Abstract
Aim: Doxorubicin-eluting beads transarterial chemoembolization (DEB-TACE) is reported to improve survival and tolerability when compared with conventional lipiodol-TACE (cTACE) for the treatment of hepatocellular carcinoma (HCC). The aim of this study was to evaluate tolerability and long-term survival in patients treated with cTACE or DEB-TACE in a real-life setting.
Methods: Incidence of adverse events and overall survival in HCC patients treated with either cTACE or DEB-TACE at Karolinska University Hospital 2004–2012 were analyzed retrospectively. Median follow-up was 7.1 years. Patients were censored when transplanted or at the end of follow-up. Patients receiving both cTACE and DEB-TACE, or treated with resection or ablation post-TACE were excluded from the survival analysis.
Results: A total of 202 patients (76 cTACE and 126 DEB-TACE) were eligible for analysis of adverse events, and 179 patients (69 cTACE and 110 DEB-TACE) were included in the survival analysis. cTACE patients were younger and had fewer tumors but higher BCLC stage than DEB-TACE. Child-Pugh and ECOG performance status were similar between groups. Adverse events (abdominal pain, nausea and vomiting, fever, fatigue) were significantly less common in the DEB-TACE group. Median survival was 17.1 months in the cTACE group and 19.1 months in the DEB-TACE (NS). In multivariate Cox regression analysis, portal vein thrombosis and tumor size were associated with increased, and sorafenib treatment post-TACE with decreased mortality.
Conclusion: In this retrospective real-life analysis, DEB-TACE had better tolerability compared to cTACE, but overall survival did not differ between the two treatments. Portal vein thrombosis, tumor size and sorafenib treatment after TACE influence survival.
Introduction
Hepatocellular carcinoma (HCC) is the sixth most common cancer worldwide, and the third leading cause of cancer mortality [Citation1]. Despite the use of ultrasound surveillance in at-risk populations, about 60% of patients with HCC are diagnosed at an intermediate or advanced stage [Citation2]. Curative treatments, such as hepatic resection, liver transplantation or ablative treatments offer a 5-year survival of 40–70%, but are only accessible for patients with early stage HCC, i.e. one single nodule, or up to 3 nodules less than 3 cm in diameter according to the Barcelona Clinic Liver Cancer classification system (BCLC stage 0 or A) [Citation3]. Patients with unresectable HCC who have preserved liver function without ascites, or absence of vascular invasion or extrahepatic spread, and without cancer-related symptoms, are classified as intermediate stage HCC (BCLC stage B) for which transarterial chemoembolization (TACE) is the recommended standard of care [Citation4]. In clinical practice, TACE is also used to treat early stage HCC, in cases having contraindications to curative treatment, or as a bridge to liver transplantation [Citation5].
TACE is based on the intra-arterial injection of a chemotherapeutic agent (e.g. doxorubicin) followed by embolization of the feeding artery, leading to the combination of a cytotoxic effect and ischemia [Citation6]. Injection is usually continued until stasis is achieved. TACE is a heterogeneous procedure in which different cytotoxic compounds, embolic agents, patient inclusion criteria and treatment schedules are applied [Citation7], resulting in varying efficacy outcomes [Citation8]. The most widely used technique is conventional TACE (cTACE) in which an emulsion of the cytotoxic drug and lipiodol (a di-iodinated long-chain ethyl ester of fatty acids) is injected in the feeding arteries to the HCC, followed by the embolic agent, e.g. gelatin sponge (gelfoam). HCCs are known to retain lipiodol, which in theory will facilitate the retention of the cytotoxic compound as well. However, a significant passage of chemotherapeutic compound into the systemic circulation has been demonstrated after cTACE [Citation9]. To overcome this systemic effect, and to standardize the embolic procedure, polyvinyl alcohol microparticles loaded with doxorubicin, so called drug-eluting beads (DEB) were developed [Citation10]. The particles embolize the feeding arteries while slowly releasing doxorubicin into the tumor, leading to sustained ischemia [Citation11] and prolonged drug delivery [Citation12].
Comparison between cTACE and DEB-TACE has been the object of several studies on overall survival (OS), tumor response, toxicity and side effects. Some retrospective studies indicate improved survival [Citation13–15] and increased tolerability [Citation9,Citation16–18] of DEB-TACE when compared with cTACE. Burrel et al. reported a median survival time exceeding 4 years after DEB-TACE in a well-selected patient cohort [Citation19]. A survival benefit of DEB-TACE could however not be confirmed in other retrospective [Citation18,Citation20,Citation21] or prospective [Citation22–25] trials. Five prospective studies [Citation10,Citation22–25] could not show a significant improvement in overall or progression-free survival. In the largest randomized controlled trial [Citation23] on 212 patients, the primary endpoint of improved tumor response 6 months after DEB-TACE was not met, although patients with more advanced disease showed a significantly better objective response.
One reason for the diverging results may be the fact that both cTACE and DEB-TACE generally lack standardized protocols [Citation11,Citation26]. Variables like patient selection, preparation of the emulsion, embolization endpoint, bead size, and whether the supernatant in the DEB syringe is used may differ from one center to the other.
Many previous retrospective real-life studies were performed on small cohorts or with a short follow-up time [Citation13,Citation15,Citation16,Citation18,Citation27]. Therefore, we conducted a study to compare long-term survival, adverse events and complications after treatment with cTACE and DEB-TACE in a real-life setting. At Karolinska University Hospital, cTACE was used in clinical routine until 2009, after which DEB-TACE was introduced. Both methods have thus been performed in the same interventional angiographic setting during the time studied. The aim of the study was to evaluate whether the switch from cTACE to DEB-TACE influenced long-term survival or the frequency of treatment-related adverse events.
Materials and methods
Patient selection
The study design was a retrospective, observational, single-center study on patients treated with cTACE or DEB-TACE at Karolinska University hospital between 1 January 2004 and 15 January 2013. Patients were identified by a computer search on the ICD-10 code C220 (liver cell carcinoma) and the intervention registry code PCT20 (transarterial chemoembolization). Data was retrieved until 28 November 2016, after which patients who were alive were censored.
Patient baseline data were collected at the time of decision on TACE treatment at the multidisciplinary therapy (MDT) conference. The diagnosis of HCC was based on radiological criteria consistent with HCC according to current guidelines at the time of diagnosis [Citation28–30]. In non-cirrhotic livers, tumor biopsy had been performed to establish the diagnosis of HCC.
Smoking was defined as none or current/former smoker. Body mass index (BMI) was calculated as (weight [kg]/height [m]2). Alcohol overconsumption was defined as having a diagnosis of alcoholic liver disease or statement of alcohol overconsumption in the patient charts; or an intake above 30 g/day (or 14 units per week) for males or 20 g/day (or 10 units per week) for females. Moderate alcohol consumption was defined as at least one unit per week. In case alcohol intake was not registered in the files the consumption was denoted as “unknown”.
Type 2 diabetes mellitus (T2DM) in a subject was defined when having a registered diagnosis in patient chart or having any anti-diabetic medication prescribed. Hypertension was similarily defined as a registered diagnosis or having any anti-hypertensive medication prescribed.
A diagnosis of cardiovascular disease (CVD) was given to subjects with a registered diagnosis of ischemic heart disease, cardiac myopathy, stroke, heart failure, or coronary valve disease in the patient files. Hypertension alone was not considered CVD.
Liver cirrhosis was defined as a registered diagnosis in the patient charts, based on previous liver biopsy, elastography value >15 kPa [Citation31] or typical imaging findings on radiology showing irregular liver parenchyma and portal hypertension.
In patients with cirrhosis, the Child-Pugh score was calculated at the time of the MDT conference. Performance status according to the The Eastern Cooperative Oncology Group (ECOG) was registered as reported on the MDT conference.
Patient charts were then scrutinized regarding post-treatment adverse events, side-effects and complications after the initial TACE treatment. If repeated TACE treatments were performed, only data from the first treatment were registered. Patients with insufficient documentation or incomplete procedures were excluded from the adverse event analysis.
Survival was evaluated from the time of the first TACE treatment until death or censoring (whichever occurred first). Reasons for censoring included liver transplantation or end of follow-up (28 November 2016). All subjects who had received treatment with both cTACE and DEB-TACE at different occasions were excluded from the survival analysis. Furthermore, subjects who had received treatment post-TACE with local ablation or liver resection were excluded.
Chemoembolization methods
In cTACE, an emulsion of lipiodol and doxorubicin was injected super-selectively or segmentally until a high grade of stasis was reached or a maximum dose of 50 mg doxorubicin was administered. Embolization was then obtained by injecting Gelfoam sponge fragments up to one millimeter in size. DEB-TACE was performed super-selectively as a rule, but in cases when this was not possible, it was performed segmentally. In the DEB-TACE group, the drug-eluting embolic agent was DC Beads® (Biocompatibles UK Ltd., Surrey, United Kingdom). These particles used were in the size of 100–300 and 300–500 µm. Each treatment was stopped when either complete stasis or a maximum doxorubicin dose of 150 mg was reached. In the cases in which the maximum doxorubicin dose was reached without complete stasis, unloaded 300–500 µm DC Beads particles were injected to reach complete embolization. Patients in both treatment groups underwent a computerized tomography (CT) scan or magnetic resonance imaging (MRI) 4 weeks after TACE to evaluate treatment effect according to WHO, EASL or mRECIST criteria depending on the preference of the radiologist at the time of evaluation, and the need of an additional TACE treatment was estimated. If residual tumor was found or a new nodule was detected, the patient was planned for additional TACE treatment. The decision to perform an additional TACE was made at the MDT conference where the post-treatment CT or MRI was demonstrated. In case of progressive disease, further TACE treatment was not performed. If complete response was achieved, the patient was planned for another CT or MRI control after 3 months.
Statistics
Pretreatment patient characteristics were compared between the treatment groups using Fisher’s exact test (binomial ordinal values), Chi2-test (polynomial ordinal values), independent samples t-test (means of numeric values) or Mann–Whitney U test (medians of numeric values). The presence of adverse effects after treatments was compared using Fisher’s exact test.
Cox regression models were used to estimate hazard ratios for all-cause mortality. The following factors were studied in the univariate analysis: age (years), sex (male), BMI (kg/m2), diabetes, liver function, tumor characteristics and given treatment (cTACE or DEB-TACE). Factors that showed a significant effect on survival in univariate survival analysis were included in a multivariate analysis together with a priori chosen factors suspected to be associated with increased mortality. The chosen factors were sorafenib treatment, bilobar disease, extrahepatic spread of HCC, macroscopic vessel growth, increased ECOG score, increased Child-Pugh class and alcohol overconsumption. The final model was corrected for age and sex. Kaplan-Meier analysis was used to calculate survival curves which were tested using log-rank test for significant differences. All statistics were calculated with SPSS version 23.0 for Macintosh.
Ethical approval
The study was approved by the regional ethics committee in Stockholm (reg no. 2010/1225-31/1).
Results
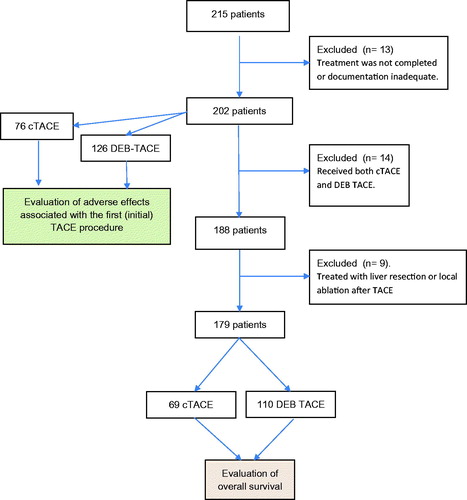
Patient enrollment is demonstrated in . We identified 215 patients treated with cTACE or DEB-TACE during the inclusion period. Of these, 13 patients were excluded due to inadequate documentation or incomplete treatment. Adverse events and side effects associated with the first TACE treatment were thus analyzed in 202 patients. Another 14/202 patients were excluded from the survival analysis since they had received both cTACE and DEB-TACE and 9 patients excluded due to treatment with liver resection or local ablation. Thus, the overall survival analysis was performed on 179 patients.
Figure 1. Enrollment of patients. Adverse events were evaluated after the first TACE procedure on 202 patients, and overall survival calculated on 179 patients who received either cTACE or DEB-TACE treatment (those receiving resection or ablation after TACE were excluded).
Patient characteristics are shown in . Patients in the DEB-TACE group were older and had a higher number of tumors (p < .05) compared to those in the cTACE group, whereas patients in the cTACE group had a more advanced stage of HCC. This included a larger proportion of patients having BCLC-class C, partially due to macrovascular invasion or extrahepatic spread, in the cTACE group compared to the DEB-TACE group (p < .05). Underlying diagnosis, presence of liver cirrhosis, Child-Pugh class and ECOG performance status were similar between the treatment groups.
Table 1. Baseline characteristics of the patient cohort evaluated for survival (n = 179).
Treatment characteristics are shown in . The mean time from diagnosis to start of TACE treatment was shorter, and the number of treatment sessions was higher, in the DEB-TACE group (p < .05). Pre-TACE ablation was more common in the cTACE group compared with the DEB-TACE group.
Table 2. Treatment characteristics of the patient cohort evaluated for survival (n = 179).
Adverse events are summarized in . Abdominal pain, nausea and vomiting, fever and fatigue were significantly less common in patients treated with DEB-TACE compared to cTACE (p < .05). There was no significant difference in the overall frequency of serious complications as bleeding, sepsis, ascites or jaundice (p = .15), but there was a trend for less bleeding from the puncture site in the DEB-TACE group (p = .06).
Table 3. Adverse events and treatment-related complications after the first TACE procedure in the cohort evaluated for adverse events (n = 202).
demonstrates similar all-cause mortality for the first 5 years of follow-up between patients treated with DEB-TACE when compared with cTACE (log-rank test p = .33). The median overall survival was 17.1 months in the cTACE group and 19.1 months in the DEB-TACE group (NS). Univariate Cox analysis of hazard ratios predictive of all-cause mortality in the whole patient cohort is demonstrated in . Total tumor size and number of tumors predicted a decreased overall survival. In the multivariate analysis (), adjusted hazard ratios demonstrated that tumor size and portal vein thrombosis were associated with decreased overall survival and sorafenib treatment after TACE with increased overall survival.
Figure 2. Kaplan–Meier survival curve for the first 5 years after treatment of the cTACE and DEB-TACE groups (log rank p = .33).
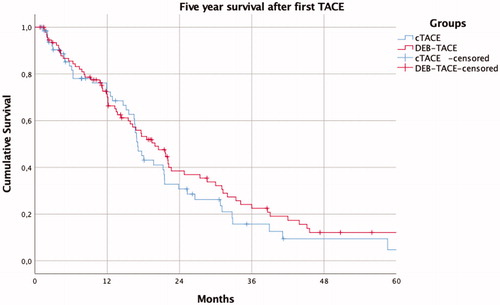
Table 4. Univariate Cox analysis of hazard ratios predictive of all-cause mortality.
Table 5. Multivariate Cox analysis of all-cause mortality.
Discussion
In this retrospective, observational, single-center study we compared survival and treatment-related adverse events in HCC patients who underwent treatment with either cTACE or DEB-TACE. The overall survival was similar between the treatment groups. Adverse events were significantly less common in subjects undergoing DEB-TACE, whereas the rate of complications (infection, ascites and jaundice) was similar. Large tumor size and portal vein thrombosis were associated with poor survival and patients treated with sorafenib after TACE had a somewhat better survival.
Both retrospective [Citation13–18,Citation20,Citation21,Citation27,Citation32,Citation33] and prospective [Citation22–25] studies have previously compared outcomes and side-effects between cTACE and DEB-TACE. Two retrospective studies [Citation13,Citation15] demonstrated improved survival after treatment with DEB-TACE, both of which were smaller and had a shorter follow-up time than the present one. Other comparative retrospective studies [Citation18,Citation20,Citation27,Citation32] showed a similar survival between cTACE and DEB-TACE, including two recent ones with longer follow-up and larger cohorts [Citation20,Citation32]. However, median survival differed greatly between these latter studies, from 14 to 16 months [20] to 37 months [Citation32]. These dissimilarities probably reflect different selection criteria and underlying etiologies [Citation34]. In fact, the use of very strict selection criteria have been shown to increase survival after TACE to up to 4 years [Citation19]. Three prospective, randomized studies failed to show a survival benefit [Citation22,Citation24] or improved tumor response [Citation23] for DEB-TACE. In contrast, two meta-analyses [Citation35,Citation36] found DEB-TACE to improve tumor response and survival compared to cTACE, whereas three other meta-analyses found similar response and survival following the two treatments [Citation37–39]. In the prospective Precision V trial [Citation23] and in a meta-analysis by Martin et al. [Citation40], DEB-TACE improved tumor response in the more advanced patients (Child-Pugh B, ECOG 1). Taken all these data together, there is no evidence in the current literature that DEB-TACE in general results in a survival benefit compared to cTACE, and our results from a real-life setting are well in line with this conclusion.
Most studies have reported an improved tolerability for DEB-TACE [Citation17,Citation18,Citation22,Citation23,Citation27,Citation40]. DEB-TACE leads to a reduced plasma peak concentration of doxorubicin and smaller area under the curve (AUC), possibly mitigating treatment-related adverse events [Citation9,Citation12,Citation25]. Our data also shows milder side effects and increased tolerability. There was a trend for a lower frequency of bleeding from the puncture site in the DEB-TACE group, which was probably due to the use of a closure device in the later years, instead of compression only.
Previous studies indicate that the embolization procedure is of greater importance for inducing tumor necrosis than the cytotoxic drug per se [Citation41,Citation42]. This suggests that the delivery system of the cytotoxic drug is of less importance when an embolization is performed. In the present study, doxorubicin was combined with embolization in both the cTACE and DEB-TACE procedures, why a potential additive effect of doxorubicin to embolization could not be evaluated.
The major strength of the present study is the long follow-up, allowing us to evaluate survival for 5 years after TACE treatment. Another strength is the real-life setting, which enables evaluation of the two methods in clinical practice.
Our study has some limitations. The first is the lack of randomization between groups, which differed regarding age, tumor size, and BCLC stage. Another limitation is the recruitment of the two cohorts during two different time periods, as a consequence of the switch from lipiodol-TACE to doxorubin-loaded bead-TACE in clinical practice at our hospital in 2009. However, the treatment procedures were comparable with respect to the angiographic setting and equivalent indications for treatment. In spite of this, we cannot exclude some bias due to improved skills, better administrative routines and technical improvements, such as the performance of the CT equipment, which would speak in favor of DEB-TACE. Despite this, we could not demonstrate an improved survival using DEB-TACE in this setting. Thus, the major improvement for our patients after the introduction of DEB-TACE is a reduced frequency of treatment-related adverse events.
In this study we could not compare tumor response after the two treatments, since response evaluation mainly had been performed with CT and not MRI. Parts of residual tumor tissue with arterial enhancement can be masked by the radiopaque lipiodol in the CT evaluations. Therefore, tumor response was not included in the current analysis.
Interestingly, sorafenib treatment after TACE was associated with an improved survival. This is possibly due to selection bias, since patients whose liver function detereriorate during TACE treatment will not be eligible for sorafenib treatment. Similar findings have been reported in previous observational studies evaluating the effect of sequential sorafenib treatment after TACE [Citation43].
In conclusion, we demonstrate that in a real-life setting DEB-TACE has better tolerability and fewer side-effects than lipiodol-TACE for the treatment of HCC, but long-term survival post-treatment does not differ. Large total tumor size and portal vein thrombosis are associated with a poorer survival.
Author’s contributions
P.S., S.W., and E.S. contributed in study conception and design. A.K., M.H, E.S., and P.S. contributed in acquisition of data. JT and MH contributed in statistical analysis. A.K., J.T., M.H., E.S., P.S., and R.A. contributed in analysis and interpretation of data. A.K., E.S., P.S., and R.A. contributed in drafting of manuscript. All contributed in critical revision. P.S. is the guarantor of article. All authors approved the final version of the article, including the authorship list.
| Abbreviations | ||
| BCLC | = | Barcelona Clinic Liver Cancer |
| cTACE | = | conventional transarterial chemoembolization |
| DEB-TACE | = | drug eluting beads transarterial chemoembolization |
| HCC | = | hepatocellular carcimona |
| OS | = | overall survival |
| PFS | = | progression-free survival |
Disclosure statement
The authors report no conflicts of interest.
Additional information
Funding
References
- Kulik L, El-Serag HB. Epidemiology and management of hepatocellular carcinoma. Gastroenterology 2019;156:477–491.e1.
- Edenvik P, Davidsdottir L, Oksanen A, et al. Application of hepatocellular carcinoma surveillance in a European setting. What can we learn from clinical practice? Liver Int. 2015;35:1862–1871.
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines: management of hepatocellular carcinoma. J Hepatol. 2018;69:182–236.
- Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet. 2018;391:1301–1314.
- Facciorusso A, Licinio R, Muscatiello N, et al. Transarterial chemoembolization: evidences from the literature and applications in hepatocellular carcinoma patients. WJH. 2015;7:2009–2019.
- Lencioni R, Petruzzi P, Crocetti L. Chemoembolization of hepatocellular carcinoma. Semin Inter Rad. 2013;30:3–11.
- Marelli L, Stigliano R, Triantos C, et al. Transarterial therapy for hepatocellular carcinoma: which technique is more effective? A systematic review of cohort and randomized studies. Cardiovasc Inter Rad. 2007;30:6–25.
- Raoul JL, Sangro B, Forner A, et al. Evolving strategies for the management of intermediate-stage hepatocellular carcinoma: available evidence and expert opinion on the use of transarterial chemoembolization. Cancer Treat Rev. 2011;37:212–220.
- Varela M, Real MI, Burrel M, et al. Chemoembolization of hepatocellular carcinoma with drug eluting beads: efficacy and doxorubicin pharmacokinetics. J Hepatol. 2007;46:474–481.
- Reyes DK, Vossen JA, Kamel IR, et al. Single-center phase II trial of transarterial chemoembolization with drug-eluting beads for patients with unresectable hepatocellular carcinoma: initial experience in the United States. Cancer J. 2009;15:526–532.
- Lencioni R, de Baere T, Burrel M, et al. Transcatheter treatment of hepatocellular carcinoma with Doxorubicin-loaded DC Bead (DEBDOX): technical recommendations. Cardiovasc Inter Rad. 2012;35:980–985.
- Lilienberg E, Dubbelboer IR, Karalli A, et al. In vivo drug delivery performance of lipiodol-based emulsion or drug-eluting beads in patients with hepatocellular carcinoma. Mol Pharm. 2017;14:448–458.
- Dhanasekaran R, Kooby DA, Staley CA, et al. Comparison of conventional transarterial chemoembolization (TACE) and chemoembolization with doxorubicin drug eluting beads (DEB) for unresectable hepatocelluar carcinoma (HCC). J Surg Oncol. 2010;101:476–480.
- Nicolini D, Svegliati BG, Candelari R, et al. Doxorubicin-eluting bead vs conventional transcatheter arterial chemoembolization for hepatocellular carcinoma before liver transplantation. WJG. 2013;19:5622–5632.
- Wiggermann P, Sieron D, Brosche C, et al. Transarterial Chemoembolization of Child-A hepatocellular carcinoma: drug-eluting bead TACE (DEB TACE) vs. TACE with cisplatin/lipiodol (cTACE). Med Sci Monit. 2011;17:CR189–95.
- Arabi M, BenMousa A, Bzeizi K, et al. Doxorubicin-loaded drug-eluting beads versus conventional transarterial chemoembolization for nonresectable hepatocellular carcinoma. Saudi J Gastroenterol. 2015;21:175–180.
- Liu YS, Ou MC, Tsai YS, et al. Transarterial chemoembolization using gelatin sponges or microspheres plus lipiodol-doxorubicin versus doxorubicin-loaded beads for the treatment of hepatocellular carcinoma. Korean J Radiol. 2015;16:125–132.
- Megias Vericat JE, Garcia Marcos R, Lopez Briz E, et al. Trans-arterial chemoembolization with doxorubicin-eluting particles versus conventional trans-arterial chemoembolization in unresectable hepatocellular carcinoma: a study of effectiveness, safety and costs. Radiologia 2015;57:496–504.
- Burrel M, Reig M, Forner A, et al. Survival of patients with hepatocellular carcinoma treated by transarterial chemoembolisation (TACE) using drug eluting beads. implications for clinical practice and trial design. J Hepatol. 2012;56:1330–1335.
- Kloeckner R, Weinmann A, Prinz F, et al. Conventional transarterial chemoembolization versus drug-eluting bead transarterial chemoembolization for the treatment of hepatocellular carcinoma. BMC Cancer 2015;15:465.
- Scartozzi M, Baroni GS, Faloppi L, et al. Trans-arterial chemo-embolization (TACE), with either lipiodol (traditional TACE) or drug-eluting microspheres (precision TACE, pTACE) in the treatment of hepatocellular carcinoma: efficacy and safety results from a large mono-institutional analysis. J Exp Clin Cancer Res. 2010;29:164.
- Golfieri R, Giampalma E, Renzulli M, et al. Randomised controlled trial of doxorubicin-eluting beads vs conventional chemoembolisation for hepatocellular carcinoma. Br J Cancer. 2014;111:255–264.
- Lammer J, Malagari K, Vogl T, et al. Prospective randomized study of doxorubicin-eluting-bead embolization in the treatment of hepatocellular carcinoma: results of the PRECISION V study. Cardiovasc Inter Rad. 2010;33:41–52.
- Sacco R, Bargellini I, Bertini M, et al. Conventional versus doxorubicin-eluting bead transarterial chemoembolization for hepatocellular carcinoma. J Vasc Interv Radiol. 2011;22:1545–1552.
- van Malenstein H, Maleux G, Vandecaveye V, et al. A randomized phase II study of drug-eluting beads versus transarterial chemoembolization for unresectable hepatocellular carcinoma. Onkologie. 2011;34:368–376.
- Cheng AL, Amarapurkar D, Chao Y, et al. Re-evaluating transarterial chemoembolization for the treatment of hepatocellular carcinoma: Consensus recommendations and review by an International Expert Panel. Liver Int. 2014;34:174–183.
- Massani M, Stecca T, Ruffolo C, et al. Should we routinely use DEBTACE for unresectable HCC? cTACE versus DEBTACE: a single-center survival analysis. Updates Surg. 2017;69:67–73.
- Bruix J, Sherman M, Llovet JM, et al. Clinical management of hepatocellular carcinoma. conclusions of the Barcelona-2000 EASL conference. European Association for the Study of the Liver. J Hepatol. 2001;35:421–430.
- Bruix J, Sherman M, Practice Guidelines Committee A. Management of hepatocellular carcinoma. Hepatology. 2005;42:1208–1236.
- Forner A, Vilana R, Ayuso C, et al. Diagnosis of hepatic nodules 20 mm or smaller in cirrhosis: prospective validation of the noninvasive diagnostic criteria for hepatocellular carcinoma. Hepatology. 2008;47:97–104.
- Bonder A, Afdhal N. Utilization of FibroScan in clinical practice. Curr Gastroenterol Rep. 2014;16:372
- Liu YS, Lin CY, Chuang MT, et al. Five-year outcome of conventional and drug-eluting transcatheter arterial chemoembolization in patients with hepatocellular carcinoma. BMC Gastroenterol. 2018;18:124
- Song MJ, Chun HJ, Song DS, et al. Comparative study between doxorubicin-eluting beads and conventional transarterial chemoembolization for treatment of hepatocellular carcinoma. J Hepatol. 2012;57:1244–1250.
- Fako V, Wang XW. The status of transarterial chemoembolization treatment in the era of precision oncology. Hepat Oncol. 2017;4:55–63.
- Huang K, Zhou Q, Wang R, et al. Doxorubicin-eluting beads versus conventional transarterial chemoembolization for the treatment of hepatocellular carcinoma. J Gastroenterol Hepatol. 2014;29:920–925.
- Zou JH, Zhang L, Ren ZG, et al. Efficacy and safety of cTACE versus DEB-TACE in patients with hepatocellular carcinoma: a meta-analysis. J Dig Dis. 2016;17:510–517.
- Facciorusso A. Drug-eluting beads transarterial chemoembolization for hepatocellular carcinoma: current state of the art. WJG. 2018;24:161–169.
- Gao S, Yang Z, Zheng Z, et al. Doxorubicin-eluting bead versus conventional TACE for unresectable hepatocellular carcinoma: a meta-analysis. Hepatogastroenterology. 2013;60:813–820.
- Han S, Zhang X, Zou L, et al. Does drug-eluting bead transcatheter arterial chemoembolization improve the management of patients with hepatocellular carcinoma? A meta-analysis. PloS One. 2014;9:e102686.
- Martin R, Geller D, Espat J, et al. Safety and efficacy of trans arterial chemoembolization with drug-eluting beads in hepatocellular cancer: a systematic review. Hepatogastroenterology. 2012;59:255–260.
- Brown KT, Do RK, Gonen M, et al. Randomized trial of hepatic artery embolization for hepatocellular carcinoma using doxorubicin-eluting microspheres compared with embolization with microspheres alone. JCO. 2016;34:2046–2053.
- Llovet JM, Real MI, Montana X, et al. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet. 2002;359:1734–1739.
- Liu F, Meng Z, Shao G, et al. Patterns of sorafenib and TACE treatment of unresectable hepatocellular carcinoma in a Chinese population: subgroup analysis of the GIDEON study. Mol Biol Rep. 2017;44:149–158.
