Abstract
Objectives: Fecal immunochemical test (FIT) is used in colorectal cancer (CRC) screening, but evaluations of multiple sample strategies in colonoscopy screening cohorts are rare. The aim of this study was to assess accuracy of FIT for advanced neoplasia (AN) with two fecal samples in a colonoscopy screening cohort.
Materials and methods: The study comprised 1155 participants of the colonoscopy arm in SCREESCO (Screening of Swedish Colons, NCT02078804), a randomized controlled study on CRC screening of 60-year-olds from the Swedish average-risk population. Participants provided two FIT samples prior to colonoscopy. First sample, mean of two, and any of the two samples above cut off level were assessed. Colonoscopy findings (CRC, advanced adenoma (AA), AN (CRC + AA) and adenoma characteristics) were evaluated in uni- and multivariable analysis in relation to FIT positivity (at ≥10 µg hemoglobin (Hb)/g).
Results: Of 1155 invited, 806 (416 women, 390 men) participated. CRC, AA and non-AA were found in one (0.1%), 80 (9.9%) and 145 (18%), respectively. Sensitivity and specificity for AN were 20%/93%, 25%/92% and 26%/89% for first, mean of two and any of the two samples respectively at cut off level 10 µg/g, corresponding to 60 (74%)–65 (80%) participants with missed AN. The difference in sensitivity between screening strategies was non-significant. The specificity for first sample was significantly higher than for any of the two samples at cut off 10 µg/g (p = .02) and 20 µg/g (p = .04). FIT positivity in participants with adenoma was associated with pedunculated shape (p = .007) and high-risk dysplasia (p = .009).
Conclusions: In an average-risk colonoscopy screening cohort of 60-year-olds, sensitivity for AN was modest and did not increase when using two samples instead of one. FIT predominantly detected adenomas with pedunculated shape and high-risk dysplasia, and participants with flat or broad based adenomas may thus be missed in screening.
Introduction
Colorectal cancer (CRC) is a large global health problem with a better prognosis if discovered at early stage, and screening with guaiac-based fecal occult blood test (gFOBT) has shown to reduce CRC mortality [Citation1].
In many countries, CRC screening with fecal immunochemical test (FIT) is established, typically inviting 50–70 year-olds biennially [Citation2,Citation3]. FIT is a quantitative test for measurement of fecal hemoglobin (f-Hb) that allows for adapting the cut off level for a positive test and hence the follow-up colonoscopy requirements [Citation4]. Choosing cut off level for a positive FIT is a balance between available colonoscopy resources and maximal detection of advanced neoplasia (AN) and varies among screening programs [Citation5,Citation6]. A low cut off level or multiple samples in a screening round leads to a higher sensitivity and lower specificity than a single sample or higher cut off level [Citation4,Citation7]. As colonic lesions may bleed intermittently, there can be a rationale for using two samples [Citation8]. Previous studies on FIT sensitivity and specificity for AN with multiple samples have used a qualitative FIT (Hemosure), were conducted in symptomatic participants, or in colonoscopy screening programs in tertiary care settings [Citation4,Citation9,Citation10]. Other studies on FIT performance with multiple samples were conducted in FIT-positive cohorts [Citation8,Citation11–13].
Colonoscopic removal of precursors to CRC can reduce the CRC incidence [Citation14], thus it is important that advanced adenomas (AAs) are not missed in FIT screening. Previous studies have indicated that accuracy of FIT is dependent on adenoma characteristics such as size, localization, shape, histology [Citation9,Citation15,Citation16] and gender [Citation17]. Age can also affect FIT accuracy [Citation18]. Most of these studies were conducted in populations of mixed age.
Screening of Swedish Colons (SCREESCO) is a randomized population-based study carried out in Sweden since 2014. We have recently published data from a cohort of the participants in the FIT group [Citation19], and we now wanted to explore neoplastic findings in participants with negative FIT.
Thus, the aim of this study was to assess advanced colonoscopy findings and performance of two FIT samples in an age-specific, population-based cohort undergoing colonoscopy screening, and to evaluate adenoma characteristics in those with positive and negative FIT at a low cut off.
Methods
The study is part of the ongoing SCREESCO (NCT02078804) trial which is a population-based national randomized controlled study on mortality in CRC, comparing screening with colonoscopy vs. FIT or non-screening [Citation20]. The study comprises in total 283,000 60-year-olds, and all Swedish counties participate except Stockholm-Gotland and Västernorrland. No exclusion criteria were applied other than previous CRC or procto-colectomy.
In the FICO (FIT-colonoscopy) study, 1155 persons who had accepted participation in the colonoscopy arm of SCREESCO were invited to send in two FIT. Inclusion period was one year starting from March 2016.
FIT
The participant was instructed to provide two FIT (O.C. Sensor Diana, Eiken, Tokyo, Japan) samples from two consecutive bowel movements prior to colonoscopy and bowel preparation. The date of the sample was registered by the participant. The participants were further requested to store the test tubes in refrigerator and as soon as possible return them by mail to the study laboratory, where they were developed according to the manufacturer’s instructions. If the two samples were despite the instructions taken on the same date, a random sample was chosen as the first sample at the laboratory unit.
Endoscopy
The colonoscopy was carried out in one of the 33 endoscopy units participating in SCREESCO, and performed by a gastroenterologist, surgeon or endoscopist nurse with a self-reported experience of at least 1000 procedures, a minimum of 100 endoscopies per year and a caecal intubation rate of at least 90%. Bowel preparation was done with Laxabon®. Colonoscopy quality parameters, i.e., bowel preparation according to the Boston scale, caecal intubation, caecal withdrawal time, were recorded in the study database. Caecum to splenic flexure was defined as proximal and descendens to rectum as distal. If the colonoscopy was incomplete due to poor bowel preparation, the participant was rescheduled for a new examination, and if incomplete due to for example luminal stricture the participant was offered CT colonography. At the endoscopy unit, the participant also completed a questionnaire on current medication and weekly dosage of non-steroidal inflammatory drugs (NSAIDs) and acetylsalicylic acid (ASA), and the height and weight were measured.
Classification of findings
The participant was classified according to the most advanced lesion. Findings at colonoscopy were registered in the study database and polyps and biopsies from suspected malignancies sent for histopathological examination. Polyps were registered according to number, macroscopic size (<5 mm, 5–9 mm, 10–19 mm and ≥20 mm), anatomical localization in colon, shape (flat/broad based or pedunculated), method of removal, and the histopathological classification (high grade or low grade dysplasia (LGD), tubular, tubulovillous or villous architecture or sessile serrated adenoma (SSA) with or without dysplasia) was reported.
CRC was defined as invasion beyond muscularis mucosae. Advanced adenoma was defined as adenomas ≥10 mm, with high grade dysplasia (HGD) or presence of dysplasia in a SSA, villous or tubulovillous growth or ≥3 adenomas without advanced features. Advanced neoplasia was CRC and AA. Non-advanced adenoma (non-AA) was defined as ≤2 tubular adenomas <10 mm with LGD or SSA <10 mm without dysplasia. Adenomas with HGD or SSA with dysplasia were defined as high-risk dysplasia. All adenoma and CRC findings were verified against the pathology report. Macroscopic polyp size was given precedence over histology, thus hyperplastic polyps (HPs) <10mm were classified as normal colonoscopy, and HP ≥10 mm were included in the analysis. Other findings included hemorrhoids, diverticular disease, inflammation or angiodysplasia. Participants with only proximal or distal lesions were classified as such, whereas those with both proximal and distal lesions were classified as ‘both’.
Statistical analysis
Findings at colonoscopy were evaluated at cut off levels 10, 20, 40, 60 and 80 µg hemoglobin (Hb)/g for the first FIT sample, any of the two samples and mean of two samples. Confidence intervals for positivity, sensitivity and specificity were calculated using the Clopper–Pearson method. Associations between binary variables were assessed by Chi2-test and McNemar’s test, and Mann–Whitney’s U test was used for continuous variables (total number of adenomas). The positive predictive value (PPV) for AN was defined as the proportion of participants with AN among FIT positives, and the negative predictive value (NPV) as the proportion of FIT-negatives among participants without AN. The false negative rate was defined as the proportion of negatives among participants with AN (1 – sensitivity). The false positive rate was defined as the proportion of positives among those without AN (1 – specificity).
For those with adenoma, a multivariable logistic regression analysis was conducted to assess what covariates are associated with positive FIT test. In this analysis, a cut off of 10 µg Hb/g for any of the two FIT sample was used. The included covariates were: localization (proximal, distal or both), total number of adenomas, histology (tubular, tubulovillous/villous, SSA or synchronous SSA and tubular adenoma), shape (flat/broad based or pedunculated), size, grade of dysplasia (high- or low-risk dysplasia) and gender. For the categorical variables with more than two categories, a Wald test was used to test all categories at once.
The regional ethics committee approved the study (ethical approval number 2012/2058-31/3) and informed consent was considered when participants sent in FIT-samples.
Results
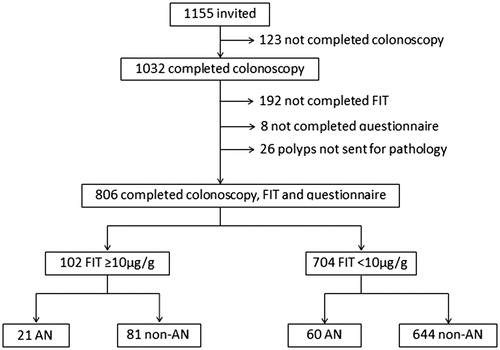
Of 1155 invited to participate in FICO, 806 completed colonoscopy, questionnaire and FIT ().
Figure 1. Flowchart of study participants. AN: advanced neoplasia (adenocarcinoma and advanced adenoma); non-AN: non-advanced adenoma, other findings or normal colonoscopy.
FIT
Of 806 participants, six sent only one FIT sample and were therefore excluded from calculations regarding mean FIT. Forty-eight (6.0%) participants had the same date on both tests and were included in the analysis, a random sample of the two was used as first sample. FIT ranged from 0.0 to 2187 µg/g. Of 806 participants, 102 (12.7%) had ≥10 µg/g in at least one of two samples; 46 of 416 women (11%) and 56 of 390 men (14%) ().
Table 1. Basic variables in 806 colonoscopy screening participants.
Findings
In 806 participants (390 men and 416 women) CRC, AA and non-AA was found in one (0.1%), 80 (9.9%) and 145 (18%), respectively. The CRC was a rectal cancer pT3bN1b. Details of 806 participants and of the 80 participants with AA are listed in , with separate columns for those that were FIT negative (both FIT samples <10 µg/g) and positive. As is seen in , 60 (8.5%) with AA and 131 (19%) with non-AA were FIT-negative.
FIT performance
FIT positivity rate ranged from 1.6 (0.7–2.5) to 12.7 (10.4–15.0) %, depending on cut off level and number of samples (). Sensitivity and specificity for AN ranged from 7.4–25.9% to 88.8–99.0%, respectively, the most sensitive being at least one of two samples ≥10 µg/g, and the most specific first sample at cut off ≥60 µg/g (). For each cut off level, there was no significant difference in sensitivity for first, mean of two or any of the two samples (p>.05). Specificity was higher for first sample than any of the two samples at cut off 10 µg/g (p = .02) and 20 µg/g (p = .04) (). Thirty participants had both FIT samples ≥10 µg/g: one with CRC, 11 with AA and 18 with non-AN, corresponding to a sensitivity of 14.8% and a specificity of 97.5% for AN (data not shown).
Table 2. Sensitivity and specificity for advanced neoplasia at different fecal immunochemical test (FIT) cut off levels and number of samples in 806 screening participants.
The PPV for AN was between 21 and 52% depending on cut off level and number of samples used, and the NPV was 91–92% (). For different cut off levels and number of samples, 60 (74%)–75 (93%) participants with AN were missed ().
Adenoma characteristics
Adenoma (AA or non-AA) was diagnosed in 225 (28%) participants, of whom 160 (20%) had only one adenoma (). Applying a screening strategy with at least one of two samples at cut off 10 µg/g for a positive test, a significantly higher proportion of distal, pedunculated, large, HGD adenomas and adenomas in men were FIT positive in univariate analysis (). Those with tubular adenomas were FIT positive in 11%, SSA in 9% and small adenomas (<10 mm) in 10% (). In those with AA, FIT was more often positive in men than in women (, p = .021). The sensitivity for AA was 34.8% (21.4–50.3) and 11.8% (3.3–27.5) (p = .021), and specificity was 88.4% (84.5–91.6) and 89.0% (85.4–92.0) (p = .8) in men and women, respectively.
Table 3. Univariate analysis of adenoma characteristics and FIT positivity in 225 colonoscopy screening participants with adenomas, of which 160 had only one adenoma.
In multivariable analysis of participants with adenoma pedunculated shape, high-risk dysplasia and male gender were independently associated with FIT positivity (). Among those with adenoma, more men than women were on ASA medication 13 vs. 3, p = .018. A sensitivity analysis was made on 198 participants (data not shown) excluding those with ASA or NSAID medication, and high-risk dysplasia and pedunculated shape remained associated with FIT positivity, but not gender.
Table 4. Odds ratio for FIT positivity (at least one of two samples ≥10 µg hemoglobin/g) in 225 screening participants with adenoma.
Discussion
This study on average-risk 60-year-old colonoscopy screening participants and two FIT samples showed that FIT detected a minority of those with AN, with a sensitivity of 20–26% at a specificity of 89–93% at cut off 10 µg/g depending on number of samples used. At cut off 10 µg/g for any of the two samples, a significantly higher rate of adenomas in men and adenomas with high-risk dysplasia or pedunculated shape were FIT positive.
FIT performance
In our cohort, there was only one CRC case, corresponding to 0.1%, which is within the same range as in other colonoscopy screening studies [Citation21–23]. Our results on FIT sensitivity of 20% for AN for the first sample at cut off ≥10 µg/g and 15% at cut off 20 µg/g are lower than previous studies from the Netherlands (38% at cut off 10 µg/g) and from Korea (32% for one test at cut off 20 µg/g) [Citation4,Citation7]. Taking the any of the two samples in consideration raised sensitivity to 24% at cut off 20 µg/g in our study as compared to 38% in the Korean study.
The main reason for the lower number in our study may be the difference in study population. It is known that FIT sensitivity is lower in women than in men [Citation17], and the aforementioned studies included a higher rate of men than ours (51% compared to 48%). Another explanation might be related to the younger age of the participants in our study (60-year-olds). Morikawa et al. [Citation24] found that FIT sensitivity (Magstream 1000/Hem SP, Fujirebio) for small adenomas (<10 mm) increased with age in men, but possibly due to other patient related factors than the age itself. Some previous studies have shown a higher FIT sensitivity for participants with ASA and/or non-steroidal anti-inflammatory drugs (NSAIDs) medication, because ASA/NSAID-exposed adenomas may be more prone to bleeding [Citation25,Citation26]. In a younger cohort, the rate of ASA-users is likely to be lower than in studies on participants aged up to 75 years; indeed in the Dutch cohort the reported ASA medication rate was 16% as compared to 8% in the present study [Citation18]. Moreover, the adenoma characteristics differ between study populations and might influence test performance: the rate of pedunculated adenomas was 11% (24 of 225), that of large adenomas (≥10 mm) was 6% (49 of 806), and that of distal AAs was 33% (26 of 80) in the present study, which is lower than in previous studies [Citation4,Citation7,Citation15,Citation27].
Another circumstance that could affect test performance is the difference in study settings: the Korean study at four tertiary medical centers, the Dutch at two centers with colonoscopy performed by gastroenterologists and polyps evaluated by expert GI pathologists, and the present at 33 different endoscopy units throughout the country with varying uptake, colonoscopist and pathologist specialization. However, the AN detection rate was comparable between cohorts, and a high rate of caecal intubation and clean bowel was achieved.
Typically, FIT screening programs have a positivity rate of 2–5% [Citation6,Citation13]. In our cohort, this would correspond to cut off levels 20–40 for first sample, 20–60 for mean of two, or 40–80 for any of the two samples. The modest sensitivity in our study yield a false negative rate of 84–90% for participants with AN and a false positive rate of 1–3% at 2–5% positivity. Applying a screening strategy with cut off 10 µg/g for any of the two samples, the number of participants with missed ANs is lowered to 60 (74%), but at the expense of a FIT positivity rate of 12.7% and a false positive rate of 11%. In our study, there was no significant gain in sensitivity adding one sample, but instead a loss in specificity at low cut off levels, which would advocate a one sample screening strategy. Although a high rate of false negatives, missed lesions could be detected with biennial or annual repeated testing. In a previous randomized study, the cumulative AN detection over repeated screening rounds was similar between one-sample and two-sample strategy, likewise concluding that the one-sample strategy was to prefer [Citation11].
Adenoma characteristics
In our study with two samples at low cut off, there was a significantly higher rate of FIT-positive pedunculated adenomas as compared to flat or broad based. One cause for FIT to be positive in these lesions could be that the protruding shape is exposed to shear stress from stool and bowel movements and hence more prone to bleeding. Haug et al. [Citation15] evaluated single sample FIT (Ridascreen) and also found that test sensitivity was associated with pedunculated shape. We did not find an independent association between adenoma localization and FIT positivity, and the results from previous studies are diverse. Some have found a higher sensitivity for distal than proximal neoplasias [Citation9,Citation16] but in the previously cited German study only for low cut off values (at specificities <90%) [Citation15]. Others have found an equal sensitivity for left- and right-sided neoplasias [Citation7]. Rozen et al. [Citation10] found that FIT-level (OC Sensor, Eiken, Tokyo, Japan) correlated to size and number of adenomas, but not to distal or proximal localization. In the Korean study, the mean FIT was higher in proximal than in distal neoplasia, but in their cohort the proximal lesions were larger than the distal lesions [Citation4].
In our study, applying a low cut off and two FIT samples allows for detection of high risk adenomas that according to Swedish polyp surveillance program require follow-up colonoscopy [Citation28]; HGD and dysplasia in SSA – defined as high-risk dysplasia, was independently associated to FIT positivity. Similar, Morikawa et al. [Citation16] found that FIT (Magstream) detected HGD adenomas at a higher sensitivity than large adenomas, especially in proximal colon. In FIT-positive cohorts on the other hand, previous studies have found that grade of dysplasia is associated to size rather than to FIT level itself [Citation5,Citation6,Citation19]. There was a tendency towards an association between adenoma size and FIT positivity in our study, but it did not reach statistical significance in multivariable analysis probably due to small sample size, as only 34 participants with adenoma were FIT positive at the chosen cut off.
Previous studies have shown a low sensitivity for FIT in detecting SSA [Citation29] and small adenomas [Citation24], in line with our findings that about 10% of participants with small adenomas or SSA are FIT positive.
Significantly more women with AA were FIT negative; only 12% of women with AA were FIT positive, and FIT sensitivity was significantly lower in women than in men at cut off 10 µg/g. When taking all adenomas into account, gender was independently associated with FIT positivity. A possible explanation for this could be that in those with adenomas, ASA medication was more common in men. In fact when restricting the analysis to those without ASA and NSAID medication, gender was no longer associated with FIT positivity in those with adenomas. We did not find an association of FIT positivity and total number of adenomas which was another adenoma characteristic that differed between gender (Supplementary Table 1). Neither could the lower rate of FIT positives among women with AA be explained by adenoma size, as there was an equal distribution of adenoma size in men and women (Supplementary Table 1), in contrast to previous studies [Citation17].
Previous studies on gender differences in FIT screening showed higher prevalence of AN in men, and an equal performance in both gender measured as PPV [Citation30]. Equal performance is also supported by evaluation of multiple rounds of FIT screening programs in which the rate of FIT interval CRCs did not significantly differ by gender [Citation31,Citation32].
Strengths and limitations
The strengths are that the study is nationwide and population-based and that participants underwent FIT testing and colonoscopy simultaneously, which enabled evaluation of FIT negatives.
This study has limitations. The small sample size makes conclusions regarding some subgroups uncertain. In the cohort, only one CRC was detected, thus we were unable to make separate analysis of CRC and FIT accuracy. Also the setting of the study with 33 colonoscopy units and different dedication of endoscopists and pathologists is not optimal for evaluation of all polyps, especially detection and diagnosis of serrated and proximal polyps, but rather mimics a screening situation when implemented nationally in Sweden.
Conclusions
In an average-risk colonoscopy screening cohort of 60-year-olds, sensitivity for AN was modest and did not significantly increase when adding a second sample. FIT predominantly detected adenomas with pedunculated shape and high-risk dysplasia, and participants with flat or broad based adenomas may thus be missed in FIT screening.
Supplemental Material
Download PDF (46.3 KB)Acknowledgements
Eiken Chemical generously supported the study with test kits and materials for analysis. Eva Berglund, Research Nurse, and Kristina Eckes, Biomedical Scientist, are thanked for practical assistance with the study.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Holme O, Bretthauer M, Fretheim A, et al. Flexible sigmoidoscopy versus faecal occult blood testing for colorectal cancer screening in asymptomatic individuals. Cochrane Database Syst Rev. 2013;(9):CD009259. DOI:10.1002/14651858.CD009259.pub2.
- Altobelli E, D'Aloisio F, Angeletti PM. Colorectal cancer screening in countries of European Council outside of the EU-28. World J Gastroenterol. 2016;22:4946–4957.
- Benson VS, Atkin WS, Green J, et al. Toward standardizing and reporting colorectal cancer screening indicators on an international level: the International Colorectal Cancer Screening Network. Int J Cancer. 2012;130:2961–2973.
- Park DI, Ryu S, Kim YH, et al. Comparison of guaiac-based and quantitative immunochemical fecal occult blood testing in a population at average risk undergoing colorectal cancer screening. Am J Gastroenterol. 2010;105:2017–2025.
- Ciatto S, Martinelli F, Castiglione G, et al. Association of FOBT-assessed faecal Hb content with colonic lesions detected in the florence screening programme. Br J Cancer. 2007;96:218–221.
- Digby J, Fraser CG, Carey FA, et al. Faecal haemoglobin concentration is related to severity of colorectal neoplasia. J Clin Pathol. 2013;66:415–419.
- de Wijkerslooth TR, Stoop EM, Bossuyt PM, et al. Immunochemical fecal occult blood testing is equally sensitive for proximal and distal advanced neoplasia. Am J Gastroenterol. 2012;107:1570–1578.
- van Roon AH, Wilschut JA, Hol L, et al. Diagnostic yield improves with collection of 2 samples in fecal immunochemical test screening without affecting attendance. Clin Gastroenterol Hepatol. 2011;9:333–339.
- Wong MC, Ching JY, Chan VC, et al. Diagnostic accuracy of a qualitative fecal immunochemical test varies with location of neoplasia but not number of specimens. Clin Gastroenterol Hepatol. 2015;13:1472–1479.
- Rozen P, Levi Z, Hazazi R, et al. Identification of colorectal adenomas by a quantitative immunochemical faecal occult blood screening test depends on adenoma characteristics, development threshold used and number of tests performed. Aliment Pharmacol Ther. 2009;29:906–917.
- Kapidzic A, van Roon AH, van Leerdam ME, et al. Attendance and diagnostic yield of repeated two-sample faecal immunochemical test screening for colorectal cancer. Gut. 2017;66(1):118-123.
- Guittet L, Bouvier V, Mariotte N, et al. Performance of immunochemical faecal occult blood test in colorectal cancer screening in average-risk population according to positivity threshold and number of samples. Int J Cancer. 2009;125:1127–1133.
- Grazzini G, Visioli CB, Zorzi M, et al. Immunochemical faecal occult blood test: number of samples and positivity cutoff. What is the best strategy for colorectal cancer screening? Br J Cancer. 2009;100:259–265.
- Citarda F, Tomaselli G, Capocaccia R, et al. Efficacy in standard clinical practice of colonoscopic polypectomy in reducing colorectal cancer incidence. Gut. 2001;48:812–815.
- Haug U, Kuntz KM, Knudsen AB, et al. Sensitivity of immunochemical faecal occult blood testing for detecting left- vs right-sided colorectal neoplasia. Br J Cancer. 2011;104:1779–1785.
- Morikawa T, Kato J, Yamaji Y, et al. A comparison of the immunochemical fecal occult blood test and total colonoscopy in the asymptomatic population. Gastroenterology. 2005;129:422–428.
- Brenner H, Haug U, Hundt S. Sex differences in performance of fecal occult blood testing. Am J Gastroenterol. 2010;105:2457–2464.
- Stegeman I, de Wijkerslooth TR, Stoop EM, et al. Risk factors for false positive and for false negative test results in screening with fecal occult blood testing. Int J Cancer. 2013;133:2408–2414.
- Wilen HR, Blom J, Hoijer J, et al. Fecal Immunochemical test (FIT) in colorectal cancer screening colonoscopy findings by different cut-off levels. J Gastroenterol Hepatol. 2019;34(1):103-112.
- Rolf Hultcrantz. Available from: https://clinicaltrials.gov/ct2/show/NCT02078804.
- Quintero E, Castells A, Bujanda L, et al. Colonoscopy versus fecal immunochemical testing in colorectal-cancer screening. N Engl J Med. 2012;366:697–706.
- Bretthauer M, Kaminski MF, Loberg M, et al. Population-based colonoscopy screening for colorectal cancer: a randomized clinical trial. JAMA Intern Med. 2016;176:894–902.
- Stegeman I, de Wijkerslooth TR, Stoop EM, et al. Combining risk factors with faecal immunochemical test outcome for selecting CRC screenees for colonoscopy. Gut. 2014;63:466–471.
- Morikawa T, Kato J, Yamaji Y, et al. Sensitivity of immunochemical fecal occult blood test to small colorectal adenomas. Am J Gastroenterol. 2007;102:2259–2264.
- Brenner H, Tao S, Haug U. Low-dose aspirin use and performance of immunochemical fecal occult blood tests. JAMA. 2010;304:2513–2520.
- Levi Z, Rozen P, Hazazi R, et al. Sensitivity, but not specificity, of a quantitative immunochemical fecal occult blood test for neoplasia is slightly increased by the use of low-dose aspirin, NSAIDs, and anticoagulants. Am J Gastroenterol. 2009;104:933–938.
- Stoop EM, de Haan MC, de Wijkerslooth TR, et al. Participation and yield of colonoscopy versus non-cathartic CT colonography in population-based screening for colorectal cancer: a randomised controlled trial. Lancet Oncol. 2012;13:55–64.
- Thorlacius H, Björk J, Öst Å, et al. Available from: http://www.lakartidningen.se/Klinik-och-vetenskap/Klinisk-oversikt/2017/05/Riktlinjer-for-endoskopisk-kontroll-efter-kolorektal-polypektomi/
- Chang LC, Shun CT, Hsu WF, et al. Fecal immunochemical test detects sessile serrated adenomas and polyps with a low level of sensitivity. Clin Gastroenterol Hepatol. 2017;15(6):872-879.e1.
- Kapidzic A, van der Meulen MP, Hol L, et al. Gender differences in fecal immunochemical test performance for early detection of colorectal neoplasia. Clin Gastroenterol Hepatol. 2015;13:1464–1471e4.
- van der Vlugt M, Grobbee EJ, Bossuyt PMM, et al. Interval colorectal cancer incidence among subjects undergoing multiple rounds of fecal immunochemical testing. Gastroenterology. 2017;153:439–447e2.
- Digby J, Fraser CG, Carey FA, et al. Interval cancers using a quantitative faecal immunochemical test (FIT) for haemoglobin when colonoscopy capacity is limited. J Med Screen. 2016;23:130–134.
