Abstract
Objectives: Crohn’s disease is characterized by a gut dysbiosis with decreased abundance of butyrate producers such as Faecalibacterium prausnitzii. Although F. prausnitzii secretes anti-inflammatory molecules, few studies have addressed the importance of F. prausnitzii in a longitudinal setting. We aimed to examine the relationship between temporal profiles of F. prausnitzii, the C. leptum group, overall butyrate production, and inflammatory activity.
Material and methods: Fecal samples (n = 59) were collected every third month from nine patients with ileal Crohn’s disease. The abundance of F. prausnitzii and C. leptum was quantified relative to the total amount of bacteria using quantitative-PCR. To assess butyrate production of gut microbiota, gene copy numbers of the butyryl-CoA:acetate-CoA transferase (BCoAT) gene were quantified by qPCR. The inflammatory activity was defined by fecal (f)-calprotectin.
Results: No correlation between the relative abundance of F. prausnitzii, the C. leptum group, or copy numbers of the BCoAT gene, and f-calprotectin was observed in the total sample set. By analyzing alterations between consecutive samples, a negative correlation between changes in the relative abundance of F. prausnitzii and f-calprotectin was observed (R = −0.39; p = .009). Changes in C. leptum (R = −0.18, p = .23) and number of copies of the BCoAT gene (R = −0.12; p = .42) did not correlate with f-calprotectin.
Conclusions: There was an inverse correlation between temporal changes in the relative abundance of F. prausnitzii, but not overall butyrate producing capacity, and changes in inflammatory activity in ileal Crohn’s disease. These findings indicate that F. prausnitzii may play a role in gut homeostasis, even though causality is still to be demonstrated.
Introduction
Crohn’s disease is a complex immune-mediated disease characterized by chronic gastrointestinal inflammation. The disease is associated with periods of remission and episodes of increased inflammatory activity, i.e. flares in the disease. The inflammation is driven by complex interactions between genetic predisposition and exposure to environmental factors. It has long been postulated that the gut microbiome plays a key role in the pathogenesis of the disease [Citation1]. Recent advances have revealed that the disease is characterized by a dysbiosis with reduced diversity [Citation2]. Although it is evident that the microbiome is an important factor in disease pathogenesis [Citation3,Citation4], it still remains to be determined whether the inflammation is induced by specific bacterial taxa, and the causative mechanisms are yet to be defined [Citation5,Citation6].
We recently published a longitudinal study demonstrating an increased volatility of the gut microbiome in patients with IBD [Citation2]. However, we were not able to identify any significant correlations between inflammatory activity and specific taxa based on 16S rRNA sequencing. Several studies indicate that 16S profiling is less sensitive for quantification of specific bacterial species compared to quantitative-PCR (qPCR), especially for low-abundant species [Citation7–9].
A reduced abundance of several butyrate producing taxa within the Clostridium leptum (C. leptum) group, such as Eubacterium spp., Ruminococcus spp., and Faecalibacterium prausnitzii (F. prausnitzii), has been observed in patients with Crohn’s disease and especially in those with ileal disease [Citation3,Citation10]. Butyrate is a short-chain fatty acid synthesized from non-absorbed carbohydrates and constitutes the main energy source for the epithelial cells in the gut. Interestingly, in vitro studies have shown that butyrate also inhibits intestinal inflammation by nuclear factor κB (NF-κB) inhibition and by regulation of oxidative stress, mucosal barrier function, and transepithelial fluid transportation [Citation11,Citation12]. Although butyrate may be produced by many different bacteria, most of them generate butyrate through the butyryl-CoA pathway [Citation13]. Quantification of the butyryl-CoA:acetate-CoA transferase (BCoAT) gene by qPCR may, therefore, be used as a proxy of the overall butyrate producing capacity [Citation13]. Recently, Laserna-Mendieta et al. used this approach to show that Crohn’s disease is associated with a reduced butyrate producing capacity relative to healthy controls [Citation14].
F. prausnitzii produces high amounts of butyrate and has been identified as a key player in ileal Crohn’s disease-associated dysbiosis. Intriguingly, low abundance of F. prausnitzii has been associated with an increased risk of future flares [Citation15]. Although the literature suggests that F. prausnitzii and butyrate production are important regulators of intestinal inflammation in Crohn´s disease, few studies have addressed these aspects from a longitudinal point of view [Citation16,Citation17].
We hypothesized that alterations in the abundance of F. prausnitzii, the C. leptum group or overall gut microbiota butyrate production, correlate with changes in inflammatory activity in patients with ileal Crohn’s disease.
Therefore, we aimed to investigate the relationship between temporal profiles of F. prausnitzii, temporal profiles of the C. leptum group, overall gut microbiota butyrate production, and the inflammatory activity of Crohn’s disease, by analyzing consecutively collected fecal samples from a cohort of patients with ileal Crohn’s disease who were followed over time.
Materials and methods
Study population and data collection
Patients with ileal Crohn’s disease in the present study were included from a previously described cohort of IBD patients [Citation18]. In short, those patients who attended the outpatient colitis clinic, Örebro University Hospital, Sweden were invited to participate. The diagnosis of Crohn’s disease was based on internationally accepted clinical, endoscopic, radiological, and histological criteria [Citation19]. The Montreal classification was used to define disease phenotype [Citation20].
After written informed consent had been obtained, patients were asked to answer a questionnaire on patient-reported outcomes, IBD-related medication, and the use of antibiotics, and also to provide a fecal sample. The patients were then followed prospectively for up to two years and were asked to provide a fecal sample and answer the questionnaire every third month. The fecal samples were collected by the patients in sterile plastic containers, sent the same day by mail to the study center, and stored at −80 °C until extraction. To reduce the risk of a delay, patients were instructed to only collect fecal samples during the first three days of the week (Monday, Tuesday or Wednesday). In general, samples were estimated to arrive to the study center within 24 h. To examine the inflammatory activity, fecal calprotectin was extracted and analyzed after all patients had ended the follow‐up, i.e. after all samples had been collected. In total, 59 fecal samples were obtained from nine patients. The medical records were scrutinized retrospectively to identify any non-self-reported use of antibiotics. To examine the relationship between temporal profiles of microbiota-related variables and inflammation, we selectively included patients with ileal Crohn’s disease who had provided fecal samples on more than four occasions and had shown fluctuating f-calprotectin concentrations over time.
Study design
This was a longitudinal study to investigate whether specific alterations in the microbial composition over time were associated with disease activity. We specifically measured the relative abundances of F. prausnitzii and of the C. leptum group in each sample. Correspondingly, copy numbers of the BCoAT gene were measured and used as a proxy of overall butyrate producing capacity. To assess the deviation of the microbial composition from the average composition in healthy individuals, available information on variation of the gut microbiome from the healthy state (i.e. “distance to the healthy plane”), was obtained [Citation2]. Alterations in the microbiota-related variables, i.e. relative abundance of F. prausnitzii and the C. leptum group, and copy numbers of the BCoAT gene, between samples were correlated with changes in the inflammatory activity, using f-calprotectin levels (EK-CAL; Bühlmann Laboratories AG, Schönenbuch, Switzerland) as a proxy of inflammatory activity. To contextualize the findings, “distance to the healthy plane” and the Shannon diversity index were correlated to the study variables post-hoc.
DNA extraction
A modified version of the “Repeated Bead Beating plusColumn” method was used for DNA extraction [Citation21]. Briefly, the extraction was performed according to the protocol with the modification that samples were stored at −20 °C overnight before the purification process was performed, which included removal of RNA and proteins. After extraction, DNA concentrations and absorbance ratios at different wavelengths were measured with a NanoDrop 2000 spectrophotometer using the software NanoDrop ND-1000 v.3.3 (Coleman Technologies Inc, Orlando, FL, USA).
qPCR
All samples were amplified in triplicate on an Applied Biosystems 7900HT Fast Real-Time PCR system (Life Technologies). The Hot FIREPol EvaGreen qPCR Supermix was used for detection of PCR products. Fifty nanograms of template DNA was used per PCR reaction. The primer sequences for F. prausnitzii [Citation22], C. leptum [Citation23], the BCoAT gene [Citation13], and Eubacteria [Citation24] are described elsewhere. Information on thermal profiles is given in . Absolute quantifications of the BCoAT gene copy numbers were measured from a standard curve as previously described [Citation13]. F. prausnitzii and the C. leptum group were measured in relation to the total bacterial abundance using the ΔΔ-method [Citation25,Citation26].
Table 1. Thermal profiles for quantitative PCR.
Distance to the healthy plane
Distance to the healthy plane is a newly developed metric to assess the degree to which an individual’s microbial composition deviates from the normal microbial variation in healthy individuals [Citation2].
For the present study, we obtained previously published data on distance to the healthy plane for the same subjects. However, measurements of distance to the healthy plane were only available for the first four time points for each patient [Citation2].
Shannon index
After removing non-bacteria taxa and subsampling to an even sequencing depth, the Shannon diversity was calculated [Citation27,Citation28].
Statistics
To assess the relative abundance of F. prausnitzii and the C. leptum group, the fold change for each respective target sequence was calculated in relation to the total presence of bacteria, as previously described [Citation25]. Absolute quantification of the BCoAT gene was performed from a standard curve according to the procedure of Louis et al. [Citation13].
Samples from each patient were collected at inclusion and then at 3-month intervals until the end of follow-up, i.e. at two years, resulting in time-series data with a maximum of nine time-points per patient. After visual inspection of the scatter plots, we decided to utilize the Pearson’s correlation coefficient to calculate the linear relationship between variables in the total sample set.
To examine the dynamic relationship of the microbiota-related variables and f-calprotectin, Δ values between adjacent time-points were assessed for all variables. Time-points were defined as adjacent if the duration between two subsequent samples was within 60‒120 days. Samples that did not meet the criterion for adjacency were excluded from all analyses of Δ values. Correlations between changes in microbiota-related variables and f-calprotectin levels were assessed using Pearson’s correlation coefficient for each set (pair) of variables.
In order to preclude bias, a sensitivity analysis was conducted in which all samples obtained within 4 weeks from prescription of antibiotics or from initiation of a new IBD-treatment (i.e. oral aminosalicylates, systemic corticosteroids, thiopurines, or biologics) were excluded.
Any p-value<.05 was considered statistically significant. R was used for the analysis of the Shannon index (phyloseq and vegan packages). GraphPad Prism v.7.0 was used for all other statistical analyses.
Ethical considerations
The study was approved by the Regional Ethics Committee (2007-291).
Results
Cohort of Crohn’s disease patients
The basic demographics and clinical characteristics of the cohort are presented in . Each patient provided fecal samples at 4‒9 time points during follow-up, giving a mean follow-up time of 19 months. The mean alteration in f-calprotectin concentrations between subsequent time points was 194.8 µg/g, detailed information on temporal alterations in f-calprotectin is provided inSupplementary Figure 5.
Table 2. Demographics and clinical characteristics of patients (n = 9) with Crohn´s disease.
F. prausnitzii and the C. leptum group were associated with the capacity of the microbiota to produce butyrate
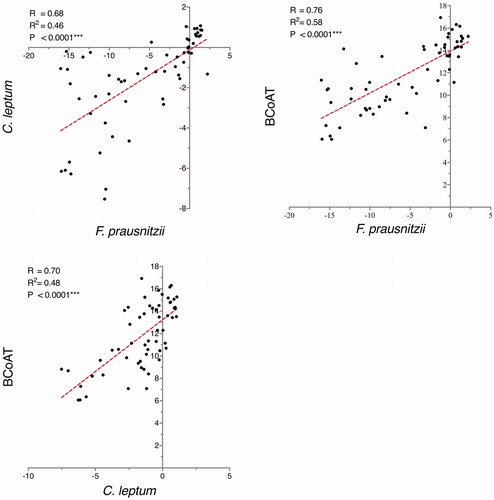
There was a correlation between the relative abundance of F. prausnitzii and the copy numbers of the BCoAT gene (R = 0.76; p < .0001, ). Furthermore, the relative abundance of the C. leptum group, representing several butyrate producers (including F. prausnitzii) correlated with the copy numbers of the BCoAT gene (R = 0.70; p < .0001). Consistently, there was a correlation between the relative abundance of F. prausnitzii and the relative abundance of the C. leptum group (R = 0.68; p < .0001).
Figure 1. Correlations between the abundance of F. prausnitzii and the C. leptum group and the copy numbers of the butyryl-CoA:acetate-CoA (BCoAT) transferase gene. The abundance of F. prausnitzii and the C. leptum group are expressed as fold change in relation to the total bacterial count, while BCoAT is presented as gene copy numbers. F. prausnitzii, the C. leptum group, and BCoAT are presented on the log-2 scale.
Comparisons of microbiota-related variables and inflammation
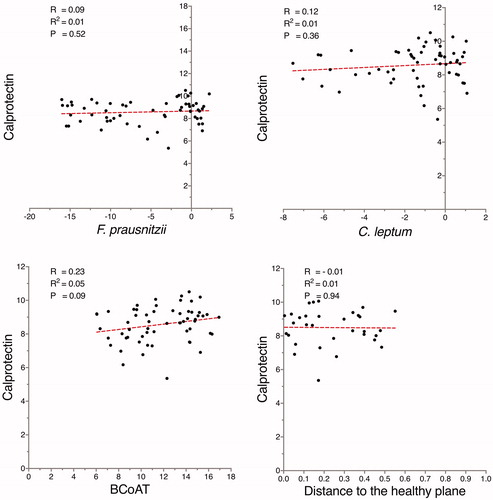
The concentration of f-calprotectin did not correlate with either the relative abundance of F. prausnitzii or the relative abundance of the C. leptum group, () Correspondingly, the distance to the healthy plane, the Shannon index and the copy numbers of the BCoAT gene did not correlate with the levels of f-calprotectin ( and Supplementary Figure 6).
Figure 2. When analyzing the total sample set, no correlations were found between the microbiota-related variables and calprotectin (µg/g). The abundance of F. prausnitzii and the C. leptum group are expressed as fold change in relation to the total bacterial count, while butyryl-CoA:acetate-CoA transferase (BCoAT) is presented as gene copy numbers. F. prausnitzii, the C. leptum group, BCoAT, and calprotectin are presented on the log-2 scale.
Dynamic comparisons of microbiota-related variables and inflammation
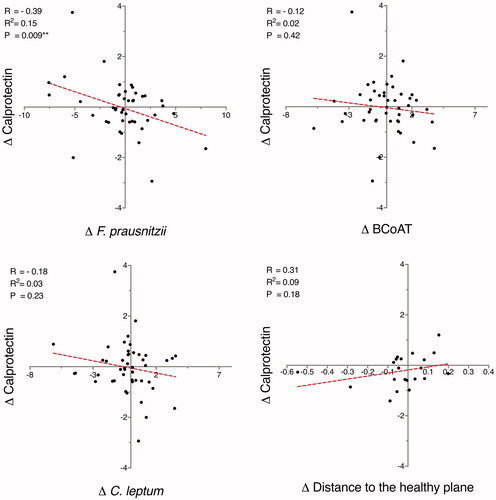
By analyzing alterations between adjacent samples, a negative correlation was observed between the relative abundance of F. prausnitzii and the concentration of f-calprotectin (R = −0.39; p = .009) (). The difference in relative abundance of F. prausnitzii between two subsequent samples accounted for 15% of the variation in f-calprotectin concentrations (R2 = 0.15). When alterations between consecutive samples were assessed, no significant correlation between the total capacity of microbiota to produce butyrate (copy numbers of the BCoAT gene) and f-calprotectin concentrations was observed (R = −0.12; p = .42) ().
Figure 3. By analyzing alterations between consecutive samples, a negative correlation between Δ F. prausnitzii and Δ calprotectin (µg/g) was observed. Δ values were defined as the difference between two adjacent time-points. The abundance of F. prausnitzii and the C. leptum group are expressed as fold change in relation to the total bacterial count, while butyryl-CoA:acetate-CoA transferase (BCoAT) is presented as gene copy numbers. F. prausnitzii, the C. leptum group, BCoAT and calprotectin are presented on the log-2 scale.
Comparisons of F. prausnitzii, the C. leptum group, and distance to the healthy plane
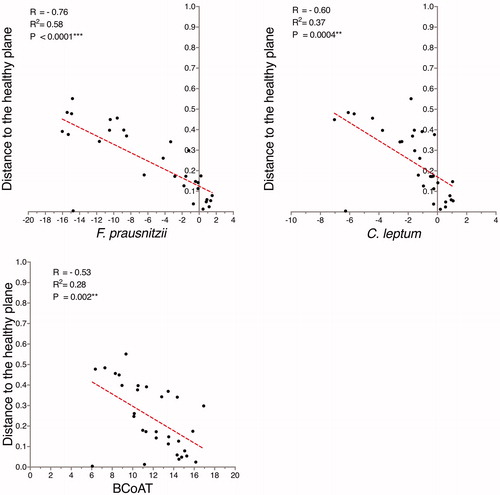
There was a negative correlation between the relative abundance of both F. prausnitzii (R = −0.76; p < .0001) and the C. leptum group (R = −0.60; p = .0004) and the distance to the healthy plane (). Similarly, there was a negative correlation between copy numbers of the BCoAT gene and the distance to the healthy plane (R = −0.53; p < .002) ().
Figure 4. There was a correlation between the abundance of F. prausnitzii and the C. leptum group and the distance to the healthy plane. Correspondingly, the gene copy numbers for butyryl-CoA:acetate-CoA transferase (BCoAT) were also correlated to the distance to the healthy plane, although this correlation was weaker. The abundance of F. prausnitzii and the C. leptum group are expressed as fold change in relation to the total bacterial count, while BCoAT is presented as gene copy numbers. F. prausnitzii, the C. leptum group, and BCoAT are presented on the log-2 scale.
Sensitivity analysis
In order to preclude bias due to use of antibiotics or changes in anti-inflammatory treatment, samples that were obtained within 4 weeks of initiation of antibiotics or introduction of a new anti-inflammatory drug were excluded (n = 5). The correlation coefficients for the dynamic comparisons of microbiota-related variables and inflammatory activity remained similar when these samples were excluded (Supplementary Figure 3), although the correlation between alterations in the relative abundance of F. prausnitzii and the concentration of f-calprotectin did not remain statistically significant (p = .06). In a consistent way, the sensitivity analyses did not change any of the other results and only resulted in small variations in the correlation coefficients and the R2-values (Supplementary Figures 1, 2, and 4).
Discussion
In this longitudinal study, we investigated the dynamic relationships between selected bacterial taxa, butyrate production, and the inflammatory activity in Crohn’s disease, by analyzing fecal samples that were collected over a period of up to two years. We found that temporal alterations in the relative abundance of F. prausnitzii, rather than overall butyrate production, were inversely correlated to changes in the inflammatory activity.
To our knowledge, this is the first study to report a temporal association between F. prausnitzii and f-calprotectin levels in Crohn’s disease. Although this association does not prove causation, it provides some support for the commonly stated hypothesis that F. prausnitzii is an important regulator of intestinal inflammation. As early as 2008, Sokol et al. reported a reduced abundance of F. prausnitzii in patients with Crohn’s disease [Citation15]. Since then, dysbiosis with a low abundance of F. prausnitzii has been confirmed in numerous Crohn’s disease populations from various geographic regions [Citation23,Citation29,Citation30], even though some data indicate that this mostly applies to patients with ileal Crohn’s disease [Citation22,Citation31]. However, this association should be interpreted with caution, since the dysbiosis may be an effect of the inflammation and a causal relationship between Crohn’s disease-associated dysbiosis and inflammation in humans is yet to be proven.
Although a recent meta-analysis indicated that the decrease in F. prausnitzii may be most pronounced in patients with clinically active Crohn’s disease, a correlation between abundance of specific bacterial taxa and inflammatory activity in patients with Crohn’s disease has not yet been shown. Most previous studies have been based on cross-sectional comparisons of cases and controls. Our longitudinal approach allowed us to analyze alterations in F. prausnitzii and f-calprotectin levels in individuals, based on consecutively collected samples. By using this design, we found that an increase in the relative abundance of F. prausnitzii was associated with a decrease in f-calprotectin levels. In contrast to the temporal association observed, we found no correlation between the relative abundance of F. prausnitzii and the concentration of f-calprotectin when single measurements were compared. This discrepancy may be explained by the fact that pronounced inter-individual differences have been reported for both f-calprotectin and microbial composition [Citation32,Citation33]. Compared to cross-sectional comparisons, our approach of analyzing temporal alterations in individuals will be independent of such subject-specific differences.
The significant correlation (regarding within-patient changes) observed between the relative abundance of F. prausnitzii and the gene copy numbers of BCoAT confirms that F. prausnitzii is a main contributor to overall butyrate production. Interestingly, there was no significant association between changes in the capacity of the microbiota to produce butyrate and changes in f-calprotectin levels. This finding is supported by a recent study by Laserna-Medieta et al., where no significant difference in the gene copy numbers of BCoAT was observed when Crohn’s disease patients were classified as active or inactive based on concentrations of f-calprotectin levels and using > 100µg/g as threshold for defining active disease [Citation14]. Similarly, we did not observe any association between changes in the relative abundance of the C. leptum group and changes in f-calprotectin levels. This may be due to dilution, since the C. leptum group, apart from F. prausnitzii, includes several other community members. Taken together, these findings indicate that the ability to hamper inflammation might be exclusive to F. prausnitzii and not shared by all other members of the C. leptum group, and that this ability is mediated by mechanisms other than butyrate production. This conclusion is also supported by data based on Caco-2 cells and in vivo 2,4,6-trinitrobenzene sulfonic acid (TNBS)-induced colitis in mice [Citation15]. Sokol et al. reported that the supernatant from F. prausnitzii both inhibited NF-κB activation in IL-1β-stimulated Caco-2 cells and effectively prolonged survival in the mouse model [Citation15]. When the Caco-2 cells and the mice were treated with butyrate in concentrations that corresponded to the butyrate-levels in F. prausnitzii supernatant, no effects on NF-κB-activity and survival rates were seen [Citation15]. This elegant experiment provides further support for the theory that molecules other than butyrate are responsible for the anti-inflammatory capacity of F. prausnitzii.
Intriguingly, Quévrain et al. recently identified a previously unknown protein (microbial anti-inflammatory molecule (MAM)) produced by F. prausnitzii [Citation34]. Transfection of epithelial cells with MAM cDNA inhibited NF-κB activation in a dose-dependent manner. Furthermore, MAM delivered by Lactococcus lactis had a protective effect on TNBS-induced colitis. This protein was also recently found to be present in human fecal samples [Citation35]. However, recent data indicate that butyrate may also play an important immunoregulatory role in IBD. Zhou et al. demonstrated that F. prausnitzii could regulate T helper 17 cell (Th17)/regulatory T-cell (Treg) differentiation in the TNBS-colitis model and that this effect was mediated by butyrate production [Citation36].
F. prausnitzii is one of the most prevalent species in the healthy microbiota and accounts for 2–15% of the total bacterial count [Citation37]. The representativeness of F. prausnitzii as a marker of a healthy gut microbiome was also illustrated by the correlation found between the relative abundance of this species and the distance to the healthy plane. Even in individuals with a low abundance of F. prausnitzii, the bacterium may contribute significantly to gut homeostasis through a compensatory increase in transcriptional activity [Citation38]. However, one may question whether reduced amounts of, or even depletion of a single species can have functional implications. Some researchers have proposed that F. prausnitzii is only a sensitive marker of dysbiosis, and that the immune response can be explained by a broader shift in microbial composition [Citation39]. However, no correlation between dysbiosis, defined as distance to the healthy plane, and f-calprotectin concentrations was found in our cohort. Although this may have been due to low statistical power, these findings further support the idea that it is F. prausnitzii and not dysbiosis per se that has an impact on intestinal inflammation.
The present study had several important limitations. Although 59 samples were analyzed, only nine patients with Crohn’s disease were included and absence of associations may, therefore, be explained by poor statistical power and may not necessarily reflect true negative findings. The abundance of bacterial taxa was assessed by analysis of fecal samples and not mucosal biopsies, since it was not possible to obtain ileal biopsies every third month. For the same reason, we had to rely on f-calprotectin as a proxy for intestinal inflammation instead of endoscopic evaluation. The study may also have been limited by the use of copies of the BCoAT gene as an indication of overall butyrate production, since the copy numbers may not truly reflect the luminal concentration of butyrate. However, direct measurement of butyrate has been shown to be less valid since active IBD interferes with butyrate absorption, and may result in an accumulation of butyrate in the gut lumen [Citation40–42]. In this study we used distance to healthy plane to assess dysbiosis, which has the advantage of clearly defining the normal volatility of the microbiota in healthy controls. However, the validity of this dysbiosis index is yet to be confirmed in independent study cohorts. Previous disease history, including surgical resections, and ongoing IBD-treatment may have influenced our results. Thus, the results of the present study should be validated in a larger cohort of treatment-naive patients who are included at diagnosis and followed over time.
In conclusion, we found that temporal changes in the relative abundance of F. prausnitzii, but not overall butyrate producing capacity, were inversely correlated to changes in the inflammatory activity in patients with ileal Crohn’s disease. Although these findings do not prove any causal relationship, they support the hypothesis that F. prausnitzii plays a key role in gut homeostasis and that this effect is mediated by mechanisms other than butyrate production.
Supplemental Materials
Download Zip (3.3 MB)Acknowledgments
A portion of this research was conducted under the Laboratory Directed Research and Development Program at PNNL, a multi- program national laboratory operated by Battelle for the U.S. Department of Energy under contract DE-AC05-76RL01830.
Disclosure statement
Jonas Halfvarson has received consultant/lecture fees from Abbvie, Celgene, Ferring, Hospira, Janssen, Medivir, MSD, Pfizer, Sandoz, Shire, Takeda, Tillotts Pharma, Janssen, MSD, Takeda, Tillotts and Vifor Pharma and grant support from Janssen, MSD and Takeda. Olle Björkqvist, Dirk Repsilber, Maike Seifert, Colin Brislawn, Janet Jansson, Lars Engstrand and Ignacio Rangel do not have any competing interests to disclose.
Additional information
Funding
References
- Sykes P. Small-bowel microflora in intestinal obstruction and Crohn's disease. Proc R Soc Med. 1976;69:325.
- Halfvarson J, Brislawn CJ, Lamendella R, et al. Dynamics of the human gut microbiome in inflammatory bowel disease. Nat Microbiol. 2017;2:17004.
- Gevers D, Kugathasan S, Denson LA, et al. The treatment-naive microbiome in new-onset Crohn’s disease. Cell Host Microbe. 2014;15:382–392.
- Schaubeck M, Clavel T, Calasan J, et al. Dysbiotic gut microbiota causes transmissible Crohn’s disease-like ileitis independent of failure in antimicrobial defence. Gut. 2016;65:225–237.
- Harper PH, Lee EC, Kettlewell MG, et al. Role of the faecal stream in the maintenance of Crohn's colitis. Gut. 1985;26:279–284.
- Zhulina Y, Hahn-Strömberg V, Shamikh A, et al. Subclinical inflammation with increased neutrophil activity in healthy twin siblings reflect environmental influence in the pathogenesis of inflammatory bowel disease. Inflamm Bowel Dis. 2013;19:1725–1731.
- Sillanpää S, Kramna L, Oikarinen S, et al. Next-generation sequencing combined with specific PCR assays to determine the bacterial 16S rRNA gene profiles of middle ear fluid collected from children with acute otitis media. mSphere. 2017;2:e00006–17.
- Thorburn F, Bennett S, Modha S, et al. The use of next generation sequencing in the diagnosis and typing of respiratory infections. J Clin Virol Off Publ Pan Am Soc Clin Virol. 2015;69:96–100.
- Smith CJ, Osborn AM. Advantages and limitations of quantitative PCR (Q-PCR)-based approaches in microbial ecology. FEMS Microbiol Ecol. 2009;67:6–20.
- Takahashi K, Nishida A, Fujimoto T, et al. Reduced abundance of butyrate-producing bacteria species in the fecal microbial community in Crohn's disease. Digestion. 2016;93:59–65.
- Segain JP, Raingeard de la Blétière D, Bourreille A, et al. Butyrate inhibits inflammatory responses through NFkappaB inhibition: implications for Crohn's disease. Gut. 2000;47:397–403.
- Canani RB, Costanzo MD, Leone L, et al. Potential beneficial effects of butyrate in intestinal and extraintestinal diseases. World J Gastroenterol. 2011;17:1519–1528.
- Louis P, Flint HJ. Development of a semiquantitative degenerate real-time PCR-based assay for estimation of numbers of butyryl-coenzyme A (CoA) CoA transferase genes in complex bacterial samples. Appl Environ Microbiol. 2007;73:2009–2012.
- Laserna-Mendieta EJ, Clooney AG, Carretero-Gomez JF, et al. Determinants of reduced genetic capacity for butyrate synthesis by the gut microbiome in Crohn’s disease and ulcerative colitis. J Crohns Colitis. 2018;12:204–216.
- Sokol H, Pigneur B, Watterlot L, et al. Faecalibacterium prausnitzii is an anti-inflammatory commensal bacterium identified by gut microbiota analysis of Crohn disease patients. Proc Natl Acad Sci USA. 2008;105:16731–16736.
- Wang Y, Gao X, Ghozlane A, et al. Characteristics of faecal microbiota in paediatric Crohn’s disease and their dynamic changes during infliximab therapy. J Crohns Colitis. 2018;12:337–346.
- Shaw KA, Bertha M, Hofmekler T, et al. Dysbiosis, inflammation, and response to treatment: a longitudinal study of pediatric subjects with newly diagnosed inflammatory bowel disease. Genome Med. 2016;8:75.
- Zhulina Y, Cao Y, Amcoff K, et al. The prognostic significance of faecal calprotectin in patients with inactive inflammatory bowel disease. Aliment Pharmacol Ther. 2016;44:495–504.
- Lennard-Jones JE. Classification of inflammatory bowel disease. Scand J Gastroenterol. 1989;24:2–6.
- Silverberg MS, Satsangi J, Ahmad T, et al. Toward an integrated clinical, molecular and serological classification of inflammatory bowel disease: report of a Working Party of the 2005 Montreal World Congress of Gastroenterology. Can J Gastroenterol. 2005;19:5A–36A.
- Salonen A, Nikkilä J, Jalanka-Tuovinen J, et al. Comparative analysis of fecal DNA extraction methods with phylogenetic microarray: effective recovery of bacterial and archaeal DNA using mechanical cell lysis. J Microbiol Methods. 2010;81:127–134.
- Willing B, Halfvarson J, Dicksved J, et al. Twin studies reveal specific imbalances in the mucosaassociated microbiota of patients with ileal Crohn’s disease. Inflamm. Bowel Dis. 2009;15:653–660.
- Sokol H, Seksik P, Furet JP, et al. Low counts of Faecalibacterium prausnitzii in colitis microbiota. Inflamm Bowel Dis. 2009;15:1183–1189.
- Barman M, Unold D, Shifley K, et al. Enteric salmonellosis disrupts the microbial ecology of the murine gastrointestinal tract. Infect Immun. 2008;76:907–915.
- Rangel I, Ganda Mall J-P, Willén R, et al. Degree of colitis correlates with microbial composition and cytokine responses in colon and caecum of Gαi2-deficient mice. FEMS Microbiol Ecol. 2016;92:fiw098.
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408.
- McMurdie PJ, Holmes S. Phyloseq: an R package for reproducible interactive analysis and graphics of microbiome census data. Plos One. 2013;8:e61217.
- Oksanen J, Guillaume Blanchet F, Kindt R, et al. Community Ecology Package [Internet]. 2015. Available from: http://cran.r-project.org, https://github.com/vegandevs/vegan.
- Swidsinski A, Loening-Baucke V, Vaneechoutte M, et al. Active Crohn’s disease and ulcerative colitis can be specifically diagnosed and monitored based on the biostructure of the fecal flora. Inflamm Bowel Dis. 2008;14:147–161.
- Joossens M, Huys G, Cnockaert M, et al. Dysbiosis of the faecal microbiota in patients with Crohn's disease and their unaffected relatives. Gut. 2011;60:631–637.
- Willing BP, Dicksved J, Halfvarson J, et al. A pyrosequencing study in twins shows that gastrointestinal microbial profiles vary with inflammatory bowel disease phenotypes. Gastroenterology. 2010;139:1844–1854.e1.
- Bashan A, Gibson TE, Friedman J, et al. Universality of human microbial dynamics. Nature. 2016;534:259–262.
- Sandborn WJ, Panés J, Zhang H, et al. Correlation between concentrations of fecal calprotectin and outcomes of patients with ulcerative colitis in a phase 2 trial. Gastroenterology. 2016;150:96–102.
- Quévrain E, Maubert MA, Michon C, et al. Identification of an anti-inflammatory protein from Faecalibacterium prausnitzii, a commensal bacterium deficient in Crohn’s disease. Gut. 2016;65:415–425.
- Quévrain E, Maubert M-A, Sokol H, et al. The presence of the anti-inflammatory protein MAM from Faecalibacterium prausnitzii, in the intestinal ecosystem. Gut. 2016;65:882–882.
- Zhou L, Zhang M, Wang Y, et al. Faecalibacterium prausnitzii produces butyrate to maintain Th17/Treg balance and to ameliorate colorectal colitis by inhibiting histone deacetylase 1. Inflamm Bowel Dis. 2018;24:1926–1940.
- Louis P, Flint HJ. Diversity, metabolism and microbial ecology of butyrate‐producing bacteria from the human large intestine. FEMS Microbiol Lett. 2009;294:1–8.
- Schirmer M, Franzosa EA, Lloyd-Price J, et al. Dynamics of metatranscription in the inflammatory bowel disease gut microbiome. Nat Microbiol. 2018;3:337–346.
- Hedin CR, van der Gast CJ, Stagg AJ, et al. The gut microbiota of siblings offers insights into microbial pathogenesis of inflammatory bowel disease. Gut Microbes. 2017;8:359–365.
- De Preter V, Arijs I, Windey K, et al. Impaired butyrate oxidation in ulcerative colitis is due to decreased butyrate uptake and a defect in the oxidation pathway. Inflamm Bowel Dis. 2012;18:1127–1136.
- Duffy MM, Regan MC, Ravichandran P, et al. Mucosal metabolism in ulcerative colitis and Crohn's disease. Dis Colon Rectum. 1998;41:1399–1405.
- Vogt JA, Wolever T. Fecal acetate is inversely related to acetate absorption from the human rectum and distal colon. J Nutr. 2003;133:3145–3148.
