recurrence. Hepatocyte ballooning and lobular inflammation were found in eight and six of nine patients with NASH recurrence, respectively. There were no differences regarding recipient’s and donor’s ages, sex and BMI at the time of LT between patients who developed NASH recurrence and those who did not (). The prevalence of pre-LT insulin-dependent diabetes was significantly higher in patients who developed NASH recurrence than those who did not (89 vs. 31%, p = .009). HbA1c level at 1-year post-LT and the increase of HbA1c level until 1-year post-LT were higher in patients who developed recurrence (HbA1c level at 1-year post-LT, 53 vs. 43 mmol/mol; the increase of HbA1c, 15 vs. 5 mmol/mol), although the differences did not reach statistical significance (HbA1c level at 1-year post-LT, p = .12; the increase of HbA1c, p = .05). The increase in body weight and BMI after LT was comparable between the patients who developed NASH recurrence and those who did not. Among these patients, macrovesicular steatosis of ≥20% at the time of LT was only identified in three patients and was not associated with NASH recurrence. There were no differences regarding the use of statins and mTOR inhibitors between groups with or without NASH recurrence.
Table 5. Comparison of patients who developed NASH recurrence with those who did not.
Discussion
The main findings of our study were as follows: (i) NASH patients, who constituted 4.6% of adult patients who underwent primary LT, presented with higher BMI and higher prevalence of pre-LT diabetes; (ii) 1-year protocol biopsy revealed that as many as half of NASH and ALD patients developed post-LT macrovesicular steatosis and/or fibrosis; (iii) multivariate analysis revealed that older recipient age and higher BMI at 1-year post-LT were independent risk factors for post-LT fatty liver disease development; and (iv) insulin-dependent diabetes was only a factor that was statistically associated with NASH recurrence. HbA1c level at 1-year post-LT and the increase of HbA1c value were higher in patients who developed NASH recurrence than those who did not, but the differences did not reach levels of statistical significance.
In this study, NASH patients constituted nearly 5% of all adult LT patients during the last 10 years. According to the analysis of the United Network for Organ Sharing database, the proportion of NASH-related cirrhosis as an indication increased dramatically from 1.2% in 1997–2003 to 7.4% in 2010 [Citation17]. Although the proportion of NASH-related cirrhosis in our cohort remains relatively lower than that reported from the USA, the proportion and presence of NASH-related cirrhosis are expected to increase, considering that a quarter of adult recipients at our institution, were already obese (BMI ≥30) during the last 5 years. Regarding the rates of NASH recurrence, our study showed a relatively high recurrence rate (41% at 1-year post-LT). In the study by Malik et al. 25% of patients developed NASH recurrence, which was diagnosed based on Brunt’s criteria, at a mean of 18 months [Citation9]. In the study by Bhagat et al., the recurrence rate of NASH was 33% diagnosed using liver biopsy specimens obtained at any time after 6 months post-LT [Citation8]. The difference in recurrence rates between studies was mainly caused by the difference in the patient population included for analysis, the timing of biopsy and the criteria used for diagnosis. In our study, NASH recurrence was diagnosed by histological findings on 1-year protocol biopsy based on Younossi’s criteria, which was recently reported to be closely associated with liver-related mortality [Citation16].
The pre-LT BMI in our patients was 29.4 kg/m2, which was comparable to that in several previous studies by Yalamanchili et al. (29.0 kg/m2) [Citation7] and Contos et al. (28.7 kg/m2) [Citation18]. The prevalence of type 2 diabetes was 40% in our cohorts, which was also within the range shown by previous studies (33–53%) [Citation7,Citation18,Citation19]. Not surprisingly, BMI was significantly higher at any time point in NASH patients than in ALD patients. Regarding the association of BMI and post-LT fatty liver disease development, pre-LT BMI, post-LT BMI and the increase in BMI were reported to be associated with the development of post-LT fatty liver disease and steatohepatitis [Citation14,Citation20,Citation21]. In our study, BMI at 1-year post-LT was an independent risk factor for post-LT fatty liver disease, but pre-LT BMI was not, which indicates that post-LT weight control would have beneficial effects on suppressing steatosis of transplanted livers.
Sprinzl et al. reported that dyslipidemia was an independent risk factor, together with obesity, for post-LT NAFLD development [Citation21]. In our study, pre-LT triglyceride levels were significantly higher in NASH patients compared with ALD patients. However, neither pre-LT nor post-LT triglycerides were associated with post-LT fatty liver disease or NASH recurrence. This inconsistency might be explained by the difference in the etiology of patients included and the definition of dyslipidemia. In Sprinzl’s study, adult patients with all the etiology were included and dyslipidemia was defined by high-density lipoprotein concentration as well as triglyceride level. These results might also be influenced by the use of statins although there were no differences regarding the frequency of statin use between NASH and ALD groups. Since post-LT dyslipidemia is a common complication strongly affected by immunosuppressive medication, the effects of dyslipidemia on the development of NAFLD and NASH need to be further investigated in future studies.
Regarding the NASH recurrence, only pre-LT insulin-dependent diabetes was found to be a risk factor for it. BMI at any time point was not a risk factor for NASH recurrence. Lim et al. showed that higher pre-LT and post-LT BMI and increase in BMI were associated with de novo steatohepatitis [Citation14]. In contrast, Malik et al. reported that BMI at the time of biopsy and the increase of BMI were not associated with NASH recurrence [Citation9]. In their study, pre-LT BMI was inversely associated with NASH recurrence, indicating that the effects of pre-LT obesity on NASH recurrence remain controversial. In our study, BMI was an independent risk factor for fatty liver disease development, but not for NASH recurrence. Conversely, pre-LT diabetes was not associated with post-LT fatty liver disease development. These results indicate that the weight control alone seems to be insufficient to prevent NASH recurrence, although avoiding obesity is a key factor in suppressing fatty liver disease development. In addition to the higher prevalence of insulin-dependent diabetes, the increase in HbA1c level was higher in the NASH recurrence group (p = .05). These results lead to several speculations on the mechanism of post-LT NASH recurrence. Severe hyperglycemia and/or insulin resistance might play a role in the pathogenesis of NASH development. Fluctuation of blood glucose levels related to steroid-induced severe diabetic state treated with insulin injections might have an effect on NASH recurrence. In this study, we were not able to investigate the importance of insulin dependence because all eight of the diabetic patients in the NASH recurrence group were insulin-dependent. All previous studies on risk factors for NASH recurrence including our own study have been performed on relatively small number of patients. Therefore, further studies are needed to elucidate the specific effects of BMI and insulin-dependence on NASH recurrence.
In this study, the rate of patients with hypertriglyceridemia and hypercholesterolemia increased after LT both in NASH and ALD patients. Gisbert et al. showed that the prevalence of dyslipidemia increased after LT, and that hypertriglyceridemia was more common than hypercholesterolemia (hypertriglyceridemia, 59%; hypercholesterolemia, 19%) [Citation22]. Dehghani et al. demonstrated the increased prevalence of hypertriglyceridemia (from 18.2 to 70%) and hypercholesterolemia (from 2.9 to 15.3%) at 6 months post-LT [Citation23]. The result of our study was consistent with the results of these two studies regarding the increased prevalence of hypertriglyceridemia after LT and the fact that hypertriglyceridemia is more common than hypercholesterolemia. On the other hand, the triglyceride level was significantly higher and cholesterol level was significantly lower in NASH patients than ALD patients before and after LT. The explanation for these results was not investigated in this study, mainly due to the small sample size. Therefore, a larger sample size study is needed to address this question further.
This study has some limitations worth mentioning. This was a retrospective single-center study, and pre-LT diagnosis of liver disease was identified based on our registry data and/or clinical records including histopathological findings of explant livers. Due to its retrospective nature, donor and recipient ethnicity was not fully evaluated in this study, although these factors could have some effect on post-LT NASH recurrence [Citation7,Citation16]. Furthermore, the diagnosis of alcohol relapse was partially based on patient self-reports and there was some possibility of missing alcohol relapse in some patients. On the other hand, intraoperative and 1-year post-LT biopsies were routinely performed for all LT recipients, which could be a major advantage of this study in investigating post-LT fatty liver disease development and NASH recurrence.
In summary, higher BMI was shown to be an independent risk factor for post-LT fatty liver disease development, indicating that avoiding post-LT obesity seems to be recommended in order to prevent post-LT steatosis. In contrast, NASH recurrence was not associated with BMI at any time point neither with the increase in post-LT body weight. Insulin-dependent diabetes was only a factor statistically associated with NASH recurrence. According to these results, weight control alone seems to be insufficient in order to prevent NASH recurrence. Optimal glycemic control should be recommended to keep NASH patients from disease recurrence, but further prospective studies are needed in order to prove its preventive effect on NASH recurrence.
Disclosure statement
Kazuaki Tokodai: Concept/design, data collection/analysis/interpretation, statistics, and drafting of the article. Ahmad Karadagi: Data analysis/interpretation, critical revision of the article, and approval of the article. Felicia Kjaernet: Data collection/analysis, critical revision of the article, and approval of the article. Antonio Romano: Data collection/analysis, critical revision of the article, and approval of the article. Bo-Göran Ericzon: Concept/design, critical revision of the article, and approval of the article. Greg Nowak: Concept/design, data analysis/interpretation, statistics, critical revision of the article, and approval of the article.
References
- Younossi ZM, Stepanova M, Afendy M, et al. Changes in the prevalence of the most common causes of chronic liver diseases in the United States from 1988 to 2008. Clin Gastroenterol Hepatol. 2011;9:524–530 e1.
- Vernon G, Baranova A, Younossi ZM. Systematic review: the epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment Pharmacol Ther. 2011;34:274–285.
- Williams CD, Stengel J, Asike MI, et al. Prevalence of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis among a largely middle-aged population utilizing ultrasound and liver biopsy: a prospective study. Gastroenterology. 2011;140:124–131.
- Ong JP, Younossi ZM. Epidemiology and natural history of NAFLD and NASH. Clin Liver Dis. 2007;11:1–16.
- Angulo P. Nonalcoholic fatty liver disease and liver transplantation. Liver Transpl. 2006;12:523–534.
- Holmer M, Melum E, Isoniemi H, et al. Nonalcoholic fatty liver disease is an increasing indication for liver transplantation in the Nordic countries. Liver Int. 2018;38:2082–2090.
- Yalamanchili K, Saadeh S, Klintmalm GB, et al. Nonalcoholic fatty liver disease after liver transplantation for cryptogenic cirrhosis or nonalcoholic fatty liver disease. Liver Transpl. 2010;16:431–439.
- Bhagat V, Mindikoglu AL, Nudo CG, et al. Outcomes of liver transplantation in patients with cirrhosis due to nonalcoholic steatohepatitis versus patients with cirrhosis due to alcoholic liver disease. Liver Transpl. 2009;15:1814–1820.
- Malik SM, Devera ME, Fontes P, et al. Recurrent disease following liver transplantation for nonalcoholic steatohepatitis cirrhosis. Liver Transpl. 2009;15:1843–1851.
- Sourianarayanane A, Arikapudi S, McCullough AJ, et al. Nonalcoholic steatohepatitis recurrence and rate of fibrosis progression following liver transplantation. Eur J Gastroenterol Hepatol. 2017;29:481–487.
- Bhati C, Idowu MO, Sanyal AJ, et al. Long-term outcomes in patients undergoing liver transplantation for nonalcoholic steatohepatitis-related cirrhosis. Transplantation. 2017;101:1867–1874.
- Ong J, Younossi ZM, Reddy V, et al. Cryptogenic cirrhosis and posttransplantation nonalcoholic fatty liver disease. Liver Transpl. 2001;7:797–801.
- Bedossa P, Consortium FP. Utility and appropriateness of the fatty liver inhibition of progression (FLIP) algorithm and steatosis, activity, and fibrosis (SAF) score in the evaluation of biopsies of nonalcoholic fatty liver disease. Hepatology. 2014;60:565–575.
- Lim LG, Cheng CL, Wee A, et al. Prevalence and clinical associations of posttransplant fatty liver disease. Liver Int. 2007;27:76–80.
- El Atrache MM, Abouljoud MS, Divine G, et al. Recurrence of non-alcoholic steatohepatitis and cryptogenic cirrhosis following orthotopic liver transplantation in the context of the metabolic syndrome. Clin Transplant. 2012;26:E505–E512.
- Younossi ZM, Stepanova M, Rafiq N, et al. Pathologic criteria for nonalcoholic steatohepatitis: interprotocol agreement and ability to predict liver-related mortality. Hepatology. 2011;53:1874–1882.
- Afzali A, Berry K, Ioannou GN. Excellent posttransplant survival for patients with nonalcoholic steatohepatitis in the United States. Liver Transpl. 2012;18:29–37.
- Contos MJ, Cales W, Sterling RK, et al. Development of nonalcoholic fatty liver disease after orthotopic liver transplantation for cryptogenic cirrhosis. Liver Transpl. 2001;7:363–373.
- Charlton M, Kasparova P, Weston S, et al. Frequency of nonalcoholic steatohepatitis as a cause of advanced liver disease. Liver Transpl. 2001;7:608–614.
- Seo S, Maganti K, Khehra M, et al. De novo nonalcoholic fatty liver disease after liver transplantation. Liver Transpl. 2007;13:844–847.
- Sprinzl MF, Weinmann A, Lohse N, et al. Metabolic syndrome and its association with fatty liver disease after orthotopic liver transplantation. Transpl Int. 2013;26:67–74.
- Gisbert C, Prieto M, Berenguer M, et al. Hyperlipidemia in liver transplant recipients: prevalence and risk factors. Liver Transpl. 1997;3:416–422.
- Dehghani SM, Taghavi SA, Eshraghian A, et al. Hyperlipidemia in Iranian liver transplant recipients: prevalence and risk factors. J Gastroenterol. 2007;42:769–774.