Abstract
Objective: The occurrence of Helicobacter pylori (HP) and clarithromycin resistant HP (crHP) have not been investigated longitudinally in the Swedish childhood population. The aims were to study the prevalence of gastric HP-DNA and crHP strains in a cohort of children living in the southernmost parts of Sweden who were investigated with upper endoscopy between 2005 and 2016.
Methods: HP colonisation and crHP in 1768 children who underwent 1887 endoscopic procedures with gastric biopsies, of whom 393 were referred for abdominal pain, gastritis, ulcer or gastrointestinal bleeding (Group I) and 1494 were referred for other reasons (Group II). The occurrence of HP-DNA from gastric biopsies was given as a global prevalence as information on previous eradication were missing.
Results: The global prevalence of HP-DNA was 222/1887 (11.8%; 95% CI 10.4%–13.3%) of which 46/222 (20.7%; 95% CI 15.9%–26.5%) were crHP. The prevalence of HP-DNA in Group I was 141/393 (35.9%; 95% CI 31.3%–40.7%), which was higher compared with that of 81/1494 (5.4%; 95% CI 4.4%–6.7%) in Group II (p < .0001). crHP strains occurred equally frequent in the biopsies in both groups and found in 29/141 (20.6%; 95% CI 14.7%–28.0%) in Group I and 17/81 (21.0%; 95% CI 13.5%–31.1%) in Group II, respectively (p > .9999).
Conclusions: More than one in every ten (12%) children investigated with upper endoscopy in the southernmost parts of Sweden were gastric HP-DNA positive of which 21% were crHP regardless of indication for investigation. Clarithromycin is therefore not recommended as first line empirical treatment for eradicating an HP infection in children.
Introduction
Antibiotic resistance in Sweden is expected to be low as compared to many other European countries, presumably due to a more restrictive prescription of antibiotics in the general population [Citation1,Citation2]. Clarithromycin is commonly used in the first line antibiotic treatments of Helicobacter pylori (HP) gastritis in Sweden [Citation3–5]. However, the prevalence of clarithromycin-resistant HP (crHP) strains is rising worldwide [Citation6]. In a European multicentre study comprising of 18 countries, the prevalence of crHP was estimated at 32% in children and 18% in adults, although the prevalence was lower in adults in Northern Europe at 8% [Citation7]. A recent study from Iceland found a prevalence of crHP in treatment of naïve patients at 6% [Citation8]. The estimates of crHP strains in adults have been estimated to 2.8% in 1998 [Citation9] and 1.5% in 2006 [Citation10], respectively.
The Swedish guidelines for treating HP in children [Citation5], which closely follow the joint guidelines of the European Society for Paediatric Gastroenterology Hepatology and Nutrition (ESPGHAN) and North American Society for Paediatric Gastroenterology, Hepatology and Nutrition (NASPGHAN) [Citation4], recommend that no eradication is commenced before an upper endoscopy with biopsies is performed to confirm diagnosis of HP. Albeit crHP strains is expected to be low in Sweden, the prevalence still remains unknown.
The objectives of this study were to estimate the global prevalence of HP as well as the occurrence of crHP in children living in the southernmost parts of Sweden who were referred to a tertiary department of paediatrics for an investigation with an upper endoscopy between 2005 and 2016.
Methods
Study population
Scania is the southernmost county of Sweden, with a population of 1.3 million including 290 000 children and adolescents [Citation11]. The Department of Paediatrics at Scania University Hospital in Malmö, Sweden, is a tertiary clinic that serves 170,000 children in the southwestern part of Scania. In the present study, medical records of children who had been investigated with an upper endoscopy between January 2005 and December 2016 were retrospectively reviewed with regard to HP-DNA presence and clarithromycin resistance in biopsies. During this time period, 2085 upper endoscopies were performed on 1866 children at a median 10.1 years of age (range 0.6–18.0). Excluded were investigations with an upper endoscopy without biopsies taken for HP (n = 164), those with failed microbiological analysis of HP (n = 7), of which four were due to insufficient tissue in biopsies and three failed with no reason stated. Children who were investigated more than once within the same calendar year had their second endoscopic investigation excluded from the analysis (n = 27), leaving 1887 completed upper endoscopies with biopsies for HP analysis (90.5%) performed on 1768 individual children for the final year by year analysis, some of which performed additional endoscopies with biopsies during the study period. The number of annual endoscopic investigations given were equal to the number of unique children, but as some children had additional endoscopies being performed in subsequent years, the overall number of endoscopies for the whole period exceeded the total number of unique children.
The primary indication stated by the referring doctor was used to stratify the material into two groups. The first group (Group I, n = 393), constituted of endoscopic investigations primarily referred for abdominal pain (n = 191), gastritis (n = 157), ulcer (n = 20) or gastrointestinal bleeding (n = 25) at median 12.3 (range 1.0–17.9) years of age (224 females, 169 males). The second group served as a non-HP disease control group (Group II) and constituted of endoscopic investigations referred for other reasons (n = 1494) at a median age 9.7 (range 0.6–18.0) years of age (823 females, 671 males) of which coeliac disease, gastroesophageal reflux disease (GERD) and inflammatory bowel disease (IBD) were most common. Since children in Group II were not expected to be colonised by HP in a higher degree than the general population, it was used as an approximation of the paediatric population in the region.
As it can be argued that the prevalence of HP colonisation and crHP only should include each child once, we also analysed the data including only the first endoscopy for each child, thereby excluding 119 subsequent investigations, leaving 1768 investigations on 1768 unique children, of which 375 were performed in Group I and 1393 in Group II, respectively.
All children in the study were anonymized by means of random numbers and assigned an identification number (ID), and the file with the key connecting them to the ID is kept under lock and key, protecting their privacy. Ethical approval to retrieve this information from the medical records was granted by the Regional Ethical Review Board at Lund University.
Endoscopic procedures and detection of Helicobacter pylori
All parents to children scheduled for endoscopy were instructed to stop giving their children PPI at least two weeks prior to the procedure. Standard upper endoscopy was performed under general anaesthesia or deep sedation with Propofol biopsies for HP analysis were collected from the ventricle of which two were from the antrum and two were from the corpus, respectively. Biopsies were analysed with the polymerase chain (PCR), with amplification and detection of H. pylori DNA and known resistance genes as previously described [Citation12]. The DNA extraction was performed with Magna Pure LC (Roche Diagnostics, Tokyo, Japan) and the amplification and melting analysis was performed using the LightCycler® (Roche Diagnostics, Tokyo, Japan). The primers used (CRFL-1 and CRRL-2), had identical sequences as the 23S rRNA reported in GenBank (accession number U27270) for HP. Wild type DNA was detected with wild-type specific probes (MP-W), and A to G mutations in nucleotide positions 2143 and 2144 were detected with specific probes (MP-2143 and MP-2144). The LIASON H. pylori Stool Antigen (HPSA) test, a chemiluminescence immunoassay, was used for detecting H. pylori in stool samples [Citation13].
Statistical analysis
Group comparisons were evaluated with Fisher’s exact test on frequencies, and Mann-Whitney U test on age differences. Linear regression was used to investigate if a trend over time existed in the prevalence of HP infection and crHP, respectively. Confidence intervals were calculated with the Wilson-Brown method recommended in the statistical software. The data was analysed using the GraphPad Prism 8 statistical software. A p-value <.05 was considered significant.
Results
HP-DNA was overall detected in 222/1887 (11.8%; 95% CI 10.4%–13.3%) biopsies of the completed investigations (). In Group I, HP-DNA was found in 141/393 (35.9%; 95% CI 31.3%–40.7%), which was higher compared with 81/1494 (5.4%; 95% CI 4.4%–6.7%) in Group II (p < .0001). In the investigations found positive for HP-DNA, 46/222 (20.7%; 95% CI 15.9%–26.5%) were clarithromycin resistant. Among those, 29/141 (20.6%; 95% CI 14.7%–28.0%) were found in Group I compared with 17/81 (21.0% (95% CI 13.5%–31.1%) in Group II (p > .9999). Using linear regression by year of the investigation, there was neither a difference in overall prevalence of HP-DNA nor prevalence of crHP over time (). There were no gender differences in prevalence of HP-DNA, which was detected in 116/1047 (11.1%; 95% CI 9.3%–13.1%) female cases and in 106/840 (12.6%; 95% CI 10.5%–15.0%) male cases (p = .3147). Furthermore, crHP was found in 30/116 (25.9%; 95% CI 18.8%–34.5%) in the female cases compared to 16/106 (15.1%; 95% CI 9.5%–23.1%) in the male cases (p = .0675).
Figure 1. Prevalence of helicobacter pylori (HP) and clarithromycin-resistant HP (crHP) strains detected in gastric biopsies of children investigated with upper endoscopy between 2005 and 2016. Children in Group I were primarily investigated for abdominal pain, gastritis, ulcer and gastrointestinal bleeding and children in Group II were investigated for other indications. (A) Number of investigations. (B) Overall prevalence of H. pylori . (C) Prevalence of H. pylori in Group I and II. (D) Prevalence clarithromycin-resistant H. pylori. HP or H. pylori: Helicobacter pylori; HP–: not colonised with HP; HP+: colonised with HP; Subscripts: Sens: clarithromycin-sensitive strain; Res: clarithromycin-resistant strain.
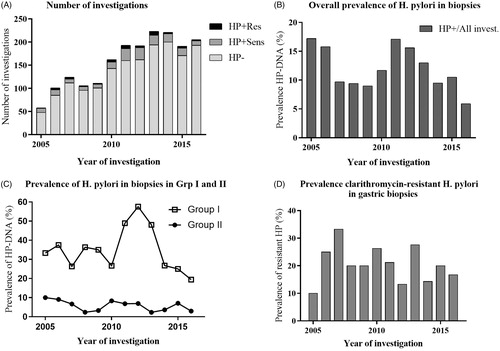
In the subset excluding all but the first endoscopic investigation for each child for the entire period 2005–2016, HP-DNA was detected in 213/1768 (12.0%; 95% CI 10.6%–13.6%) of the children. In Group I, HP-DNA was present in 135/375 (36.0%; 95% CI 31.3%–41.0%) compared with 78/1393 (5.6%; 95% CI 4.5%–6.9%) in Group II (p < .0001).
In the children found positive for HP-DNA, 43/213 (20.2%; 95% CI 15.3%–26.1%) were crHP. Among those children, 26/135 (19.3%; 95% CI 13.5%–26.7%) were found in Group I compared with 17/78 (21.8% (95% CI 14.1%–32.2%) in Group II, respectively (p > .9999). Data of prior HPSA testing were available from children investigated between 2013 and 2016 and had been performed in 224/840 (26.7%; 95% CI 23.8%–29.8%) of the investigations, whereof 124/180 (68.9%; 95% CI 61.8%–75.2%) in Group I compared with 100/660 (15.2%; 95% CI 12.6%–18.1% in Group II, respectively (p < .0001). There were a total of 82 endoscopies with positive HP-DNA between 2013 and 2016, of which 57 had a prior positive HPSA (69.5%; 95% CI 58.9%–78.2%), whereof 54/56 (96.4%; 95% CI 87.9%–99.4%) in Group I compared with 3/26 (11.5%; 95% CI 4.0%–29.0%) in Group II, respectively (p < .0001).
Discussion
The present study demonstrated a 12% prevalence of gastric HP-DNA positivity in children living in the southernmost parts of Sweden referred to a tertiary university hospital for an upper endoscopy between 2005 and 2016, which is in line with reports from North of Stockholm (11.3% in histology data) [Citation14]. We further found a 21% global prevalence of crHP regardless of indication for endoscopy, and this level was consistent between the groups. A 21% prevalence of crHP in children is higher than previously reported on adults from the West coast of Sweden (2.8%) [Citation9], and from the northern part of Sweden (1.5%) [Citation10]. It could be speculated that this difference in prevalence of crHP may be attributed to an increasing trend over time consistent with findings in many countries worldwide [Citation6]. Another plausible explanation could be a regional variation in occurrence of HP in Sweden due to demography and a higher antibiotic prescription rate in the South of Sweden [Citation15].
Despite a high prevalence of crHP in the southernmost part of Sweden, there was no significant change over time, as opposed to the increase seen in other parts of the World [Citation6]. A stricter adherence to guidelines with upper endoscopy and microbial susceptibility determination before eradication of HP in children may be contributing factors to maintain a low prevalence of crHP. Using clarithromycin as empirical treatment for eradicating HP is not recommended to populations with a prevalence of crHP exceeding 15% [Citation16]. In accordance with the Swedish national guidelines for adults [Citation17], other antimicrobials such as metronidazole should instead be considered as a first line treatment for eradication along with amoxicillin and a proton pump inhibitors in select cases. For successful treatment, it is therefore recommended to do HP eradication according to the antibiotic resistance pattern from gastric biopsies and not merely based non-invasive testing in order to achieve a high successful eradication rate.
The strength of the study is the high number of consecutively investigated children analysed for gastric HP-DNA over a long period (90% of all the endoscopic investigations), which makes the results very accurate for this group. A limitation of the study is the risk of selection bias not mirroring the general population, which makes it difficult to estimate the true prevalence of HP in the general population. As a proxy of the general population, we therefore attempted to estimate the prevalence of HP in children not primarily investigated for HP (Group II), however, the result of prior HPSA testing seems to be a determining factor for the indication chosen by the referring doctor, and the prevalence in Group II is therefore probably an underestimation, and for Group I, an overestimation is likely based on the fact that more than 95% of those with HP in biopsies had a prior positive HPSA test result between 2013 and 2016. The prevalence of gastric HP-DNA amongst children not primarily investigated for HP-related indications and estimated at 5.4% is close to the prevalence of 6.3% in histology data found in individuals aged 20–45 years from Stockholm [Citation14]. Still, it is probably biased despite our efforts to avoid that, and the overall prevalence of 11.8% in our study is most likely a better approximation, compared with the all age groups prevalence of 11.3% in histology in the Stockholm study [Citation14]. Another weakness is that lack of information about previous antibiotic use, including eradication, and the migrant status of the participating children originating from other countries, which both would select for increase in crHP [Citation18]. As previous antibiotic treatment was unknown, primary or secondary resistance could not be determined. Thus, all reported prevalence rates for crHP were considered as global and regarded with caution. Yet, only two other previous Swedish studies on prevalence of crHP have been performed, none of which addressed children or the southern part of Sweden.
Conclusion
The present study found more than one in every ten (12%) children investigated with upper endoscopy in the southernmost parts of Sweden to be positive for gastric HP-DNA of which 21% were crHP regardless of indication for investigation. This is well over the threshold for using clarithromycin as first line empirical treatment for eradicating HP in children. Clarithromycin is therefore, without microbial susceptibility determination, not recommended as first line empirical treatment for eradicating an HP infection in children.
Acknowledgments
The study was logistically supported (e.g., office space and access to secure computers) by the Faculty of Medicine, Lund University and the Skåne County Council of Research and Development, but no monetary grant was issued.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- European Centre for Disease Prevention and Control. Antimicrobial consumption – Annual Epidemiological Report for 2016. Stockholm: ECDC; 2018 [cited 2019 Jun 27]. Available from: https://ecdc.europa.eu/en/publications-data/antimicrobial-consumption-annual-epidemiological-report-2016#no-link.
- Molstad S, Lofmark S, Carlin K, et al. Lessons learnt during 20 years of the Swedish strategic programme against antibiotic resistance. Bull World Health Organ. 2017;95:764–773.
- Chey WD, Leontiadis GI, Howden CW, et al. ACG clinical guideline: treatment of Helicobacter pylori infection. Am J Gastroenterol. 2017;112:212–239.
- Jones NL, Koletzko S, Goodman K, et al. Joint ESPGHAN/NASPGHAN guidelines for the management of Helicobacter pylori in children and adolescents (Update 2016). J Pediatr Gastroenterol Nutr. 2017;64:991–1003.
- Casswall T, Arnell H. Vårdprogram för Helicobacter pylori hos barn [Guidelines for Helicobacter pylori treatment in children] [pdf]. Svenska Föreningen För Pediatrisk Gastroenterologi, Hepatologi Och Nutrition (SPGHN); 2013 [cited 2019 Jun 27]. Available from: http://www.gastro.blf.net/vardprogram/vardprogram_reg.html
- Thung I, Aramin H, Vavinskaya V, et al. Review article: the global emergence of Helicobacter pylori antibiotic resistance. Aliment Pharmacol Ther. 2016;43:514–533.
- Megraud F, Coenen S, Versporten A, et al. Helicobacter pylori resistance to antibiotics in Europe and its relationship to antibiotic consumption. Gut. 2013;62:34–42.
- Gunnarsdottir AI, Gudjonsson H, Hardardottir H, et al. Antibiotic susceptibility of Helicobacter pylori in Iceland. Infect Dis (Lond). 2017;49:647–654.
- Jaup BH, Brandberg A, Stenquist B, et al. Antibiotic resistance among strains of Helicobacter pylori in Gothenburg. Bacteria resistant to metronidazole. Lakartidningen. 1998;95:279–281.
- Storskrubb T, Aro P, Ronkainen J, et al. Antimicrobial susceptibility of Helicobacter pylori strains in a random adult Swedish population. Helicobacter. 2006;11:224–230.
- Statistics Sweden. Population in 1-year age intervals for children 0-17 in Scania County 2017. 2018. Statistics Sweden. [cited 2019 Jun 27]. Available from: http://www.statistikdatabasen.scb.se/sq/57937.
- Matsumura M, Hikiba Y, Ogura K, et al. Rapid detection of mutations in the 23S rRNA gene of Helicobacter pylori that confers resistance to clarithromycin treatment to the bacterium. J Clin Microbiol. 2001;39:691–695.
- Ramirez-Lazaro MJ, Lite J, Lario S, et al. Good diagnostic accuracy of a chemiluminescent immunoassay in stool samples for diagnosis of Helicobacter pylori infection in patients with dyspepsia. J Investig Med. 2016;64:388–391.
- Agreus L, Hellstrom PM, Talley NJ, et al. Towards a healthy stomach? Helicobacter pylori prevalence has dramatically decreased over 23 years in adults in a Swedish community. United European Gastroenterol J. 2016;4:686–696.
- The Public Health Agency of Sweden. Patientsäkerhetssatsning 2014 – Utvärdering av antibiotikaförskrivning och landstingens arbete för ökad följsamhet till lokala behandlingsrekommentationer. The Public Health Agency of Sweden. 2014 [cited 2019 Jun 27]. Available from: https://www.folkhalsomyndigheten.se/contentassets/c7382894996946c3a90f7dbb514cd9f2/patientsakerhetssatsning-2014.pdf.
- Graham DY, Lee YC, Wu MS. Rational Helicobacter pylori therapy: evidence-based medicine rather than medicine-based evidence. Clin Gastroenterol Hepatol. 2014;12:177–186.e3; Discussion e12-3.
- Agreus L, Lööf L, Simren M, et al. Nationell riktlinje 2016: Outredd dyspepsi, okomplicerade duodenal- och ventrikelsr samt funktionell dyspepsi [National guidelines 2016: Uninvestigated dyspepsia, uncomplicated duodenal and ventricular ulcer and functional dyspepsia]. 2016 [cited 2019 Jun 27]. Available from: http://www.svenskgastroenterologi.se/sites/default/files/outredd_dyspepsi_h_pylori_ulkus_161118.pdf
- Koletzko S, Richy F, Bontems P, et al. Prospective multicentre study on antibiotic resistance of Helicobacter pylori strains obtained from children living in Europe. Gut. 2006;55:1711–1716.
